Abstract
Paraquat (PQ) is an agrochemical agent commonly used worldwide, which can cause acute lung injury (ALI) and death. Hyperbaric oxygen treatment (HBOT) is a therapeutic method, but the mechanisms of the protective effect of HBOT on ALI remain elusive. The purpose of this study was to evaluate the effect of HBOT on acute lung injury induced by PQ in rats. Wistar Albino rats (n=21) were separated into three groups of seven animals each: control (C), PQ, and PQ + HBOT groups. 20 mg/kg PQ was administered intraperitoneally in PQ and PQ + HBOT groups to induce experimental lung injury. Three days after PQ treatment, PQ + HBOT group was administered 100% O2 at 2.0 ATA for 1 hour per day, for five consecutive days. At the end of the study, lung tissue was obtained for determining total oxidant status (TOS), total antioxidant status (TAS), oxidative stress index (OSI) and histopathological determination. Tumor necrosis factor-alpha (TNF-α), interleukin 6 (IL-6), basic fibroblast growth factor (bFGF), transforming growth factor (TGF)-β1 mRNA levels were assessed by quantitative reverse transcription-polymerase chain reaction. In addition, the inducible nitric oxide synthase (iNOS) level in the plasma was determined. Plasma iNOS, OSI, tissue TNF-α, TGF-β1 and bFGF mRNA levels, and histological injury scores in PQ + HBOT group were significantly lower than PQ group. TAS level in PQ + HBOT group was significantly higher than PQ group. The findings suggest that HBOT could effectively ameliorate PQ-induced lung injury in rats.
Keywords: Paraquat, lung injury, hyperbaric oxygen treatment, oxidant, antioxidant
Introduction
Paraquat (PQ) is an agrochemical agent commonly used worldwide, which is allied to potential risks of intoxication. Paraquat, a widely used herbicide, is well known to exhibit oxidative stress and lung injury. Paraquat a highly toxic herbicide causes fatal pulmonary damage. Although liver, kidney, heart and central nervous system are affected, lung damage and pulmonary fibrosis are the most widespread injuries and the usual causes of death [1,2]. It is pathologically characterized by lung edema, hemorrhage, interstitial inflammation, and bronchial epithelial cell proliferation. Respiratory failure as a result of lung injury is the most common cause of death from PQ [3,4].
Hyperbaric oxygen treatment (HBOT) is a therapy based on high concentrations of partial oxygen pressure inside a compartment known as hyperbaric chamber. The oxygen used in the chamber is pure and is present at a pressure of two atmospheres [5,6]. Exposure to HBOT increases the dissolved oxygen content in blood, stimulates fibroblastic activity and collagen production, decreases the inflammatory response, and limits oedema, which improves the microvasculature [7]. HBOT has been reported to be an effective therapy for peripheral vascular disorders, crushing injury, compartment syndrome, acute traumatic peripheral ischemia [8,9] and improving chronic wounds healing [10]. Various mechanisms, including the ability to improve tissue oxygenation, metabolism, and microcirculation of ischemic tissues, have been suggested to account for the beneficial effects of HBOT [8,9].
HBOT is an established modality in the treatment of many disorders although their effects and functions are still unclear. Although HBOT has been studied on different lung injury models, the physiological effects and usefulness of HBOT on lung injury has not been well elucidated. The aim of the present study was to examine the effect of HBOT on paraquat-induced lung injury in rats and whether HBOT could prevent lung tissue injury induced by paraquat by measuring oxidant parameters, such as total oxidative status (TOS), oxidative stress index (OSI), and antioxidative parameters such as total antioxidant status (TAS) in the tissue samples of rats.
Methods
Animals
Twenty-one adult male Wistar albino rats (Dumlupınar University Experimental Animal Laboratory, Kütahya, Turkey) weighing 250-300 g were used. All rats were kept under environmentally controlled conditions in an air-conditioned room at 21°C, with appropriate humidity and a 12 h:12 h light:dark cycle and were fed standard rat chow and water ad libitum. This project was approved by the Dumlupınar University Ethics Committee of Animal Care and Usage, Kütahya, Turkey.
Experimental design
The rats were randomly divided into three groups, 7 rats each:
Group 1: served as controls and the rats were injected intraperitoneally with saline (0.9% NaCl). Group 2: designated PQ was injected intraperitoneally daily with PQ at a single dose of 20 mg/kg body weight. Group 3: designated PQ + HBOT, was injected intraperitoneally daily with PQ at a single dose of 20 mg/kg body weight and was administered 100% O2 at 2.0 ATA for 1 hour per day, for five consecutive days, three days after PQ treatment.
Lung injury was induced by paraquat as previously described [11]. Paraquat dichloride (methyl violgen, Sigma) was dissolved in saline (0.9% NaCl) and animals were given 20 mg/kg intraperitoneal injection in each case.
Hyperbaric oxygen therapy (HBOT)
Rats were placed in a hyperbaric chamber 3 days after paraquat administration and received 1 hour of hyperbaric oxygen (HBO2) at 2.0 ATA in 100% O2 each day for five consecutive days [12]. Compression air was performed at a rate of 1 kg/cm2/min to 2.0 ATA/100% oxygen and maintained for 60 minutes. The chamber was flushed with 100% oxygen at a rate of 5 L/min to avoid carbondioxide accumulation. Decompression was performed at 0.2 kg/cm2/min. Chamber temperature was maintained between 22°C and 25°C. To minimize the effects of diurnal variation, all HBO2 exposures were started at around 10:00 AM.
