Abstract
To investigate the impact of hypoxia on the expression of receptor activator of NF-kB ligand (RANKL) and osteoprotegerin (OPG) in human periodontal ligament cells (hPDLCs) in vitro. hPDLCs were incubated in a hypoxic atmosphere of 2% O2, 5% CO2, 94% N2 at 37°C for 6, 12, 24 and 48 h. After that, cell proliferation assay was determined using CCK-8 technique. SP immunocytochemistry method was performed to trace the expression of hypoxia-inducible factor 1 alpha (HIF-1α) in hPDLCs. The expression levels of RANKL and OPG were investigated using real-time PCR and ELISA. As a control, the cells were incubated at normoxic conditions of 20% O2, 5% CO2, 75% N2. All results were analyzed using one-way ANOVA at a significant level of P=0.05. OPG mRNA and protein levels were down-regulated meanwhile RANKL mRNA and soluble RANKL (sRANKL) protein levels were up-regulated after stimulated by hypoxia. The relative RANKL/OPG expression ratios were increased in both mRNA and protein levels. The expression of RANKL mRNA and sRANKL protein levels was enhanced significantly (P<0.05) under the hypoxia conditions at 12 h, 24 h and 48 h while OPG mRNA and protein were reduced significantly (P<0.05) at 12 h, 24 h and 48 h. Hypoxia can affect the expression of RANKL and OPG in hPDLCs, which constitute an important pathogenic event in the alveolar bone resorption. Lack of oxygen in periodontal tissue may accelerate the development of periodontitis.
Keywords: Hypoxia, hPDLCs, OPG, RANKL
Introduction
Hypoxia (also known as hypoxiation or anoxemia) is a condition in which the body tissues and cells are deprived of adequate oxygen supply [1]. Our previous study have showed hypoxia can change the expression of matrix metalloproteinase (MMP) and tissue inhibitors of matrix metalloproteinase (TIMP) mRNA and also lead to the imbalance of MMP-2/TIMP-2 mRNA expression, which may be closely correlated with the occurrence and development of periodontal disease and play an important role in the process of periodontal tissue destruction in periodontitis [2]. However, it is not clear whether hypoxia affects the expression of RANKL/OPG of human periodontal ligament cells which is closely associated with the destruction of alveolar bone in periodontitis [3].
Alveolar bone loss is mediated by osteoclasts. Receptor activator of NF-κB ligand/receptor activator of NFκB/osteoprotegerin (RANKL/RANK/OPG) are the key cytokines that regulate this process. RANKL can trigger osteoclast differentiation and activation via a series of enzyme cascade, through its cognate receptor or activator of nuclear factor-KB (RANK), which is the only receptor on osteoclast precursors. OPG is a secreted protein that acts as a decoy receptor for RANKL, inhibiting bone resorption through binding to RANKL, and blocking it from interacting with its receptor RANK [4]. It is hypothesized that hypoxia affects the expression levels of RANKL and OPG as well as their relative expression ratio in periodontal ligament cells (PDLCs), which may influence bone remodeling process on root surfaces. Hence, this study aims to investigate the effect of hypoxia on the expression and release of RANKL and OPG in human PDLCs.
Materials and methods
Cell culture
Healthy human premolars were extracted under local anesthesia for orthodontic treatment, and were washed thrice with phosphate buffered saline (PBS, pH 7.3). PDLs were obtained from the middle third of the root surfaces with a surgical scalpel. The primary PDLs were maintained in Dulbecco’s modified eagle’s medium (DMEM; Gibco, USA) containing 20% fetal bovine serum (FBS; Hyclone, Beijing, China) and antibiotic solution (100 U mL-1 penicillin and 100 μg ML-1 streptomycin) under a humidified atmosphere of 20% O2, 5% CO2, and 75% N2 at 37°C. After confluence, the cells were detached with 0.25% typsin (Difco laboratories, Detroit, MI, USA) and subcultured in DMEM containing 10% FBS and antibiotic solution. The medium was changed every 48 h and cultures between fifth and seventh passages were used for the experiments. The study was approved by the ethics committee of college of stomatology, Shandong university, and informed consent was obtained from each patient.
