Abstract
Acute lung injury (ALI) is a common emergency and severe case in clinic. High mobility group protein box 1 (HMGB1) can be treated as a new anti-inflammatory treatment target. Toll-like receptor 4 (TLR4) is an important receptor of HMGB1. Ketamine is a widely used intravenous anesthetic with good anti-inflammatory and immune regulating function. Whether it can protect ALI through inhibiting HMGB1 and TLR4 expression in lung tissue still needs further investigation. Male SD rats were randomly divided into control, lipopolysaccharide (LPS) group and ketamine intervention group with 15 rats in each group. The rats were euthanatized at 24 h after modeling and the bronchoalveolar lavage fluid (BALF) was collected for HMGB1 and TLR4 level detection. Western Blot was applied to analyze HMGB1 and TLR4 protein expression in the lung tissue. HMGB1 and TLR4 concentration in BALF were 5.369 ± 1.564 ng/ml and 43.980 ± 7.524 pg/ml in the control, respectively. They were 12.358 ± 4.681 ng/ml and 102.538 ± 8.412 pg/ml in LPS group, and 7.399 ± 2.346 ng/ml and 87.208 ± 7.558 pg/ml in ketamine intervention group, respectively. Their levels increased significantly in LPS group and down-regulated after ketamine intervention. HMGB1 and TLR4 protein expression in lung tissue elevated obviously in LPS group, and decreased after ketamine treatment. HMGB1 and TLR4 protein level showed positive correlation in lung tissue (r = 0.921, P < 0.001). Ketamine can inhibit HMGB1 and TLR4 expression in ALI, and alleviate LPS induced rat lung injury.
Keywords: Ketamine, acute lung injury, HMGB1, TLR4
Introduction
Acute lung injury (ALI) is caused by severe shock, infection, or trauma in lung. Multiple inflammatory cells induced pulmonary inflammatory response and inflammatory cells out of control result in pulmonary capillary injury [1]. It may lead to tissue hypoxia, necrosis and dysfunction, resulting in multiple organ dysfunction syndromes. ALI is a common serious complication of patients with critical disease with mortality as high as 35~40% that seriously affect people’s lives and health. So far, the pathogenesis of ALI has not been fully elucidated, several studies suggested that blocking inflammatory cells out of control to release inflammatory factors in time is the key to prevent ALI [2,3]. High mobility group box1 (HMGB1) is a kind of non-histone nucleoprotein that widely exists in eukaryotic cells. It is both the inflammation early starter and late promoter that involved in ALI process [4,5]. HMGB1 plays a central role in the inflammation network of ALI. It can translocate from nucleus to cytoplasm in immune cells and be actively secreted to extracellular during stimulation [6,7]. Inhibiting HMGB1 expression and release can delay ALI process. Toll-like receptor 4 (TLR4) receptor is the receptor of HMGB1 that widely expressed in vascular endothelial cells, dendritic cells and lymphocytes. Studies suggested that HMGB1 can activate NF-κB pathway through MAKP and MyD88 b binding with TLR4 to participate in ALI [8,9].
Ketamine is widely used in clinical intravenous anesthesia with good anti-inflammatory and immune regulating function. It can alleviate inflammation induced heart and lung injury by inhibiting a variety of inflammatory cytokines production, neutrophils effect and adhesion molecules expression [10,11]. The mechanism of ketamine in regulating pulmonary inflammatory factor synthesis and release is still not clearly elucidated. In this study, we tried to investigate ketamine effect in ALI by establishing LPS induced rat ALI model and detecting HMGB1 and TLR4 expression level.