Tissue preparation and blood sampling
After the final HBOT exposure, rats were fasted overnight before blood sampling was performed. Animals were anesthetized with 50 mg/kg ketamine and xylazine (10 mg/kg) (ip) and blood sample was collected from the aorta. Blood samples were centrifuged at 3000 rpm for 5 min, and the plasma was then collected for iNOS analysis and stored at -80°C until analysis. Lung samples were then collected in liquid nitrogen for analysis of oxidant, antioxidant enzymes and molecular biologic studies, and stored at -80°C until analysis. Histopatological evaluation was also performed.
Biochemical analyses
Plasma concentration of iNOS was analyzed in ELISA device, in accordance with the rat-specific kit protocols (Cusabio, China).
Total antioxidant (TAS) and total oxidant (TOS) levels, which are among the oxidative stress parameters, were measured using kits (Rell Assay, Gaziantep, Turkey) that work with spectrophotometric methods [13,14]. In Beckman Coulter AU680 analyzer (Beckman Coulter, Miami, FL, USA). Oxidative stress index (OSI=[(TOS/TAS)×100]) was calculated by using the obtained TAS and TOS data. The results were calculated dividing the concentrations of the parameters analyzed in the lung tissue by the most recently obtained total protein levels. The results were shown as concentration per gram protein or concentration milligram protein.
Real-time polymerase chain reaction (RT-PCR)
Total RNA isolation was extracted from lung tissue samples by the GeneJET RNA Purification Kit (Thermo, Cat No: # K0732) according to the manufacturer’s protocol. Total mRNA concentrations were measured at 260 nm using a Maestro Nano Micro-Volume spectrophotometer (Maestrogen Inc., Las Vegas, NV). Samples were stored at -80°C until further analysis. Complementary DNAs (cDNA) were synthesized by EasyScript™ cDNA Synthesis Kit (abm). cDNAs were stored at -20°C until used in the real-time polymerase chain reaction.
A total of 20 µL of PCR mixture containing cDNA template (equivalent to 20 ng total RNA), PCR-grade H2O, 10 µM each of forward primer, reverse primer and EvaGreen 2X qPCR Master Mix (abm), was amplified by using an LightCycler 480 Real Time PCR System (Roche Diagnostics, Germany) with an initial melt at 95°C for 10 min followed by 35 cycles at 95°C for 15 s, 58°C for 60 s and 72°C for 20 s. The following gene-specific primers were used: TNF-α (forward: 5’-CCA CCA CGC TCT TCT GTC TAC-3’; reverse:5’-GCT ACG GGC TTG TCA CTC G-3’; 148 bp expected); IL-6 (forward: 5’-CTT CCA GCC AGT TGC CTT CTT G-3’; reverse: 5’-TGG TCT GTT GTG GGT GGT ATC C-3’; 109 bp expected); bFGF (forward: 5’-GGC TAT GAA GGA AGA TGG AC-3’; reverse: 5’-GTT CGT TTC AGT GCC ACA-3’; 141 bp expected); TGF-β1 (forward: 5’-GCT AAT GGT GGA CCG CAA CAA C-3’; reverse: 5’-CAG CAG CCG GTT ACC AAG-3’; 229 bp expected); β-actin (forward: 5’-CTA TCG GCA ATG AGC GGT TCC-3’ reverse: 5’-TGT GTT GGC ATA GAG GTC TTT ACG-3’; 147 bp expected). The number of amplification steps required to reach an arbitrary intensity threshold (Ct) was computed. The relative gene expression was represented as 2 (-∆Ct), where ∆Ct=Cttarget-Ctβ-actin. Fold change for the treatment was calculated as 2-∆∆Ct, where ∆∆Ct=∆Cttreatment-∆Ctvehicle.
Histopathologic examinations
Lung tissue samples were fixed in 10% formalin, embedded in paraffin, sectioned (thickness of 4 µm), and slides were stained with hematoxylin and eosin (H&E). Slides were examined under light microscope (Olympus BX51, Tokyo, Japan) by a pathologist who was blinded to the treatment each animal had received. The pathologic slides were graded histopathologically according to the severity of lung damage by using a modified scoring system (Table 1) [15]. In this scoring system, four lung damage indices; edema (score: 0-4), hemorrhage (score: 0-4), leukocyte infiltration (score: 0-4), and alveolar septal thickening (score: 0-4) are graded for a total score of 0-16.
Table 1.
Histopathologic lung damage criteria according to modified scoring system [15]
| Scor | Edema | Hemorrhage | Leukocyte infiltration | Alveolar septal thickening |
|---|---|---|---|---|
| 0 | No edema | No hemorrhage | Less than 10 cells | No thickened alveolar walls |
| 1 | Edema in one quadrant | Hemorrhage in one quadrant | At least 10 cells | Thickened walls in one quadrant |
| 2 | Edema in two quadrant | Hemorrhage in two quadrant | At least 25 cells | Thickened walls in two quadrant |
| 3 | Edema in three quadrant | Hemorrhage in three quadrant | At least 50 cells | Thickened walls in three quadrant |
| 4 | Edema in four quadrant | Hemorrhage in four quadrant | Over 75 cells | Thickened walls in four quadrant |
Statistical analysis
Statistical analysis was done with SPSS (Statistical Package for Social Sciences, Chicago, IL, USA) 16.0 pocket program. All results were given as means ± standard error (SE). For analysis of concentration effect and time course, a multiple range of anova and Bonferroni post-hoc tests were used. Values smaller than P ≤ 0.05 were accepted as statistically significant.