Cell proliferation assay
The hPDLCs (5×103/well) were seeded in 96-well plates and cultured under non-differentiating conditions. At 6, 12, 24 and 48 h, cell proliferation was measured using Cell Counting Kit-8 (CCK-8, Dojindo, Japan). Briefly, 10 μl of CCK-8 solution was added to each well of the 96-well plates, which were incubated for 2 h in a standard incubator. Then, the absorbance was measured at 450 nm, and a proliferation curve was generated based on the absorbance and time.
Immunocytochemical detection
The cells were washed 3 times with 0.01 M PBS and fixed with 4% PFA (paraformaldehyde) for 10 min at room temperature. The cells were immersed in 3% H2O2 for 20 minutes at 37°C and incubated with 1.5% blocking serum in PBS to prevent nonspecific staining. Then, rabbit anti-human HIF-1α primary antibody (dilution 1:200; Santa Cruz Biotechnology Inc, Santa Cruz, CA) was added, and the cells were incubated for 12 hours at 4°C. Subsequently, cells were incubated with goat anti-rabbit secondary antibody for 1 hour at 37°C and were processed for enzymatic immunohistochemical staining using a broad spectrum immunoperoxidase DAB kit (Zhongshan Biotechnology Co, Beijing, China) according to the manufacturer’s protocol. Finally, the cells were counterstained with hematoxylin and examined under a microscope.
Real-time PCR analysis
Real-time PCR was used to determine the mRNA transcription levels of OPG and RANKL genes. Total RNA was extracted from the cells with RNAiso Plus (Takara Bio, Shiga, Japan) at different periods of time. First-strand complementary DNA was synthesized from 1 μg total RNA by reverse transcription using the PrimeScript RT Reagent Kit with gDNA Erase (Takara Bio). Real-time polymerase chain reaction amplifications labeled with SYBR Premix Ex Taq (Takara Bio) were performed in a Roche 480 Light Cycler (Roche, Mannheim, Germany) at 95°C for 30 s, 95°C for 5 s and 60°C for 30 s for a total of 40 cycles. The primers used were the followings: human RANKL forward primer 5’-AGGCCTTTCAAGGAGCTGTG-3’ and reverse primer 5’-CTTGCTCCTCTTGGCCAGAT-3’; human 0PG forward primer 5’-AAGGGCGCTACCTTGAGTAG-3’ and reverse primer 5’-GCAAACTGTATTTCGCTCTGGG-3’; human GAPDH forward primer 5’-GCACCGTCAAGGCTGAGAAC-3’ and reverse primer 5’-TGGTGAAGACGCCAGTGGA-3’.
ELISA assay
After incubated cultured under different conditions, the supernatant was collected with centrifugalization and used for ELISA assay. The assay was performed according to the manufacturer’s instructions. The ELISA kits for OPG and soluble RANKL were obtained from R & D Systems (Minneapolis, MN, USA).
Statistical analysis
OPG and RANKL mRNA and protein expressions were valued and presented as the mean ± SD. Differences between the experimental groups and the control group were analyzed by one-way ANOVA followed by Tukey’s post hoc test; data were considered significant at P<0.05. The relative OPG/RANKL expression ratios were calculated at each time point.
Results
Proliferation of hPDLCs
A Cell Counting Kit-8 (CCK-8) assay revealed that the hPDLCs proliferation increased in all groups in a time-dependent manner. The proliferation in hPDLCs increased under hypoxia condition. The hPDLCs proliferation in the hypoxia group was significantly higher than in the control group from 24 h to 36 h (P<0.05) (As shown in Figure 1).
Figure 1.