Materials and methods
Main reagents and instruments
LPS was bought from Sigma. Ketamine injection was purchased from Fujian Gutian Pharmaceutical co., Ltd. Rat HMGB1 ELISA kit purchased from Shanghai Yaji biological technology co., Ltd. Rat TLR4 ELISA kit was bought in Shanghai Rich biological technology co., Ltd. Total protein extraction kit was bought in Shanghai BestBio biological co., Ltd. Coomassie brilliant blue protein assay kit was got from Shanghai Major biological technology co., LTD. SDS-polyacrylamide, PBST solution, vertical electrophoresis apparatus and GIS-2020-D gel image analysis system were purchased from Sigma. HMGB1, TLR4 and GAPDH antibodies were got from Abcam. Horseradish peroxidase labeled goat anti-rabbit IgG was purchased Shanghai Cent biological co., Ltd.
Modeling
Forty five male SD rats (Animal experiment center in Wuhan province) weighted 180-220 g were divided into control, LPS group and ketamine intervention group. LPS at 5 mg/kg was injected through femoral venous to establish ALI model. In the ketamine intervention group, ketamine at 10 mg/kg was injected to the rats before modeling. Equal volume normal saline was injected in ALI and control groups.
Rats were used for all experiments, and all procedures were approved by the Animal Ethics Committee of our hospital.
ELISA
The lung was isolated after euthanatizing the rat. After ligating the right lung, 5 ml normal saline was filled into the left lung to take out the bronchoalveolar lavage fluid (BALF). The BALF was centrifuged at 3000 rpm for 10 min under 4°C. ELISA kit was applied to detect HMGB1 and TLR4 expression in BALF. Major steps include: put 50 μl diluted standard product into the corresponding reaction holes to prepare the standard curve. Add 50 μl samples to each hole. After washing the plate for 5 times, 50 μl enzyme reagent was added. The plate was washed for 5 times again after incubated at 37°C for 30 min. 50 μl agent A and 50 μl agent B were inserted to each hole and the plate was incubated at 37°C for 10 min. The reaction was terminated after adding 50 μl terminates liquid. The plate was measured at 450 nm wavelength to get the absorbance value (OD value). The sample concentration was calculated according to the OD value and standard curve.
Western blot
Total protein was extracted from lung tissue and separated by denaturing SDS-polyacrylamide gel electrophoresis. Detection was performed with chemiluminiscence and calculated with GIS-2020-D gel image analysis system. Protein levels were normalized to GAPDH.
Statistical analysis
All statistical analyses were performed using SPSS13.0 software (Chicago, IL). Pearson correlation analysis was applied to calculate correlation between HMGB1 and TLR4 expression. Inspection level α = 0.05. P < 0.05 was considered as significant difference.
Results
HMGB1 and TLR4 concentration changes in BALF
Compared with control, HMGB1 and TLR4 concentrations in LPS group obviously elevated (P < 0.001). After ketamine intervention, HMGB1 and TLR4 levels decreased significantly. Compared with LPS group, t value for HMGB1 was 19.48 (P < 0.001), while t value for TLR4 was 16.38 (P = 0.003, Table 1).
Table 1.
HMGB1 and TLR4 concentration changes in BALF
| Groups | N | HMGB1 (ng/ml) | TLR4 (pg/ml) |
|---|---|---|---|
| Control | 15 | 5.369 ± 1.564 | 43.980 ± 7.524 |
| LPS group | 15 | 12.358 ± 4.681* | 102.538 ± 8.412* |
| Ketamine intervention group | 15 | 7.399 ± 2.346** | 87.208 ± 7.558** |
P < 0.001, compared with control;
P < 0.001, compared with LPS group.
HMGB1 and TLR4 protein expression in rat lung tissue
Western blot results showed that HMGB1 and TLR4 protein expression level was low in control, while their level up-regulated in LPS group (P < 0.001). After ketamine treatment, their expression level was significantly inhibited (P < 0.001) (Figures 1, 2 and 3).
Figure 1.