Results
Effect of HBOT on tissue TAS, TOS and OSI levels
There were statistically significant differences in tissue TAS levels among the groups of C, PQ, and PQ + HBOT (P=0.001). Compared with the C and PQ + HBOT groups, tissue TAS level was decreased significantly in PQ group (P=0.017 and P=0.001). Tissue TAS level significantly increased in the PQ + HBOT group compared with the C and PQ groups (P=0.038, P=0.001) (Table 2).
Table 2.
The levels of Total Antioxidant Status (TAS), Total Oxidant Status (TOS), Oxidative Stress Index (OSI) in Control (C), Paraquat (PQ) and Paraquat + Hyperbaric Oxygen Treatment (PQ + HBOT) groups
| Groups | C (n=7) | PQ (n=7) | PQ + HBOT (n=7) | p |
|---|---|---|---|---|
| TAS | 22.6 ± 0.83 | 19.9 ± 0.21*,† | 26.1 ± 0.75* | 0.001 |
| TOS | 0.62 ± 0.05 | 0.66 ± 0.02 | 0.63 ± 0.01 | 0.560 |
| OSI | 2.78 ± 0.29 | 3.31 ± 0.14† | 2.44 ± 0.09 | 0.007 |
Data are expressed as mean± standard error. p: Shows the differences between all groups.
P ≤ 0.05 vs C group;
P ≤ 0.05 versus PQ + HBOT group.
Significant differences were not observed in the levels of tissue TOS among the groups of C, PQ, and PQ + HBOT (P=0.560). It was observed that augmentation in tissue TOS level of the PQ group compared with the C and PQ + HBOT groups was not significant (P=0.902 and P=0.535) (Table 2).
OSI levels differed a significantly among the groups of C, PQ, and PQ + HBOT (P=0.007). The high levels of OSI were found in PQ group compared with PQ + HBOT group, P=0.001 (Table 2).
Effect of HBOT on plasma iNOS
Plasma iNOS levels were observed to be statistically significant different among C (2.88 ± 0.02 IU/ml), PQ (3.32 ± 0.19 IU/ml), and PQ + HBOT (2.75 ± 0.15 IU/ml) (P=0.041). Compared with the PQ + HBOT group, the plasma iNOS levels were increased significantly in PQ group (P=0.05) (Figure 1).
Figure 1.
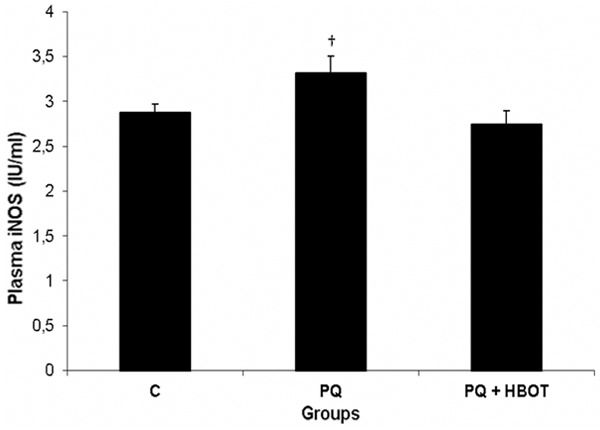
Plasma iNOS concentrations in Control (C), Paraquat (PQ), Paraquat + Hyperbaric Oxygen Treatment (PQ + HBOT) groups. Data are expressed as mean± standard error. †P ≤ 0.05 versus PQ + HBOT group. iNOS, inducible nitric oxide synthase.
Effect of HBOT on mRNA expression of TNF-α, IL-6, bFGF and TGF-β1
There were significant differences in TNF-α, IL-6, bFGF and TGF-β1 gene mRNA expression levels in the lung tissue among C, PQ, PQ + HBOT groups, (P ≤ 0.05). Rat lung expression of TNF-α, IL-6, bFGF and TGF-β1 mRNA was increased in the PQ group compared with the C group (P ≤ 0.05). HBOT produced a significant decrease in rat lung expression of TNF-α, bFGF and TGF-β1 mRNA (P ≤ 0.05) in the PQ + HBOT group. But, low levels of IL-6 gene mRNA expression were not significant in the PQ + HBOT group compared with PQ group (Figure 2).
Figure 2.
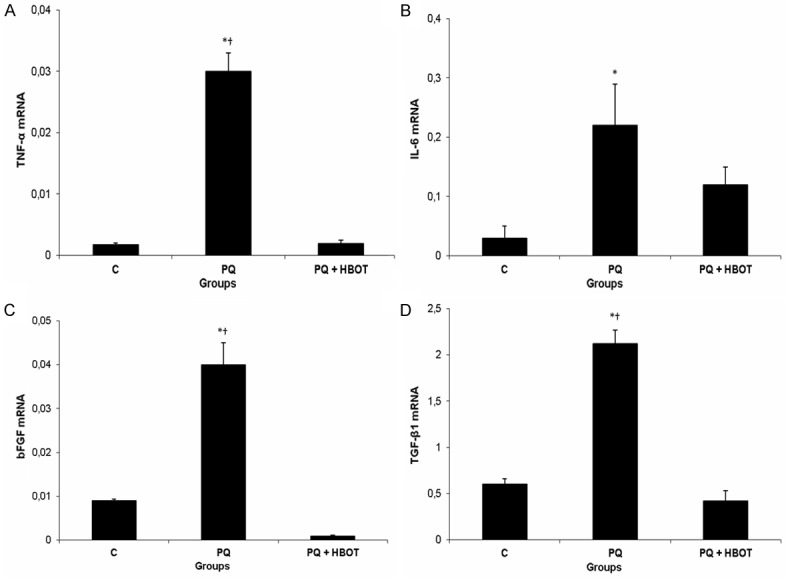
Effect of HBOT on mRNA expression of TNF-α, IL-6, bFGF and TGF-β1 in rats with paraquat-induced acute lung injury. A. The TNF-α mRNA expression for each group. B. The IL-6 mRNA expression for each group. C. The bFGF mRNA expression for each group. D. The TGF-β1 mRNA expression for each group. Groups: Control (C; n=7), Paraquat (PQ; n=7), Paraquat + Hyperbaric Oxygen Treatment (PQ + HBOT; n=7). Data were expressed as mean ± standard error. *P ≤ 0.05 vs control group, †P ≤ 0.05 vs PQ + HBOT group. TNF-α, Tumor necrosis factor-alpha; IL-6, interleukin 6; bFGF, basic fibroblast growth factor; TGF-β1, transforming growth factor-beta1.