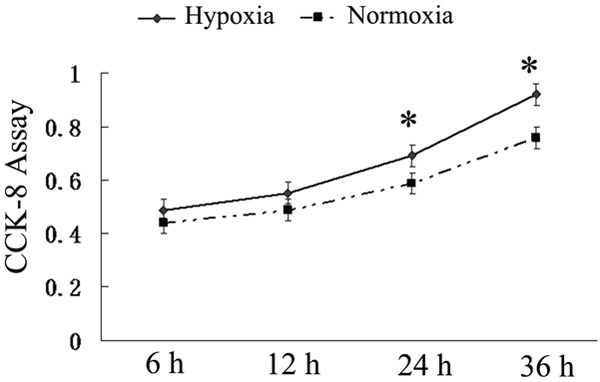
Cell counting kit-8 (CCK-8) assay in different time points. Cells were seeded in 96-well plates and cultured under different conditions. At 6, 12, 24, and 36 hours, cell proliferation was measured using a Cell Counting Kit-8. The result revealed the proliferation curves of the hypoxia groups increased sharply from 24 h to 36 h. (P<0.05).
Expression of HIF-1α protein in hPDLCs
Immunocytochemistry showed nucelus expression of HIF-1α protein in hPDLCs incubated in a hypoxic atmosphere of 2% O2, 5% CO2, 94% N2, which appeared yellow or brown (Figure 2A). Meanwhile, hPDLCs incubated in a normoxic did not express HIF-1α protein (Figure 2B).
Figure 2.
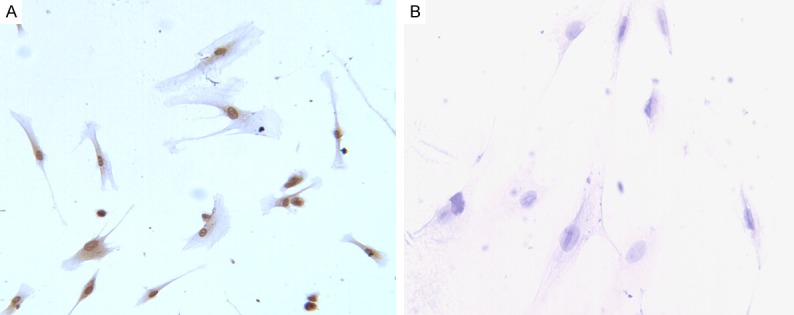
Immunocytochemical analysis of HIF-1α protein in hPDLCs. (A) Immunocytochemical location of HIF-1α protein in hPDLCs incubated in a hypoxic atmosphere of 2% O2, 5% CO2, 94% N2 at 37°C. It showed that the expression of PLAP-1 was positive in cytoplasm and nucelus of hPDLCs (A). (B) There was no brown granule in the hPDLCs incubated in a normoxic atmosphere (B).
Real-time PCR analysis
To determine whether the RANKL/OPG system was involved in the response of hPDLCs to hypoxia, we further investigated OPG mRNA, RANKL mRNA transcription by real-time PCR. The RANKL mRNA increased from 12 h to 48 h and significantly higher than in control (P<0.05) (Figure 3A). However, OPG mRNA declined gradually since 6 h to 48 h and lower than that of the control (P<0.05) (Figure 3B). Taken together, the ratio of RANKL/OPG mRNA increased significantly since 6 h to 48 h under hypoxia (P<0.05) (Figure 3C).
Figure 3.
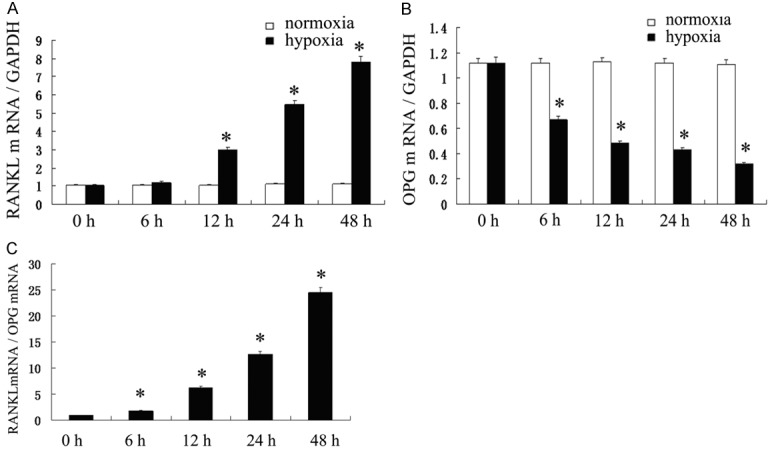
The mRNA expression levels of receptor activator of NF-jB ligand (RANKL) and osteoprotegerin (OPG) changed in a time-dependent manner. A. RANKL mRNA increased from 12 h to 48 h and significantly higher than control (P<0.05). B. The OPG mRNA declined gradually since 6 h to 48 h and lower than that of the control (P < 0.05). C. The ratio of RANKL/OPG mRNA increased significantly since 6 h to 48 h under hypoxia (P<0.05).