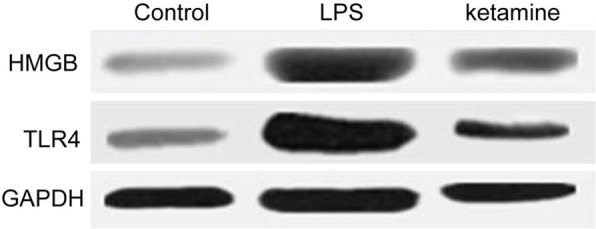
HMGB1 and TLR4 protein expression comparison in control, LPS group and ketamine group.
Figure 2.
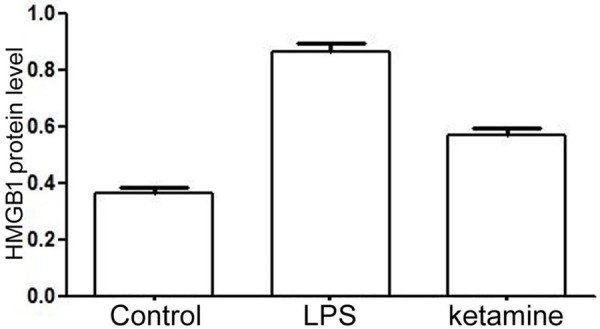
HMGB1 protein level comparison in control, LPS group and ketamine group.
Figure 3.
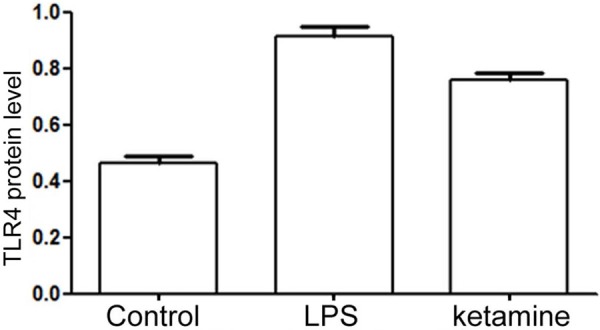
TLR4 protein level comparison in control, LPS group and ketamine group.
HMGB1 and TLR4 protein expression level correlation analysis in rat lung tissue
Pearson correlation analysis revealed that HMGB1 was positively correlated with TLR4 (r = 0.921, P < 0.001, Figure 4).
Figure 4.
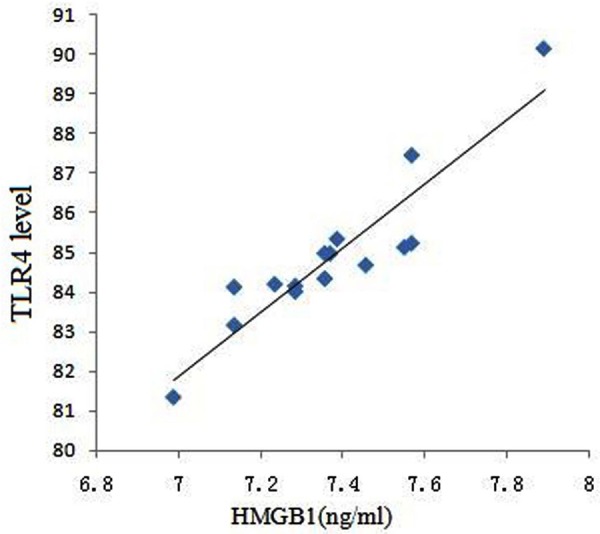
HMGB1 and TLR4 protein expression level correlation analysis in rat lung tissue.
