Abstract
It is well known that lung cancer is the 1st leading cause of death worldwide. Many reports have demonstrated that Bad, the Bcl-xL/Bcl-2-associated death promoter plays a crucial role in the intrinsic apoptosis pathway. The aim of this study was to explore the expression of Bad and its clinical significance in small cell lung carcinoma (SCLC) By analyzing the expression of Bad in 147 SCLC patient specimen, we found that Bad expression was remarkably decreased in 55.8% (82/147) cases, compared with the neighboring non-tumor tissues. Further study showed that Bad expression was correlated with adverse clinical characters such as clinical stage (P = 0.001), tumor size (P = 0.036) and tumor recurrence (P = 0.030). Furthermore, the results of Kaplan-Meier analysis showed that low Bad expression was significantly correlated to overall survival (P < 0.0001) and disease-free survival (P = 0.017) of patients with SCLC. Moreover, multivariate analyses revealed that Bad was an independent indicator of overall survival in SCLC (hazard ration = 0.620, 95% confidence interval: 0.389-0.987, P < 0.001). In summary, we can conclude that patients with SCLC represent downregulation of Bad and the latter could be served as a useful biomarker for the outcomes of SCLC.
Keywords: Bad, Bcl2, SCLC, poor prognosis
Introduction
According to the data of Global cancer statistics, lung cancer is the most common cancer worldwide and is the first leading cause of cancer-related deaths in male and female [1]. Lung cancer can be divided into two types: non-small cell lung cancer (NSCLC) and small-cell lung cancer (SCLC). The most aggressive type is small-cell lung cancer (SCLC) and it accounts for 15-18% of all lung cancer diagnoses [2]. However, the treatment for SCLC is still difficult and the disease-free survival (DFS) is short [3]. In fact, the median survival of SCLC patients is about 15-20 months and the 5-year survival is less than 15% [4]. Although surgical technique, chemotherapy and radiation therapy for treatment of SCLC have been improved, it is difficult to cure due to the propensity of SCLC to metastasis early [5].
Apoptosis or the programmed cell death is crucial in the development of multi-cellular organisms and the protection against diseases such as malignant tumor [6]. Apoptosis have been recognized as the most important type of cell death and it includes two pathways: the extrinsic pathway (death receptor mediated pathway) and the intrinsic pathway (mitochondrial mediated pathway) [7]. In the intrinsic pathway, the B-cell lymphoma 2 protein (Bcl-2) family proteins play very important role. The Bcl-2 family proteins comprise 25 pro- and anti-apoptotic members. it has been reported that the Bcl-2 family members exist genetic and epigenetic alterations in many human cancers [8]. In SCLC, the sequential accumulation of genetic/epigenetic abnormalities endows the ability to escape apoptosis by deleting the pro-apoptotic gene and amplifying the anti-apoptotic gene especially amplification of the gene Bcl2-l1 and Bc2-l2 genes [9]. Somebody had showed that the protein level of Bcl-2 was increased in more than 90% of cases with metastatic SCLC and high expression and intensity of Bcl-2 protein is associated with the chemo-resistant in SCLC cell lines [10,11]. Furthermore, Bcl-2 expression could serve as an independent prognostic marker for poor prognosis of SCLC [12]. The Bcl-xL/Bcl-2-associated death promoter (Bad) is an important member of Bcl-2 family. Bad belongs to the pro-apoptotic family members which contains only the BH3 domain [13]. Previous studies have showed that Bad as a crucial element is involved in the anti-apoptotic signaling pathways in many cancers such as colon cancer, prostate cancer, breast cancer [14-16]. While the Bad was phosphorylated at several sites such as serines 112, 136 and 155, it loses the ability to bind Bcl-2 proteins and thus become inactivity [17]. In a wide range of malignant tumors, the expression of Bad could be as a strong predictor of overall survival and superior prognostic marker. Weimin Li et al. showed that Bad expression is decreased in non-small cell lung cancer tissues compared to normal lung tissues and demonstrated that the loss of Bad may be an independent and powerful predictor of adverse prognosis in NSCLC [18]. However, Pauline R.M. Dobson et al. showed that the strong BAD expression is related to a favorable prognosis but is not an independent prognostic factor and the AKT pathway may contribute to the prognosis of invasive breast cancer [15]. However, there is no study confirmed the expression of Bad in patient with SCLC and its clinical significance. Further study should be done to investigate that Bad could be a novel biomarker and prognostic predictor for making individual therapy strategies in patients with SCLC. It is also worthy to gain a deeper understanding of the molecular events involved in SCLC progression in order to identify novel potential targets for therapy.
The aim of this study is to understand further the detection, extent and significance of Bad expression in SCLC, we examined immunohistochemically a series of SCLC paraffin samples for Bad proteins. Relationship between Bad expression and the clinicopathological features was assessed and the prognostic value of Bad in SCLC was further determined.