ELISA assay
Results of our ELISA assay documented that the release of soluble RANKL increased from 12 h to 48 h and significantly higher than that in control (P<0.05) (Figure 4A). The release of OPG was significantly decreased when the hPDLs were stimulated with hypoxic. Hypoxia reduced OPG release from hPDLCs in a hypoxic atmosphere of 2% O2, 5% CO2, 94% N2 comparing to the control (P<0.05) (Figure 4B). The ratio of sRANKL/OPG increased significantly since 12 h to 48 h under hypoxia (P<0.05) (Figure 4C).
Figure 4.
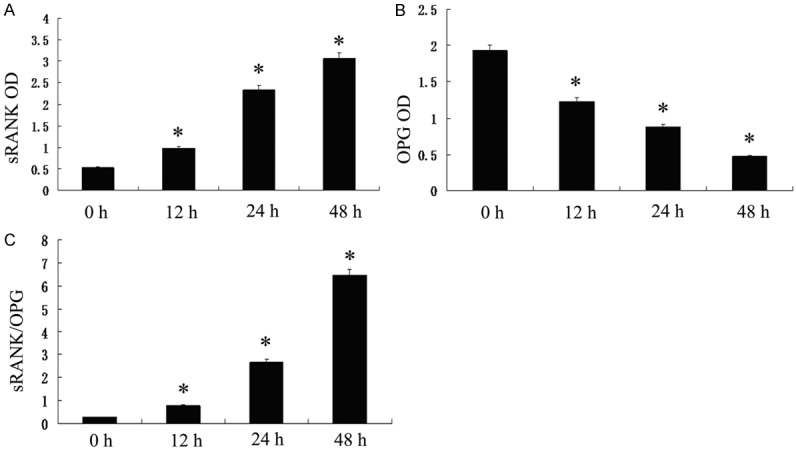
Effect of hypoxia on the release of OPG and soluble RANKL. A. Soluble RANKL increased from 12 h to 48 h and significantly higher than control (P<0.05). B. OPG declined gradually since 12 h to 48 h and lower than that of the control (P<0.05). C. The ratio of sRANKL/OPG increased significantly since 12 h to 48 h under hypoxia (P<0.05).
Discussion
Periodontitis is a chronic inflammatory disease characterized by the destruction of tooth supporting tissues and deep periodontal pockets favoring the proliferation of anaerobic bacteria [5]. When periodontal microcirculation was locally damaged for periodontitis, diabetes, cardiovascular diseases and orthodontic treatment, the periodontal tissues may suffer from hypoxia obviously [6,7]. Histopathologic studies have demonstrated evidence of mass angiogenesis in periodontitis tissues. Yet despite the observed increased blood vessel formation, the periodontal tissues have also been shown to be relatively hypoxic and ischemic [8]. Hypoxia is often a feature of inflammation and cancer [9]. Hypoxia is known to be a potent stimulus for angiogenesis in rheumatic arthritis and tumors [10,11]. The expression of VEGF in a wide spectrum of cells, including fibroblasts, is regulated by a number of cytokines, including transforming growth factor (TGF) and IL-1, as well as by hypoxia [12]. HIF-1α, a ubiquitous and highly conserved transcription factor, plays a key role in mediating cellular responses to hypoxia [13]. The expression of HIF-1α has been studied in vivo and has been demonstrated widely in the tissues of animals exposed to hypoxic conditions [14]. Our previous study has showed hypoxia changes the metabolic pathway of human periodontal ligament cells by upregulating the expression of HIF-1α and VEGF [15]. In this study, the hPDLCs proliferation in the hypoxia group was significantly higher than that in normoxia group from 24 h to 36 h. Meanwhile, the positive expression of HIF-1 protein was observed in hPDLCs under hypoxic atmosphere of 2% O2, 5% CO2, 94% N2 at 37°C, which was in according with previous researches [16,17]. The expression of HIF-1 alpha and VEGF (vascular endothelial growth factor) in human periodontal ligament cells was significantly higher in hypoxic conditions than in normoxic conditions. This transcription factor is rapidly degraded under normoxic conditions but is stabilized under hypoxic conditions, rapidly translocating to the nucleus, where it transactivates a number of genes with hypoxia-responsive elements in their promoters [18].