Effect of HBOT on histopathologic examinations
Histopathologic examinations revealed normal lung structure in the control group Severe lung injury was seen in PQ group. In the PQ + HBOT group, minimal hemorrhage, leukocyte infiltration, and alveolar septal thickening were observed (Figure 3). Detailed damage scores and comparisons between groups were shown in Table 3.
Figure 3.
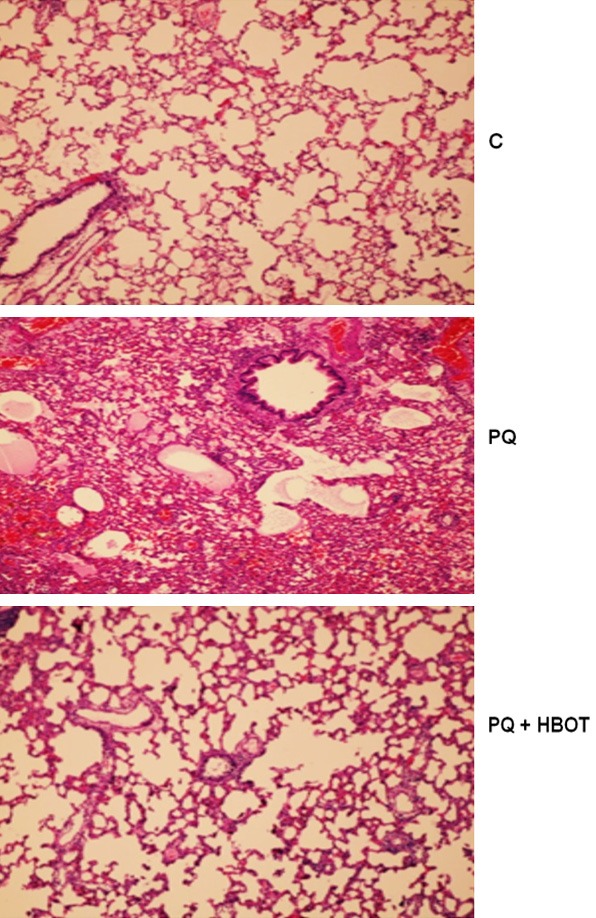
Effect of HBOT on Histopathologic Examinations. Control group demonstrated normal alveolar structure in lung. PQ group demonstrated severe edema, hemorrhage, leukocyte infiltration, and alveolar septal thickening. PQ + HBOT group demonstrated minimal hemorrhage, leukocyte infiltration, and alveolar septal thickening, H&E ×100. PQ, paraquat; HBOT, hyperbaric oxygen treatment.
Table 3.
Comparison of scored histopathological values in lung tissue samples between Control (C), Paraquat (PQ) and Paraquat + hyperbaric oxygen treatment (PQ + HBOT) groups
| Scored values | C (n=7) | PQ (n=7) | PQ + HBOT (n=7) | p |
|---|---|---|---|---|
| Edema | 0.28 ± 0.18 | 2.14 ± 0.40*,† | 0.28 ± 0.18 | 0.000 |
| Hemorrhage | 0.85 ± 0.14 | 3.00 ± 0.21*,† | 1.14 ± 0.14 | 0.000 |
| Leukocyte infiltration | 0.14 ± 0.14 | 3.85 ± 0.14*,† | 1.00 ± 0.21* | 0.000 |
| Alveolar septal thickening | 0.14 ± 0.14 | 2.57 ± 0.20*,† | 1.28 ± 0.18* | 0.000 |
| Total damage score | 0.36 ± 0.09 | 2.90 ± 0.17*,† | 0.93 ± 0.12 | 0.001 |
Data are expressed as mean ± standard error. p: Shows the differences between all groups.
P ≤ 0.05 vs C group;
P ≤ 0.05 versus PQ + HBOT group.
Discussion
PQ is a highly toxic compound for humans and animals causes fatal injury to organs upon high dose ingestion. The major cause of death in paraquat poisoning is respiratory failure. PQ accumulates in the lungs via the alveolar cells, inducing the production of intracellular reactive oxygen species (ROS) and the development of lung inflammation and fibrosis [16]. Direct exposure to PQ causes severe irritation to the eyes and skin, and ingestion of concentrated products may result in fatal injury to lungs because of edema, hemorrhage, and subsequent fibrosis as well as damage to other organs [17]. Small amounts of paraquat can cause severe and irreversible systemic damage refractory to any known treatment [18]. The mechanism of paraquat toxicity has not been fully understood.
