Abstract
Background: The detailed mechanisms of knee osteoarthritis (OA) pain have not been clarified, but involvement of inflammatory cytokines such as tumor necrosis factor-alpha (TNF) has been suggested. The present study aimed to investigate the more detailed neurological involvement of TNF in joint pain using a TNF-knockout mouse OA model. Methods: The right knees of twelve-week-old C57BL/6J wild and TNF-deficient knockout (TNF-ko) mice (n=15, each group) were given a single intra-articular injection of 10 µg monoiodoacetate in 10 mL sterile saline. The left knees were only punctured as the control. Evaluations were performed immediately after the injection (baseline) and at 7, 14, and 28 days after the injection with a subsequent intra-articular injection of neurotracer into both knees. The animals were evaluated for immunofluorescence of the lumbar dorsal root ganglia (DRG) innervating the knee joints. The injected knees were observed macroscopically and mouse pain-related behaviors were scored. Results: Macroscopic observation showed similar knee OA development in both wild and TNF-ko mice. Calcitonin gene-related peptide (CGRP, a neuropeptide identified as a inflammatory pain-related biomarker) was significantly increased in DRG neurons innervating OA-induced knee joints with significantly less CGRP expression in TNF-ko animals. Pain-related behavior scoring showed a significant increase in pain in OA-induced joints, but there was no significant difference in pain observed between the wild and TNF-ko mice. Conclusions: The result of the present study indicates the possible association of TNF-alpha in OA pain but not OA development.
Keywords: Osteoarthritis, mice model, tumor necrosis factor (TNF)-alpha, monoiodoacetate (MIA)
Introduction
Knee osteoarthritis (OA) is a common chronic degenerative disease characterized by a worn and damaged joint structure with loss of articular cartilage components and subchondral bone. Pain is a major symptom of OA that leads to considerable disability among older adults [1]. The mechanisms of OA pain have not yet been clarified. We previously reported the properties of knee OA pain using a rodent OA model developed by intra-articular injection of monoiodoacetate (MIA). MIA induces joint osteoarthritic changes by inhibiting glycolysis and causing injury to cartilage and chondrocyte sand enhanced pain behavior [2]. Previous studies suggest that joint pain can include both nociceptive pain and neuropathic pain components, with increased levels of synovial inflammatory cytokines [1-3]. Tumor necrosis factor-alpha (TNF) is one of the inflammatory cytokines which is found in progressive joint arthritis [4]. We have reported the possible association of TNF with joint pain by evaluating human synovial fluid, but TNF’s direct association with pain has not yet been evaluated in detail [5]. The present study aimed to investigate the neurological involvement of TNF in joint pain in greater detail using a TNF-knockout mouse OA model, including pain-related behavior and macroscopical OA progression.
Materials and methods
All protocols for animal procedures were reviewed and approved by the ethics committee of our institution.
Mouse knee OA model by intra-articular injection
Fifteen male twelve-week-old C57BL/6J wild mice and fifteen twelve-week-old TNF-deficient knockout mice were prepared for the study. TNF deficiency was confirmed by genotyping prior to the experiment. All mice were anesthetized with sodium pentobarbital (40 mg/kg) and treated aseptically throughout the experiment. The right knees of hind legs were treated with a single intra-articular injection of 10 µg MIA (Sigma-Aldrich, St. Louis, MO) in 10-µL sterile saline. The left knees were treated with 10 µL sterile saline as controls. The MIA solution was injected through the patellar ligament with a 30-gauge needle with the knee flexed at 90° as reported previously [2]. After the injection, the animals were returned to their cages and kept under a 12/12 h light/dark cycle with food and water ad libitum. The ipsilateral knees were subsequently identified as OA knees, while the opposite side (contralateral) was identified as control. Evaluations were performed by four senior orthopedic surgeons immediately after the injection (baseline: BL) and subsequently at 7, 14, and 28 days after the injection. Seven days prior to each evaluation, both knee joints of the mice were intra-articularly injected with the retrograde neurotracer Fluorogold (Fluorochrome, LLC, Denver, CO) 2% in 10 µL sterile saline to trace the dorsal root ganglia (DRG) neurons innervating the knee joints.