The periodontal ligament cells (PDLCs) are a group of heterogeneous cells, which mainly consist of fibroblasts and undifferentiated mesenchymal cells. The PDLCs play a pivotal role in the process of local inflammation and bone remodeling. When periapical inflammation lesions occur [19], proinflammatory cytokines interleukin-1 (IL-1) [20] and tumor necrosis factor-alpha (TNF-α) are induced in PDLCs, and positive staining of RANKL and OPG can be observed in the periodontal ligament fibroblasts [21]. Additionally, force-induced RANKL expression in PDLCs was observed during orthodontic treatment and suggested to participate in the bone remodeling process [22]. Osteoclastogenesis is primarily activated by receptor activator of nuclear factor kappaB ligand (RANKL) and is inhibited by osteoprotegerin (OPG). OPG is the soluble decoy receptor of RANKL. It can suppress the binding between RANKL and RANK and further block their effects on osteoclastogenesis and bone resorption. Local OPG gene transfer to periodontal tissue inhibits alveolar bone resorption [23]. The changes in the relative RANKL/OPG expression ratio also affect the bone remodeling process [24]. An increase in this ratio suggests the possibility of bone resorption, whilst a decrease indicates the possibility of bone formation [25].
However, there are few reports about above factors how expression under hypoxia conditions in hPDLCs even though hypoxia exists generally in periodontitis [26]. Hence, we assessed hPDLCs responses and activity under condition of hypoxia in vitro, as a model relevant to pathogenesis of periodontal diseases. To determine whether the RANKL/OPG system is involved in the response of hPDLCs to hypoxia, we further investigated OPG, RANKL mRNA transcription by real-time PCR and ELISA assay. Our result showed The OPG declined gradually since 12 h to 48 h and lower than that of the control. The RANKL mRNA increased from 12 h to 48 h and significantly higher than normoxia. Taken together, the ratio of RANKL/OPG in hPDLCs increased significantly under hypoxia.
As all know, RANKL-mediated osteoclastogenesis plays a pivotal role in inflammatory bone resorption. RANKL and OPG are key regulators of osteoclast differentiation in the bone loss seen in periodontitis [27]. Studies show that activated T and B cells can be the cellular source of RANKL for bone resorption in periodontitis tissues. RANKL expressions are distributed in lymphocytes and macrophages. OPG is observed in endothelial cells of periodontitis tissues [28]. This study indicated that hPDLCs suffered from hypoxia can disrupt the balance of RANKL/OPG. hPDLCs under hypoxia are also the cellular source of RANKL and OPG.
Summarily, hypoxia can affect the expression of RANKL and OPG in hPDLCs, which constitute an important pathogenic event in the alveolar bone resorption. Lack of oxygen in periodontal tissue may accelerate the development of periodontitis.
Acknowledgements
This work was partly supported by National Natural Science Foundation of China (81271138) awarded to Shu Li; Open Foundation of Shandong Provincial Key Laboratory of Oral Biomedicine (SDKQ201403) and Shandong Province Natural Science Foundation (ZR2015PH017) awarded to Xijiao Yu.
Disclosure of conflict of interest
None.