Oxidative stress induced by free radicals may be the primary cause of tissue injury and may occur in inflammation [19]. An imbalance between ROS and the antioxidant defence system results in oxidative stress, which is closely linked to the pathogenesis of acute and chronic lung injury. Increased levels of ROS can cause direct tissue injury and promote inflammatory responses via the regulation of diverse pro-inflammatory mediators in the lungs [20].
TAS and TOS are the additive measurement parameters of antioxidant effects that enable the measurement of all the antioxidant and oxidant parameters by being evaluated together. The TOS/TAS ratio, in other words the OSI, is an indicator of the oxidative stress degree and shows the anti-oxidation and oxidation redox balance [21]. In a study by Rifaioglu et al. [22], it was observed that TAS levels were decreased while TOS values were found to be significantly higher in the PQ (15, 30, 45) groups compared with control group [22]. Kılıç et al. [23] reported that GSH and TAS levels were significantly decreased and the OSI was higher in the bleomycin-induced lung injury rats when compared to controls [23]. In our study we found that serum TAS levels of rats in the PQ group decreased when compared with the other groups. Serum TOS values of the PQ group were higher than the C and PQ + HBOT, but the differences were not statistically significant. In addition, an increase was observed in OSI levels in the PQ group compared with PQ + HBOT group. After HBOT application, we observed significantly increased TAS levels and decreased TOS and OSI levels. There are important studies in the literature about how the HBOT application affects the antioxidant enzymes in various tissues in the organism. Sanchez and colleagues [24] applied HBOT to patients with diabetic foot, and they reported that antioxidant enzyme levels were increased in the HBOT group when compared to the antibiotic treatment group. Koca et al. [25] recently showed that inflammatory cytokines and oxidative stress decreased in HBOT groups. Harabin and colleagues [26] reported an increase in SOD activity in the lung and decrease in GSH-Px activity in brain and lung tissues in HBOT-applied rats. These results seem compatible with our study results. This demonstrates that HBOT may have a preventive effect on lung aganist oxidative injury.
iNOS that is released in any acute event (trauma, stress, acute inflammation, etc.) may have either a protective or detrimental effect on tissue. High NO concentrations produced by iNOS increase the damage [27]. In this study, an increase was observed in plasma iNOS level in the PQ group compared with the PQ + HBOT group. In the study performed by Zocrato [28], it was shown that paraquat poisoning increases endothelial iNOS expression. The cytokine TNFα has been shown to play a majör role in driving the expression of iNOS in inflammatory states [29]. In our experiments, there was increased mRNA levels of TNF-α, which is consistent with a possible role of this cytokine in the PQ group. It has been reported that TNF-α is necessary for iNOS expression and iNOS-dependent vascular dysfunction [30]. These results suggest that increasing TNF-α mRNA levels can cause a increase in levels of iNOS. These high levels of plasma iNOS and TNF-α mRNA expression may be the results of their interaction and activating each other in the PQ group.
HBOT not only increased antioxidant enzyme expression, such as Cu/Zn-superoxide dismutase, catalase, and glutathione peroxidase, but also significantly decreased pro-oxidant enzyme levels, such as iNOS [31]. Huang et al. [32], showed that protein expression of iNOS and the serum NO content were dramatically reduced in the HBOT application group as compared to the spinal cord injury group. In this study, there were significant decreases plasma iNOS in the PQ + HBOT group compared with the PQ group. HBOT may exert significant effects plasma level of iNOS and protect lung tissue from paraqut induced acute lung injury.
The inflammatory reaction plays an important role in acute lung injury (ALI) in patients following paraquat poisoning. Clinically, the expression of inflammatory cytokines such as IL-6, TNF-α in the blood is significantly elevated in patients with paraquat poisoning. Numerous basic and clinical studies have demonstrated that overexpression of TNF-α can induce acute lung injury [33]. Liu et al. [34] have demonsrated that paraquat caused a significant acute systemic inflammatory response as demonstrated by the increased serum concentrations of the pro-inflammatory mediators TNF-α, IL-1β, and IL-6. Lee et al. [35] reported that IL-6 plasma levels were not elevated at 6 and 12 hours in a PQ-intoxicated rat model (50 mg/kg), although a different study showed that IL-6 levels in lung tissues were higher 1 day after PQ injection in a rat model (18 mg/kg) [36]. In this study, TNF-α and IL-6 mRNA expression in the lung tissue were significantly increased in the PQ group and were reduced with HBOT application. But, low levels of IL-6 gene mRNA expression were not significant in the PQ + HBOT group compared with PQ group. Recent experimental reports have demonstrated that HBOT exposure significantly reduced the lung injury and proinflammatory cytokines such as TNF-α, IL-1β, IL-6 [37]. These data show that HBOT alleviates PQ-induced lung injury and inhibits TNF-α and IL-6 mRNA levels. Reduced TNF-α and IL-6 mRNA levels may, at least in part, contribute to the beneficial effect of HBOT.
bFGF is involved in many physiological and pathophysiological processes like growth, wound and bone healing, cell differentiation and proliferation, but also tumour progression and metastasis, due to its ability to stimulate the activity of fibroblasts, endothelial cells, smooth muscle cells and neurons, [38,39]. In our study, we found that lung bFGF mRNA levels of rats in the PQ + HBOT group decreased when compared with the PQ group. There has not been any study in the literature between bFGF and PQ that can be compared with the results of this study. Kang et al. [40], demonstrated that it was increased secretion of bFGF in cells after initial HBOT exposure. In another study by Jung et al. [41], it was showed that HBOT was not verified on serum concentrations of bFGF [41]. In this study and previous studies on this subject, the duration and type of administration of HBOT differ leading to the differences in the results.