Materials and methods
Patients and tissue specimens
All specimens were obtained from 147 SCLC patients who underwent surgical resection at Department of Thoracic Surgery, Zhumadian Central Hospital, between January 2000 and December 2007 and approved by the Regional Ethical Committee of Zhumadian Central Hospital. For Western blot and quantitative RT-PCR expression studies, ten paired SCLC and corresponding adjacent nontumorous tissues after surgical resection immediately stored at -80°C were subjected to western blot. The 147 patients aged from 20 to 81 years (median age is 57). The median follow-up period was 15 months (range: 1-66 months). Tumor stage was defined according to tumor-node metastasis (TNM) classification of the American Joint Committee on International Union against Cancer. Tumor differentiation was assessed according to Edmonson and Steiner grading system.
Western blot
We performed the Western blot to analyze the protein level in SCLC tissues according to previous protocols [19]. Briefly, the tissue lysates were collected and the concentrations of the protein were quantified by BCA assay kit (Thermo Fischer Scientific Inc.). Then, 30 μg of protein from each sample were examined on 10% SDS-PAGE gels and transferred onto PVDF membranes at 100 V for 60 min. After blocked by 5% nonfat milk for 1 h at room temperature, the membranes were incubated with primary antibody against Bad (at a 1:1000 dilution) or GAPDH (at a 1:1000 dilution) overnight at 4°C. After rinsed with TBST for 3 times, the membranes were incubated with peroxidase-conjugated secondary antibody (1:5000 dilution) for 1 h at room temperature. Eventually, we detected the single bands by enhanced chemiluminescence detection system (Amersham, NJ).
Immunohistochemistry
The paraffin sections were dewaxed in xylene and alcohol, boiled with EDTA (1 mmol/L; PH 8.0) and treated with 3% H2O2 for 10 minutes. The IHC analysis was performed using the envision two-step method [20]. briefly, the slides were incubated with primary antibody (Bad, at 1:500 dilution) overnight at 4°C and then washed in PBS for 3 times before the secondary antibody was added. After 1 hour at room temperature, the slides were stained with DAB and counterstained with Mayer’s hematoxylin. Slides that were not incubated with primary antibody were used as the negative controls. Stained cell proportions were scored as previous described [21]. The product of [positively stained cell proportion x stained intensity] served as the receptor score. The median value of IHC scores was 4; therefore low and high expression was set at scores of < 4 and ≥ 4, respectively [22].
Statistical analysis
Statistical analyses were performed using the SPSS 16.0 software (SPSS, Chicago, IL, USA). The Student t test was used for comparison between groups. The x2 test was performed to analyze the correlation between Bad expression and clinic pathological parameters. The Kaplan-Meier method (the log-rank test) was used for survival curves. Cox regression model with stepwise manner (forward, likelihood ratio) was utilized to perform a multivariate analysis. P < 0.05 (two-tailed) was considered statistically significant.
Results
Bad is decreased in fresh SCLC tissues
First, we determined the expression levels of Bad in 8 paired HCC tissues by western bolt. The results of western blot showed that Bad expression was significantly decreased in SCLC tissues than that in the corresponding adjacent nontumorous tissues in 75% (6/8) of cases (Figure 1A). Simultaneously, the altered expression of Bad between tumor and adjacent nontumor tissues appeared statistically significant (Figure 1B).
Figure 1.
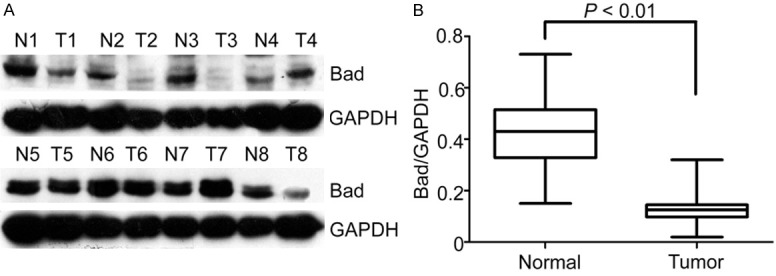
Expression of Bad in SCLC tissue samples by western blot. A. Protein levels of Bad in SCLC tissue samples by Western blot. Representative images of Bad expression were presented. The ratio of Bad/GAPDH was indicated below. B. Relative intensity of Bad normalized to GAPDH was calculated (n = 8).
Low Bad expression is associated with the poor clinic pathological parameters
For further study, we performed IHC to assess the expression of Bad in 147 paraffin-embedded SCLC tissues who received tumor resection. Our data showed that Bad was mainly located in the cytoplasm in both cancer tissues and normal tissues (Figure 2). Elsewhere, the scattered staining of Bad in nuclear was also observed in both cases. In addition, Bad expression is lower in SCLC compared to normal tissues (Figure 2) which is consisted with our previous results. After examining the relationship between Bad expression and clinicopathologic factors, we found a significant difference was observed in clinical stage (P = 0.001), tumor size (P = 0.036), NSE level (P = 0.005), and tumor recurrence (P = 0.030), but there was no statistical relationship between Bad expression and the rest clinic pathological parameters, such as age, gender or tumor location (Table 1).
Figure 2.