Immunofluorescent evaluation for sensory neurons innervating knee joints
MIA-treated mice were divided into two groups according to TNF deficiency: the wild group and the TNF-knockout (-ko) group. At each evaluation interval, five animals from each group were perfused transcardially with 0.9% saline, followed by 20 mL of 4% paraformaldehyde in phosphate buffer fixative. The fourth lumbar (L)-4 DRGs, which predominantly innervate the knee joints [1], were resected bilaterally. The specimens were immersed in the buffered paraformaldehyde fixative overnight at 4°C. They were then stored in phosphate-buffered saline (PBS) containing 20% sucrose for 20 hours at 4°C, after which each DRG was sectioned at 10-μm thickness on a cryostat. The specimens were then treated for 90 min at room temperature with a blocking solution consisting of PBS containing 0.3% Triton X-100 and 3% skim milk. The DRG specimens were then processed using a rabbit antibody against calcitonin gene-related peptide (CGRP, neuropeptide acting as a biomarker for inflammatory-pain [1], 1:1000; Immunostar, Hudson, WI). After incubation with the diluted antibodies for 20 h at 4°C, DRG sections were incubated with Alexa 488-conjugated goat anti-rabbit IgG (1:1000; Molecular Probes, NY, USA). We statistically evaluated the distribution of immunostained DRG neurons by using non-repeated ANOVA with a post hoc Student-Newman-Keuls (SNK) test.
Behavioral evaluation
Pain-related behavior was quantified using a pain score as follows based on the response to von Frey hairs: no response (score 0), withdraw the limb (score 1), and lift or lick the limb (score 2). The test was performed by evaluating the behavior when touching the plantar surface of the hind paws of awake animals placed in a chamber with a metal grid floor. The test was repeated four times and the total score was regarded as the observational pain score (up to score 8). The score was statistically evaluated with a non-repeated ANOVA with a post hoc SNK test.
Results
Ipsilateral knee joints showed gradual degenerative changes such as swelling and limited range of motion compared with the contralateral knee in both the wild and TNF-ko mice. There was no significant difference noted between the wild and TNF-ko mouse joints (Figure 1). Immunofluorescent studies revealed that the average proportion of CGRP-immunoreactive(-ir) FG-labeled DRG neurons, indicating DRG neurons innervating knee joints with the pain-related biomarker, were significantly increased in OA joints compared to the contralateral joints. The peak difference occurred at 1 week after the injection (P<0.01). However, the increase was significantly less in the TNF-ko joints (P<0.01). The proportion of CGRP-ir FG-labeled DRG neurons peaked at 1 week after the MIA injection (Figures 2, 3).
Figure 1.
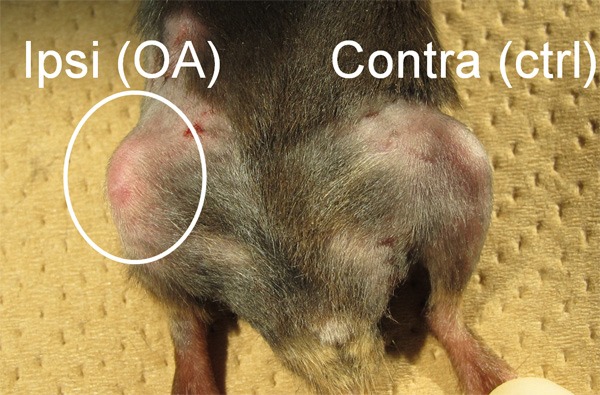
Physical evaluation showed swelling and limited range of motion in the ipsilateral knee compared with the contralateral knee. There was no significant difference between the wild type mouse and TNF-ko mouse joints.
Figure 2.
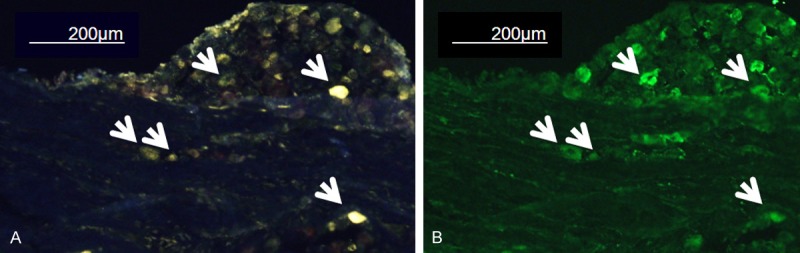
Representative immunofluorescence image of L-2 DRG. (A) FG-labeled image and (B) CGRP-immunostain imaging from the same slice. Arrows indicate the CGRP-immunoreactive FG-labeled DRG neurons. DRG: dorsal root ganglia, FG: Fluorogold (neurotracer), CGRP: calcitonin gene-related neuropeptide (biomarker for inflammatory pain).