Discussion
ALI is a kind of common emergency and severe cases in clinic. Its prevalence rate was 11~25% in multiple trauma, and it reached 25~50% during severe infection and 40% in massive transfusion. Multiple risk factors at the same time can further increase its prevalence rate. Longer risk factor duration may cause higher prevalence rate, and it reach 76%, 85% and 93% after the risk factor sustained for 24, 48 and 72 h, respectively [12,13]. Despite quickly progress of medical technology in recent years, ALI mortality remains high. HMGB1 is a kind of non-histones nucleoprotein that participates in DNA replication, transcription and translation. Wang et al. [14] in 1999 first discovered that HMGB1 involved in sepsis process as a late inflammatory mediator. Therefore, HMGB1 plays an important role in the treatment of inflammatory disease. Studies suggested that HMGB1 can cause lung injury by activating alveolar macrophages and neutrophils and stimulating proinflammatory cytokines. Its level can reflect the severity of inflammation and tissue damage [15,16]. TLR4 is an important receptor of HMGB1. Studies found that HMGB1 in neutrophils and macrophages participate in ALI through TLR4-MyD88-NF-κB signaling pathway [17,18].
Ketamine is a derivative of the phencyclidine (PCP) that can antagonize noncompetitive N-methyl-D-aspartate (NMOA) receptor. It is often used in clinical anesthesia as short-acting general anesthetics [19,20]. Recent study showed that ketamine can inhibit a variety of inflammatory cytokines production, neutrophils effect and adhesion molecules expression. It also showed obvious inhibitory effect on inflammation caused by septic shock or extracorporeal circulation. NF-κB is an important transcription factor that can activate a variety of inflammatory cytokines under stress [21,22]. HMGB1 exists in the nucleus to stabilize nuclear chromosome and regulate protein transcription and translation under normal physiological condition. It transfers to the cytoplasm, combines with TLR4, prompts NF-κB activation, and releases cytokine under pathological status. Studies suggested that ketamine can inhibit NF-κB level and activity with dose dependent. In addition, it was also indicated that ketamine terminate more inflammatory factor generation by inhibiting TLR4 expression [23,24]. ZHANG, et al. showed that ketamine dose in clinical use can inhibit NF-κB activity to some extent that plays a lung tissue protection effect on LPS induced ALI [25,26]. Thus, Ketamine has repair effect to ALI caused by inflammation and can be used to relieve clinical ALI induced inflammation damage.
This research established ALI model induced by LPS, and detected HMGB1 and TLR4 expression levels in BALF and lung tissue. It was found that HMGB1 and TLR4 overexpressed significantly in LPS group, while they reduced after ketamine treatment, suggesting that ketamine can alleviate ALI and facilitate lung injury treatment. In addition, this study also performed Pearson correlation analysis between HMGB1 and TLR4 expression. It was revealed that they have obvious linear correlation. The higher HMGB1 concentration, the higher TLR4 was. It suggested that we could inhibit inflammation and prevent ALI by regulating signaling pathway.
In brief, HMGB1 involves inflammation process caused by ALI. Ketamine can protect lung injury by regulating HMGB1 and TLR4 protein expression. However, animal experiment cannot reflect human body completely and accurately. Animal number in this study is limited, which may also bring certain influence. The mechanism of ALI is complicated and multiple factors may impact each other. Thus, it can only reflect ketamine improving ALI to a certain extent. Further investigation is needed to clarify the mechanism of ketamine effect on ALI.
Acknowledgements
Research supported by the natural science foundation of Hubei Province (NO: 2014CFC1051).
Disclosure of conflict of interest
None.