TGF-β1 is a polypeptide cytokine that promotes inflammatory responses by releasing related inflammatory cells [42]. TGF-β1 plays a critical role in tissue injury development of multiple organs, including lung [43]. Additionally, TGF-β1 has been reported to play an important role in ALI induced with PQ and hyperoxia [44]. It was discovered that the rat serum TGF-β1 levels in the paraquat groups were significant higher than that in the control group and the rat pulmonary TGF-β1 mRNA expression levels were also higher than that in the control group [45]. In our study, the TGF-β1 level increased 3 days after PQ intoxication and was reduced with HBOT application. Romero-Valdovinos et al. [46] showed that growth of fibroblasts and in the expression of TGF-β mRNA were decreased in keloid and nonkeloid fibroblast after chronic exposition to hyperbaric oxygenation compared with normal oxygen partial pressure [46]. This suggests that HBOT modulates the TGF-β1 level, thereby reducing PQ-induced lung injury.
In conclusion, results from the study showed that oxidative stress, reduction of antioxidant capacity, and inflammation play a crucial role in the PQ-induced lung injury. HBOT markedly inhibited the secretion of inflammatory mediators, decreased OSI and iNOS levels, and significantly increased TAS activity. In addition, it reduced lung oedema, haemorrhage, leukocyte infiltration and alveolar septal thickening on histopathologic examination. The histopathological findings support the biochemical findings. The data suggest that HBOT is a highly promising agent in protecting early lung tissue damage induced by paraquat exposure.
Acknowledgements
The authors are greatful to Dumlupınar Research Center (DPU-ILTEM) for using the laboratory and equipments, to Didem Ocak and Arif Soylu for their help in experimental research.
Disclosure of conflict of interest
None.
References
- 1.Rose MS, Smith LL, Wyatt I. Evidence for energy-dependent accumulation of paraquat into rat lung. Nature. 1974;252:314–5. doi: 10.1038/252314b0. [DOI] [PubMed] [Google Scholar]
- 2.Forman HJ, Aldrich TK, Posner MA, Fisher AB. Differential paraquat uptake and redox kinetics of rat granular pneumocytes and alveolar macrophages. J Pharmacol Exp Ther. 1982;221:428–33. [PubMed] [Google Scholar]
- 3.Dinis-Oliveira RJ, Duarte JA, S’anchez-Navarro A, Remi˜ao F, Bastos ML, Carvalho F. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol. 2008;38:13–71. doi: 10.1080/10408440701669959. [DOI] [PubMed] [Google Scholar]
- 4.Venkatesan N. Pulmonary protective effects of curcumin against paraquat toxicity. Life Sci. 1999;66:21–28. doi: 10.1016/s0024-3205(99)00576-7. [DOI] [PubMed] [Google Scholar]
- 5.Castro-Silva O, Sankarankutty AK, Martinelli AL, Souza FF, Teixeira AC, Feres O, Mente ED, Oliveira GR, Akita R, Muglia V, Elias J Jr, Ramalho LN, Zucoloto S. Therapeutic effect of hyperbaric oxygen in hepatic artery thrombosis and functional cholestasis after orthotopic liver transplantation. Transplant Proc. 2006;38:1913–7. doi: 10.1016/j.transproceed.2006.06.062. [DOI] [PubMed] [Google Scholar]
- 6.Lima CX, Sanches MD, Rezende Neto JB, Silva RC, Teixeira MM, Souza Dda G, Santos Gde C, Melo JR. Hyperbaric oxygen therapy aggravates liver reperfusion injury in rats. Acta Cir Bras. 2008;23:315–21. doi: 10.1590/s0102-86502008000400004. [DOI] [PubMed] [Google Scholar]
- 7.Laurila JP, Laatikainen LE, Castellone MD, Laukkanen MO. SOD3 reduces inflammatory cell migration by regulating adhesion molecule and cytokine expression. PLoS One. 2009;4:e5786. doi: 10.1371/journal.pone.0005786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yephuny SN, Lyskin GI, Fokina TS. Hyperbaric oxygenation in treatment of peripheral vascular disorders. Int Angiol. 1985;4:207–9. [PubMed] [Google Scholar]
- 9.Myers RA. Hyperbaric oxygen therapy for trauma: Crush injury, compartment syndrome, and other acute traumatic peripheral ischemias. Int Anesthesiol Clin. 2000;38:139–51. doi: 10.1097/00004311-200001000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Kranke P, Bennett M, Roeckl-Wiedmann I, Debus S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2004:CD004123. doi: 10.1002/14651858.CD004123.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Santiago VR, Rzezinski AF, Nardelli LM, Silva JD, Garcia CS, Maron-Gutierrez T, Ornellas DS, Morales MM, Capelozzi VL, Marini J, Pelosi P, Rocco PR. Recruitment maneuver in experimental acute lung injury: The role of alveolar collapse and edema. Crit Care Med. 2010;38:2207–14. doi: 10.1097/CCM.0b013e3181f3e076. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Gao C, Wang Y, Liu F, Ma L, Deng C, Niu KC, Lin MT, Wang C. Reducing pulmonary injury by hyperbaric oxygen preconditioning during simulated high altitude exposure in rats. J Trauma. 2011;71:673–9. doi: 10.1097/TA.0b013e3181f5b073. [DOI] [PubMed] [Google Scholar]