Figure 3.
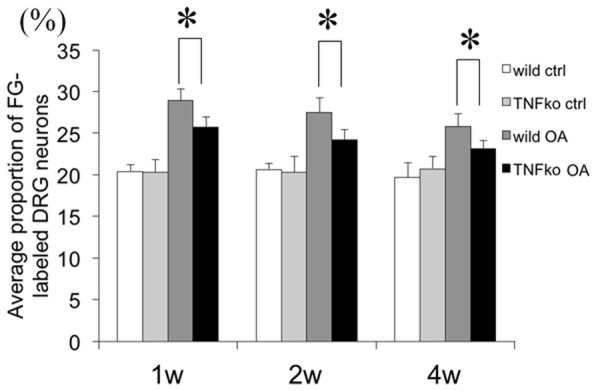
Average proportion of CGRP-immunoreactive (ir) FG-labeled DRG neurons. The proportion was significantly increased in OA joints compared with the contralateral joints at the peak 1 week after the injection, while the increase was significantly less in the joints of the TNF-ko mice. *: P<0.01.
The pain-related behavioral tests showed a significant increase in ipsilateral knee pain compared with the corresponding contralateral knee. There was no significant difference between pain in the wild mouse and TNF-ko mouse joints (Figure 4).
Figure 4.
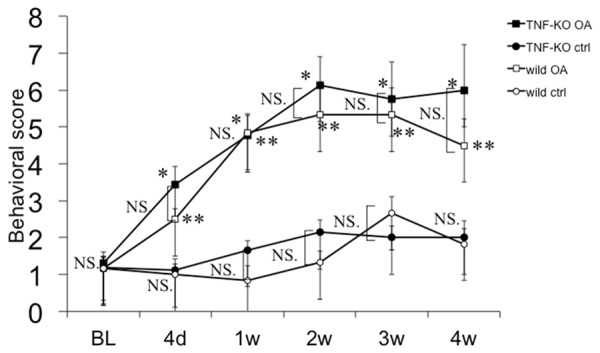
Behavioral scoring by pain reaction. OA joints showed significantly higher pain scores compared with the corresponding control. There was no significant difference in pain reaction between the wild mice and the TNF deficient mice. NS: Non significant. *, **: P<0.01 vs corresponding ctrl.
Discussion
The present study investigated the involvement of TNF in OA using a knock-out mouse knee OA model induced by MIA intra-articular injection. Macroscopic observation showed the obvious development of knee OA in a similar fashion in both the wild and TNF-ko mice. CGRP, a neuropeptide identified as a pain-related biomarker, showed a significant increase in DRG neurons innervating OA-induced knee joints. However, this increase of CGRP expression in TNF-ko animals was significantly less than in the wild type mice. Pain-related behavior scoring showed a significant increase in pain in OA-induced joints, but no significant difference was noted between the wild and TNF-ko mice.
TNF has the potential to cause long-lasting sensitization of joint nociceptors to mechanical stimuli [3]. TNF can also activate pain-related astrocytes [4]. These actions may be responsible for the results seen in our study. In the present study, TNF-deficiency resulted in insufficient CGRP expression that can be explained by a diminished response to mechanical stimuli. Additionally, our previous cytokine assay analysis using human synovial fluid from the knees of OA patients demonstrated a possible association between TNF levels and pain intensity, while interleukin-6 levels were more closely associated with joint function [5]. This partly supports the results of the present study. The present immunofluorescent findings indicate that TNF can regulate the pain-related neuronal activities of nerves innervating the knee joints. It is possible that MIA-induced intra-articular inflammation can induce increased CGRP expression via TNF enrollment, indicating the critical role of TNF in OA pain. Furthermore, CGRP may be associated with the development of arthritis, in addition to TNF, by activation of the NF-KB pathway,as reported previously in a studyof developmental dysplasia of the hip [6].
On the other hand, we did not observe a significant difference in the pain behavior scores of the wild and TNF-ko mice. Considering the fact that increased TNF induces allodynia and ahyperalgesic response [3,7], TNF deficiency should have suppressed the pain behavior in TNF-ko mice. However, our scoring system may not have been able to detect subtle changes in allodynia in a mouse model. Further pain threshold studies should be considered in the future.