References
- 1.Kao RL, Xu X, Xenocostas A, Parry N, Mele T, Martin CM, Rui T. Induction of acute lung inflammation in mice with hemorrhagic shock and resuscitation: role of HMGB1. J Inflamm (Lond) 2014;11:30. doi: 10.1186/s12950-014-0030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haitsma JJ, Lachmann B, Papadakos PJ. Additives in intravenous anesthesia modulate pulmonary inflammation in a model of LPS-induced respiratory distress. Acta Anaesthesiol Scand. 2009;53:176–182. doi: 10.1111/j.1399-6576.2008.01844.x. [DOI] [PubMed] [Google Scholar]
- 3.Liu XX, Yu DD, Chen MJ, Sun T, Li G, Huang WJ, Nie H, Wang C, Zhang YX, Gong Q, Ren BX. Hesperidin ameliorates lipopolysaccharide-induced acute lung injury in mice by inhibiting HMGB1 release. Int Immunopharmacol. 2015;25:370–376. doi: 10.1016/j.intimp.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 4.Zhou M, Zhang Y, Chen X, Zhu J, Du M, Zhou L, Zhang L, Wang W, Sun G. PTEN-Foxo1 signaling triggers HMGB1-mediated innate immune responses in acute lung injury. Immunol Res. 2015;62:95–105. doi: 10.1007/s12026-015-8639-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Entezari M, Javdan M, Antoine DJ, Morrow DM, Sitapara RA, Patel V, Wang M, Sharma L, Gorasiya S, Zur M, Wu W, Li J, Yang H, Ashby CR, Thomas D, Wang H, Mantell LL. Inhibition of extracellular HMGB1 attenuates hyperoxia-induced inflammatory acute lung injury. Redox Biol. 2014;2:314–322. doi: 10.1016/j.redox.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng G, Sun B, Li TZ. Daidzein attenuates lipopolysaccharide-induced acute lung injury via toll-like receptor 4/NF-kappaB pathway. Int Immunopharmacol. 2015;26:392–400. doi: 10.1016/j.intimp.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Dange RB, Agarwal D, Teruyama R, Francis J. Toll-like receptor 4 inhibition within the paraventricular nucleus attenuates blood pressure and inflammatory response in a genetic model of hypertension. J Neuroinflammation. 2015;12:31. doi: 10.1186/s12974-015-0242-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tumurkhuu G, Dagvadorj J, Jones HD, Chen S, Shimada K, Crother TR, Arditi M. Alternatively spliced myeloid differentiation protein-2 inhibits TLR4-mediated lung inflammation. J Immunol. 2015;194:1686–1694. doi: 10.4049/jimmunol.1402123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu W, Shan LP, Dong XS, Liu Z. Toll-like receptor 4 implicated in acute lung injury induced by paraquat poisoning in mice. Int J Clin Exp Med. 2014;7:3392–3397. [PMC free article] [PubMed] [Google Scholar]
- 10.Fastner C, Mairbaurl H, Weber NC, van der Sluijs K, Hackl F, Hotz L, Dahan A, Hollmann MW, Berger MM. Intravenous S-ketamine does not inhibit alveolar fluid clearance in a septic rat model. PLoS One. 2014;9:e112622. doi: 10.1371/journal.pone.0112622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erdem MK, Yurdakan G, Yilmaz-Sipahi E. The effects of ketamine, midazolam and ketamine/xylazine on acute lung injury induced by alpha-naphthylthiourea in rats. Adv Clin Exp Med. 2014;23:343–351. doi: 10.17219/acem/37124. [DOI] [PubMed] [Google Scholar]
- 12.Zhang X, Luan Z, Liang Y, Liu Y, Ma X. Downregulation of high mobility group box 1 attenuates the severity of acute lung injury in endotoxemic mice. Mol Med Rep. 2015;11:4513–4517. doi: 10.3892/mmr.2015.3251. [DOI] [PubMed] [Google Scholar]
- 13.Wang G, Liu L, Zhang Y, Han D, Liu J, Xu J, Xie X, Wu Y, Zhang D, Ke R, Li S, Zhu Y, Feng W, Li M. Activation of PPARgamma attenuates LPS-induced acute lung injury by inhibition of HMGB1-RAGE levels. Eur J Pharmacol. 2014;726:27–32. doi: 10.1016/j.ejphar.2014.01.030. [DOI] [PubMed] [Google Scholar]