References
- 1.Semenza GL. Life with oxygen. Science. 2007;318:62–64. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- 2.Song AM, Hou C, Chen JF, Sun J, Tian T, Li S. Effect of hypoxia on the expression of matrix metalloproteinase and tissue inhibitors of matrix metalloproteinase mRNA in human periodontal ligament fibroblasts in vitro. Zhonghua Kou Qiang Yi Xue Za Zhi. 2012;47:599–604. doi: 10.3760/cma.j.issn.1002-0098.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Crotti T, Smith MD, Hirsch R, Soukoulis S, Weedon H, Capone M, Ahern MJ, Haynes D. Receptor activator NF kappaB ligand (RANKL) and osteoprotegerin (OPG) protein expression in periodontitis. J Periodontal Res. 2003;38:380–387. doi: 10.1034/j.1600-0765.2003.00615.x. [DOI] [PubMed] [Google Scholar]
- 4.Yu X, Lv L, Zhang J, Zhang T, Xiao C, Li S. Expression of neuropeptides and bone remodeling-related factors during periodontal tissue regeneration in denervated rats. J Mol Histol. 2015;46:195–203. doi: 10.1007/s10735-015-9611-x. [DOI] [PubMed] [Google Scholar]
- 5.Gölz L, Memmert S, Rath-Deschner B, Jäger A, Appel T, Baumgarten G, Götz W, Frede S. Hypoxia and P. gingivalis synergistically induce HIF-1 and NF-κB activation in PDL cells and periodontal diseases. Mediators Inflamm. 2015;2015:438085. doi: 10.1155/2015/438085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terrizzi AR, Fernandez-Solari J, Lee CM, Martínez MP, Conti MI. Lead intoxication under environmental hypoxia impairs oral health. J Toxicol Environ Health A. 2014;77:1304–1310. doi: 10.1080/15287394.2014.938209. [DOI] [PubMed] [Google Scholar]
- 7.Jian C, Li C, Ren Y, He Y, Li Y, Feng X, Zhang G, Tan Y. Hypoxia augments lipopolysaccharide-induced cytokine expression in periodontal ligament cells. Inflammation. 2014;37:1413–1423. doi: 10.1007/s10753-014-9865-6. [DOI] [PubMed] [Google Scholar]
- 8.Gaengler P, Merte K. Effects of force application on periodontal blood circulation. A vital microscopic study in rats. J Periodontal Res. 1983;18:86–92. doi: 10.1111/j.1600-0765.1983.tb00339.x. [DOI] [PubMed] [Google Scholar]
- 9.Huang LE, Bunn HF. Hypoxia-inducible factor and its biomedical relevance. J Biol Chem. 2003;278:19575–19578. doi: 10.1074/jbc.R200030200. [DOI] [PubMed] [Google Scholar]
- 10.Wykoff CC, Pugh CW, Harris AL, Maxwell PH, Ratcliffe PJ. The HIF pathway: implications for patterns of gene expression in cancer. Novartis Found Symp. 2001;240:212–25. doi: 10.1002/0470868716.ch15. discussion 225-231. [DOI] [PubMed] [Google Scholar]
- 11.Tang N, Wang L, Esko J, Giordano FJ, Huang Y, Gerber HP, Ferrara N, Johnson RS. Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell. 2004;6:485–495. doi: 10.1016/j.ccr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 12.Hitchon C, Wong K, Ma G, Reed J, Lyttle D, El-Gabalawy H. Hypoxia-induced production of stromal cell-derived factor 1 (CXCL12) and vascular endothelial growth factor by synovial fibroblasts. Arthritis Rheum. 2002;46:2587–2597. doi: 10.1002/art.10520. [DOI] [PubMed] [Google Scholar]
- 13.Cramer T, Yamanishi Y, Clausen BE, Förster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pugh CW, Ratcliffe PJ. Regulation of angiogenesis by hypoxia: role of the HIF system. Nat Med. 2003;9:677–684. doi: 10.1038/nm0603-677. [DOI] [PubMed] [Google Scholar]
- 15.Hou C, Tang KL, Yang PS, Zhang PP, Sun J, Li S. Effect of hypoxia on the expression of HIF-1alpha and VEGF in human periodontal ligament cells in vitro. Shanghai Kou Qiang Yi Xue. 2010;19:329–334. [PubMed] [Google Scholar]