- 13.Erel Ö. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37:112–119. doi: 10.1016/j.clinbiochem.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 14.Erel Ö. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Yamanel L, Kaldirim U, Oztas Y, Coskun O, Poyrazoglu Y, Durusu M, Cinar O, Tuncer SK, Eyi YE Uysal B, Topal T, Oter S, Korkmaz A. Ozone therapy and hyperbaric oxygen treatment in lung injury in septic rats. Int J Med Sci. 2011;8:48–55. doi: 10.7150/ijms.8.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang X, Shao C, Wu Q, Huang M, Zhou Z. Pyrrolidine dithiocarbamate attenuates paraquat-induced lung injury in rats. J Biomed Biotechnol. 2009;2009:619487. doi: 10.1155/2009/619487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishii K, Adachi J, Tomita M, Kurosaka M, Ueno Y. Oxysterols as indices of oxidative stress in man after paraquat ingestion. Free Radic Res. 2002;36:163–8. doi: 10.1080/10715760290006493. [DOI] [PubMed] [Google Scholar]
- 18.Tian ZG, Ji Y, Yan WJ, Xu CY, Kong QY, Han F, Zhao Y, Pang QF. Methylene blue protects against paraquat-induced acute lung injury in rats. Int Immunopharmacol. 2013;17:309–313. doi: 10.1016/j.intimp.2013.06.022. [DOI] [PubMed] [Google Scholar]
- 19.Thomson A, Hemphill D, Jeejeebhoy KN. Oxidative stress and antioxidants in intestinal disease. Dig Dis. 1998;16:152–158. doi: 10.1159/000016859. [DOI] [PubMed] [Google Scholar]
- 20.Tasaka S, Amaya F, Hashimoto S, Ishizaka A. Roles of oxidants and redox signaling in the pathogenesis of acute respiratory distress syndrome. Antioxid Redox Signal. 2008;10:739–753. doi: 10.1089/ars.2007.1940. [DOI] [PubMed] [Google Scholar]
- 21.Torun E, Gedik AH, Cakir E, Umutoglu T, Gok O, Kilic U. Serum paraoxonase, TAS, TOS and ceruloplasmin in brucellosis. Int J Clin Exp Med. 2014;7:1592–1597. [PMC free article] [PubMed] [Google Scholar]
- 22.Rifaioglu MM, Sefil F, Gokce H, Nacar A, Dorum BA, Davarci M. Protective effects of caffeic acid phenethyl ester on the dose-dependent acute nephrotoxicity with paraquat in a rat model. Environ Toxicol. 2015;30:375–81. doi: 10.1002/tox.21915. [DOI] [PubMed] [Google Scholar]
- 23.Kilic T, Parlakpinar H, Polat A, Taslidere E, Vardi N, Sarihan E, Ermis H, Tanbag K. Protective and therapeutic effect of molsidomine on bleomycin-induced lung fibrosis in rats. Inflammation. 2014;37:1167–78. doi: 10.1007/s10753-014-9841-1. [DOI] [PubMed] [Google Scholar]
- 24.Martínez-Sánchez G, Al-Dalain SM, Menéndez S, Re L, Giuliani A, Candelario-Jalil E, Alvarez H, Fernández-Montequín JI, León OS. Therapeutic efficacy of ozone in patients with diabetic foot. Eur J Pharmacol. 2005;523:151–61. doi: 10.1016/j.ejphar.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 25.Koca K, Yurttas Y, Bilgic S, Cayci T, Topal T, Durusu M, Kaldirim U, Akgul EO, Ozkan H, Yanmis I, Oguz E, Tunay S, Korkmaz A, Basbozkurt M. Effect of preconditioned hyperbaric oxygen and ozone on ischemia-reperfusion induced tourniquet in skeletal bone of rats. J Surg Res. 2010;164:83–9. doi: 10.1016/j.jss.2010.06.030. [DOI] [PubMed] [Google Scholar]
- 26.Harabin AL, Braisted JC, Flynn ET. Response of antioxidant enzymes to intermittent and continuous hyperbaric oxygen. J Appl Physiol. 1990;69:328–35. doi: 10.1152/jappl.1990.69.1.328. [DOI] [PubMed] [Google Scholar]
- 27.Kubes P, McCafferty DM. Nitric oxide and intestinal inflammation. Am J Med. 2000;109:150–8. doi: 10.1016/s0002-9343(00)00480-0. [DOI] [PubMed] [Google Scholar]
- 28.Zocrato LB, Capettini LS, Rezende BA, Silva JF, Rodrigues-Machado Mda G, Cortes SF, Lemos VS. Increased expression of endothelial iNOS accounts for hyporesponsiveness of pulmonary artery to vasoconstrictors after paraquat poisoning. Toxicol In Vitro. 2010;24:1019–25. doi: 10.1016/j.tiv.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Kang YJ, Lee BK, Lee YS, Seo HG, Park MK, Kim HJ, Yun-Choi HS, Lee DH, Chang KC. Suppression of tumor necrosis factor-alpha and inducible nitric oxide synthase gene expression by THI 52, a new synthetic naphthyl-benzylisoquinoline alkaloid. Biochem Pharmacol. 2003;65:457–64. doi: 10.1016/s0006-2952(02)01549-6. [DOI] [PubMed] [Google Scholar]