- 14.Ma ZH, Ma QY, Wang LC, Sha HC, Wu SL, Zhang M. Effect of resveratrol on NF-kappaB activity in rat peritoneal macrophages. Am J Chin Med. 2006;34:623–630. doi: 10.1142/S0192415X06004156. [DOI] [PubMed] [Google Scholar]
- 15.Luan ZG, Zhang XJ, Yin XH, Ma XC, Zhang H, Zhang C, Guo RX. Downregulation of HMGB1 protects against the development of acute lung injury after severe acute pancreatitis. Immunobiology. 2013;218:1261–1270. doi: 10.1016/j.imbio.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Gao RJ, Zhou YH, Liu T, Han QF, Yao HC. Response to letter regarding “Increased serum HMGB1 level may predict the fatal outcomes in patients with chronic heart failure”. Int J Cardiol. 2015;187:434–435. doi: 10.1016/j.ijcard.2015.03.380. [DOI] [PubMed] [Google Scholar]
- 17.Sodhi CP, Jia H, Yamaguchi Y, Lu P, Good M, Egan C, Ozolek J, Zhu X, Billiar TR, Hackam DJ. Intestinal Epithelial TLR-4 Activation Is Required for the Development of Acute Lung Injury after Trauma/Hemorrhagic Shock via the Release of HMGB1 from the Gut. J Immunol. 2015;194:4931–4939. doi: 10.4049/jimmunol.1402490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li W, Xu Q, Deng Y, Yang Z, Xing S, Zhao X, Zhu P, Wang X, He Z, Gao Y. High-mobility group box 1 accelerates lipopolysaccharide-induced lung fibroblast proliferation in vitro: involvement of the NF-kappaB signaling pathway. Lab Invest. 2015;95:635–47. doi: 10.1038/labinvest.2015.44. [DOI] [PubMed] [Google Scholar]
- 19.Gokcinar D, Ergin V, Cumaoglu A, Menevse A, Aricioglu A. Effects of ketamine, propofol, and ketofol on proinflammatory cytokines and markers of oxidative stress in a rat model of endotoxemia-induced acute lung injury. Acta Biochim Pol. 2013;60:451–456. [PubMed] [Google Scholar]
- 20.Yang CH, Tsai PS, Wang TY, Huang CJ. Dexmedetomidine-ketamine combination mitigates acute lung injury in haemorrhagic shock rats. Resuscitation. 2009;80:1204–1210. doi: 10.1016/j.resuscitation.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 21.Trujillo KA, Zamora JJ, Warmoth KP. Increased response to ketamine following treatment at long intervals: implications for intermittent use. Biol Psychiatry. 2008;63:178–183. doi: 10.1016/j.biopsych.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Degryse B, Bonaldi T, Scaffidi P, Muller S, Resnati M, Sanvito F, Arrigoni G, Bianchi ME. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol. 2001;152:1197–1206. doi: 10.1083/jcb.152.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun XJ, Li XQ, Wang XL, Tan WF, Wang JK. Sevoflurane Inhibits Nuclear Factor-kappaB Activation in Lipopolysaccharide-Induced Acute Inflammatory Lung Injury via Toll-Like Receptor 4 Signaling. PLoS One. 2015;10:e0122752. doi: 10.1371/journal.pone.0122752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frank MG, Weber MD, Watkins LR, Maier SF. Stress sounds the alarmin: The role of the danger-associated molecular pattern HMGB1 in stress-induced neuroinflammatory priming. Brain Behav Immun. 2015;48:1–7. doi: 10.1016/j.bbi.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tianzhu Z, Shumin W. Esculin Inhibits the Inflammation of LPS-Induced Acute Lung Injury in Mice Via Regulation of TLR/NF-kappaB Pathways. Inflammation. 2015;38:1529–36. doi: 10.1007/s10753-015-0127-z. [DOI] [PubMed] [Google Scholar]
- 26.Takenaka I, Ogata M, Koga K, Matsumoto T, Shigematsu A. Ketamine suppresses endotoxin-induced tumor necrosis factor alpha production in mice. Anesthesiology. 1994;80:402–408. doi: 10.1097/00000542-199402000-00020. [DOI] [PubMed] [Google Scholar]