- 16.Wu Y, Han X, Guo Y, Wu H, Ren J, Li J, Ai D, Wang L, Bai D. Response of immortalized murine cementoblast cells to hypoxia in vitro. Arch Oral Biol. 2013;58:1718–1725. doi: 10.1016/j.archoralbio.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Amemiya K, Kaneko Y, Muramatsu T, Shimono M, Inoue T. Pulp cell responses during hypoxia and reoxygenation in vitro. Eur J Oral Sci. 2003;111:332–338. doi: 10.1034/j.1600-0722.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 18.Nair PN. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit Rev Oral Biol Med. 2004;15:348–381. doi: 10.1177/154411130401500604. [DOI] [PubMed] [Google Scholar]
- 19.Menezes R, Bramante CM, da Silva Paiva KB, Letra A, Carneiro E, Fernando Zambuzzi W, Granjeiro JM. Receptor activator NFkappaB-ligand and osteoprotegerin protein expression in human periapical cysts and granulomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:404–409. doi: 10.1016/j.tripleo.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 20.Wada N, Maeda H, Yoshimine Y, Akamine A. Lipopolysaccharide stimulates expression of osteoprotegerin and receptor activator of NF-kappa B ligand in periodontal ligament fibroblasts through the induction of interleukin-1 beta and tumor necrosis factor-alpha. Bone. 2004;35:629–635. doi: 10.1016/j.bone.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi M. RANK/RANKL/OPG during orthodontic tooth movement. Orthod Craniofac Res. 2009;12:113–119. doi: 10.1111/j.1601-6343.2009.01444.x. [DOI] [PubMed] [Google Scholar]
- 22.Nakao K, Goto T, Gunjigake KK, Konoo T, Kobayashi S, Yamaguchi K. Intermittent force induces high RANKL expression in human periodontal ligament cells. J Dent Res. 2007;86:623–628. doi: 10.1177/154405910708600708. [DOI] [PubMed] [Google Scholar]
- 23.Chen R, Kanzaki H, Chiba M, Nishimura M, Kanzaki R, Igarashi K. Local osteoprotegerin gene transfer to periodontal tissue inhibits lipopolysaccharide-induced alveolar bone resorption. J Periodontal Res. 2008;43:237–245. doi: 10.1111/j.1600-0765.2007.01021.x. [DOI] [PubMed] [Google Scholar]
- 24.Boyce BF, Xing LP. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473:139–146. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bostanci N, Ilgenli T, Emingil G, Afacan B, Han B, Töz H, Berdeli A, Atilla G, McKay IJ, Hughes FJ, Belibasakis GN. Differential expression of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin mRNA in periodontal diseases. J Periodontal Res. 2007;42:287–293. doi: 10.1111/j.1600-0765.2006.00946.x. [DOI] [PubMed] [Google Scholar]
- 26.Terrizzi AR, Fernandez-Solari J, Lee CM, Bozzini C, Mandalunis PM, Elverdin JC, Conti MI, Martínez MP. Alveolar bone loss associated to periodontal disease in lead intoxicated rats under environmental hypoxia. Arch Oral Biol. 2013;58:1407–1414. doi: 10.1016/j.archoralbio.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, Daro E, Smith J, Tometsko ME, Maliszewski CR, Armstrong A, Shen V, Bain S, Cosman D, Anderson D, Morrissey PJ, Peschon JJ, Schuh J. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412–2424. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kawai T, Matsuyama T, Hosokawa Y, Makihira S, Seki M, Karimbux NY, Goncalves RB, Valverde P, Dibart S, Li YP, Miranda LA, Ernst CW, Izumi Y, Taubman MA. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol. 2006;169:987–998. doi: 10.2353/ajpath.2006.060180. [DOI] [PMC free article] [PubMed] [Google Scholar]