The present study has several limitations. First, no further studies have confirmed the association of TNF with TNF-ko OA mice by administering TNF. Second, histological analysis was not performed, since the present study aimed mainly to evaluate pain-related innervation. Subchondral bone can be a source for inflammatory cytokines in OA [8], and histological studies should be performed in the future. Third, the MIA OA model is based on an external mediator of chemical inflammation. To overcome this, future models will need to be based on physically induced OA models such as destabilizing surgery of the medial meniscus [9].
In conclusion, we investigated the possible involvement of TNF in mouse knee OA using transgenic TNF knock-out mice in a model induced by MIA injection. Macroscopic observation of OA and related pain scores were not significantly different between wild and TNF-ko mice. However, inflammatory pain-related neuropeptide CGRP showed a significant increase in DRG neurons innervating OA-induced knee joints of wild type mice; the increase was significantly less in the TNF-ko mice. The results of the present study indicate the possible involvement of TNF in OA pain rather than the development of OA itself.
Disclosure of conflict of interest
None.
References
- 1.Schaible HG, von Banchet GS, Boettger MK, Brauer R, Gajda M, Richter F, Hensellek S, Brenn D, Natura G. The role of proinflammatory cytokines in the generation and maintenance of joint pain. Ann N Y Acad Sci. 2010;1193:60–69. doi: 10.1111/j.1749-6632.2009.05301.x. [DOI] [PubMed] [Google Scholar]
- 2.Orita S, Ishikawa T, Miyagi M, Ochiai N, Inoue G, Eguchi Y, Kamoda H, Arai G, Toyone T, Aoki Y, Kubo T, Takahashi K, Ohtori S. Pain-related sensory innervation in monoiodoacetate-induced osteoarthritis in rat knees that gradually develops neuronal injury in addition to inflammatory pain. BMC Musculoskelet Disord. 2011;12:134. doi: 10.1186/1471-2474-12-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richter F, Natura G, Loser S, Schmidt K, Viisanen H, Schaible HG. Tumor necrosis factor causes persistent sensitization of joint nociceptors to mechanical stimuli in rats. Arthritis Rheum. 2010;62:3806–3814. doi: 10.1002/art.27715. [DOI] [PubMed] [Google Scholar]
- 4.Gao YJ, Zhang L, Ji RR. Spinal injection of TNF-alpha-activated astrocytes produces persistent pain symptom mechanical allodynia by releasing monocyte chemoattractant protein-1. Glia. 2010;58:1871–1880. doi: 10.1002/glia.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Orita S, Koshi T, Mitsuka T, Miyagi M, Inoue G, Arai G, Ishikawa T, Hanaoka E, Yamashita K, Yamashita M, Eguchi Y, Toyone T, Takahashi K, Ohtori S. Associations between proinflammatory cytokines in the synovial fluid and radiographic grading and pain-related scores in 47 consecutive patients with osteoarthritis of the knee. BMC Musculoskelet Disord. 2011;12:144. doi: 10.1186/1471-2474-12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H, Zhang X, He JY, Zheng XF, Li D, Li Z, Zhu JF, Shen C, Cai GQ, Chen XD. Increasing expression of substance P and calcitonin gene-related peptide in synovial tissue and fluid contribute to the progress of arthritis in developmental dysplasia of the hip. Arthritis Res Ther. 2015;17:4. doi: 10.1186/s13075-014-0513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murata Y, Onda A, Rydevik B, Takahashi I, Takahashi K, Olmarker K. Changes in pain behavior and histologic changes caused by application of tumor necrosis factor-alpha to the dorsal root ganglion in rats. Spine (Phila Pa 1976) 2006;31:530–535. doi: 10.1097/01.brs.0000201260.10082.23. [DOI] [PubMed] [Google Scholar]
- 8.Ogino S, Sasho T, Nakagawa K, Suzuki M, Yamaguchi S, Higashi M, Takahashi K, Moriya H. Detection of pain-related molecules in the subchondral bone of osteoarthritic knees. Clin Rheumatol. 2009;28:1395–1402. doi: 10.1007/s10067-009-1258-0. [DOI] [PubMed] [Google Scholar]
- 9.Akagi R, Sasho T, Saito M, Endo J, Yamaguchi S, Muramatsu Y, Mukoyama S, Akatsu Y, Katsuragi J, Fukawa T, Takahashi K. Effective knock down of matrix metalloproteinase-13 by an intra-articular injection of small interfering RNA (siRNA) in a murine surgically-induced osteoarthritis model. J Orthop Res. 2014;32:1175–1180. doi: 10.1002/jor.22654. [DOI] [PubMed] [Google Scholar]