- 30.Aires RD, Capettini LS, Silva JF, Rodrigues-Machado Mda G, Pinho V, Teixeira MM, Cortes SF, Lemos VS. Paraquat poisoning induces TNF-α-dependent iNOS/NO mediated hyporesponsiveness of the aorta to vasoconstrictors in rats. PLoS One. 2013;8:e73562. doi: 10.1371/journal.pone.0073562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Q, Gould LJ. Hyperbaric oxygen reduces matrix metalloproteinases in ischemic wounds through a redox-dependent mechanism. J Invest Dermatol. 2014;134:237–46. doi: 10.1038/jid.2013.301. [DOI] [PubMed] [Google Scholar]
- 32.Huang H, Xue L, Zhang X, Weng Q, Chen H, Gu J, Ye S, Chen X, Zhang W, Liao H. Hyperbaric oxygen therapy provides neuroprotection following spinal cord injury in a rat model. Int J Clin Exp Pathol. 2013;6:1337–42. [PMC free article] [PubMed] [Google Scholar]
- 33.He Q, Chen HX, Li W, Wu Y, Chen SJ, Yue Q, Xiao M, Li JW. IL-36 cytokine expression and its relationship with p38 MAPK and NF-κB pathways in psoriasis vulgaris skin lesions. J Hua Zhong Univ Sci Technolog Med Sci. 2013;33:594–599. doi: 10.1007/s11596-013-1164-1. [DOI] [PubMed] [Google Scholar]
- 34.Liu MW, Su MX, Zhang W, Wang YQ, Chen M, Wang L, Qian CY. Protective effect of Xuebijing injection on paraquat-induced pulmonary injury via down-regulating the expression of p38 MAPK in rats. BMC Complement Altern Med. 2014;14:498. doi: 10.1186/1472-6882-14-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J, Kwon W, Jo Y, Suh G, Youn Y. Protective effects of ethyl pyruvate treatment on paraquat-intoxicated rats. Hum Exp Toxicol. 2008;27:49–54. doi: 10.1177/0960327108088976. [DOI] [PubMed] [Google Scholar]
- 36.Zhi Q, Sun H, Qian X, Yang L. Edaravone, a novel antidote against lung injury and pulmonary fibrosis induced by paraquat? Int Immunopharmacol. 2011;11:96–102. doi: 10.1016/j.intimp.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 37.Bao XC, Fang YQ, You P, Zhang S, Ma J. Protective role of peroxisome proliferator-activated receptor β/δ in acute lung injury induced by prolonged hyperbaric hyperoxia in rats. Respir Physiol Neurobiol. 2014;199:9–18. doi: 10.1016/j.resp.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Brattström D, Bergqvist M, Larsson A, Holmertz J, Hesselius P, Rosenberg L, Brodin O, Wagenius G. Basic fibroblast growth factor and vascular endothelial growth factor in sera from non-small cell lung cancer patients. Anticancer Res. 1998;18:1123–7. [PubMed] [Google Scholar]
- 39.Dellacono FR, Spiro J, Eisma R, Kreutzer D. Expression of basic fibroblast growth factor and its receptors by head and neck squamous carcinoma tumor and vascular endothelial cells. Am J Surg. 1997;174:540–4. doi: 10.1016/s0002-9610(97)00169-4. [DOI] [PubMed] [Google Scholar]
- 40.Kang TS, Gorti GK, Quan SY, Ho M, Koch RJ. Effect of hyperbaric oxygen on the growth factor profile of fibroblasts. Arch Facial Plast Surg. 2004;6:31–5. doi: 10.1001/archfaci.6.1.31. [DOI] [PubMed] [Google Scholar]
- 41.Jung S, Wermker K, Poetschik H, Ziebura T, Kleinheinz J. The impact of hyperbaric oxygen therapy on serological values of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) Head Face Med. 2010;22:29. doi: 10.1186/1746-160X-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pittet JF, Griffiths MJ, Geiser T, Kaminski N, Dalton SL, Huang X, Brown LA, Gotwals PJ, Koteliansky VE, Matthay MA, Sheppard D. TGF-beta is a critical mediator of acute lung injury. J Clin Invest. 2001;107:1537–44. doi: 10.1172/JCI11963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shull MM, Ormsby I, Kier AB, Pawlowskr S, Diebold RJ, Yin M, Allen R, Sidman C, Proetzel G, Calvin D. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–9. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruiz V, Ordonez RM, Berumen J, Ramirez R, Uhal B, Becerril C, Pardo A, Selman M. Unbalanced collagenases/TIMP-1 expression and epithelial apoptosis in experimental lung fibrosis. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1026–36. doi: 10.1152/ajplung.00183.2003. [DOI] [PubMed] [Google Scholar]
- 45.Kan B, Jian X, Zhou Q, Wang J, Yu G, Sun J, Gao Y. Effect of transforming growth factor-β1 on acute lung injury caused by paraquat. Mol Med Rep. 2014;9:1232–6. doi: 10.3892/mmr.2014.1938. [DOI] [PubMed] [Google Scholar]
- 46.Romero-Valdovinos M, Cárdenas-Mejía A, Gutiérrez-Gómez C, Flisser A, Kawa-Karasik S, Ortiz-Monasterio F. Keloid skin scars: the influence of hyperbaric oxygenation on fibroblast growth and on the expression of messenger RNA for insulin like growth factor and for transforming growth factor. In Vitro Cell Dev Biol Anim. 2011;47:421–4. doi: 10.1007/s11626-011-9418-3. [DOI] [PubMed] [Google Scholar]