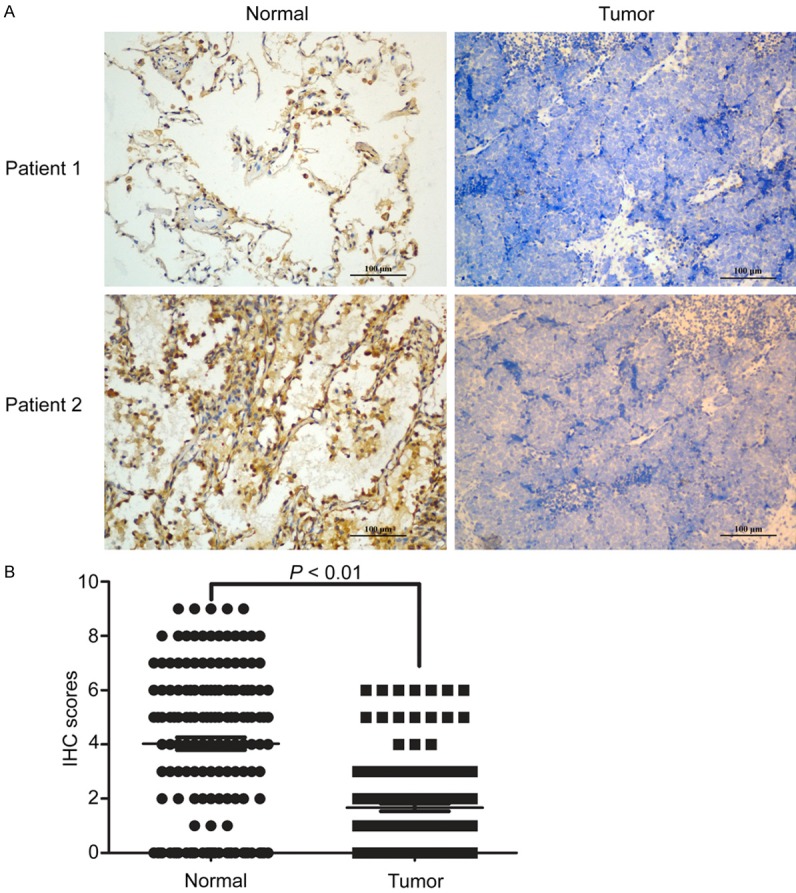
Expression of Bad in HCC tissues by IHC. A. The expression of Bad in normal lung tissues and SCLC tissues. B. ICH scores of the measurement in all 147 patients were calculated using the Wilcoxon matched paired test.
Table 1.
Correlation of clinic pathological parameters and Bad expression in the SCLC (n = 147)
| Variable | All cases | Low expression | High expression | P valuea |
|---|---|---|---|---|
| Age (years)b | 0.255 | |||
| < 57 | 55 | 34 (57.6%) | 21 (42.3%) | |
| ≥ 57 | 92 | 48 (50.0%) | 44 (50.0%) | |
| Gender | 0.712 | |||
| Male | 88 | 48 (52.4%) | 40 (47.6%) | |
| Female | 59 | 34 (65.0%) | 25 (35.0%) | |
| Lymph node metastasis | 0.490 | |||
| Yes | 87 | 45(54.3%) | 42 (45.7%) | |
| No | 42 | 19 (49.3%) | 23 (50.7%) | |
| LDH (U/L) | 0.326 | |||
| < 240 | 77 | 40 (44.7%) | 37 (55.3%) | |
| ≥ 240 | 70 | 42 (56.3%) | 28 (43.7%) | |
| NSE (µg/L) | 0.005 | |||
| ≤ 12.5 | 54 | 22 (51.8%) | 32 (48.2%) | |
| > 12.5 | 93 | 60 (62.5%) | 33 (37.5%) | |
| Tumor size (cm) | 0.036 | |||
| < 5 | 65 | 30 (41.5%) | 35 (58.5%) | |
| ≥ 5 | 82 | 52 (56.9%) | 30 (43.1%) | |
| Tumor location | 0.560 | |||
| Left | 82 | 44 (50.2%) | 38 (49.8%) | |
| Right | 65 | 38 (58.9%) | 27 (41.1%) | |
| Stage | 0.001 | |||
| I-II | 53 | 20 (47.7%) | 33 (52.3%) | |
| III-IV | 94 | 62 (60.5%) | 32 (39.5%) | |
| Tumor recurrence | 0.030 | |||
| Yes | 55 | 37 (63.8%) | 18 (36.2%) | |
| No | 92 | 45 (50.7%) | 47 (49.3%) |
Chi-square test;
Median age.
Low expression of Bad was related to the poor prognosis of SCLC patients
The association between Bad expression and the survival of SCLC patients was detected by Kaplan-Meier survival analysis. The results showed that patients with lower Bad expression were likely to be with significantly shorter overall survival (P < 0.001, Figure 3). Furthermore, a significant difference was observed between the expression of Bad and disease-free survival (P = 0.017, Figure 3B).
Figure 3.
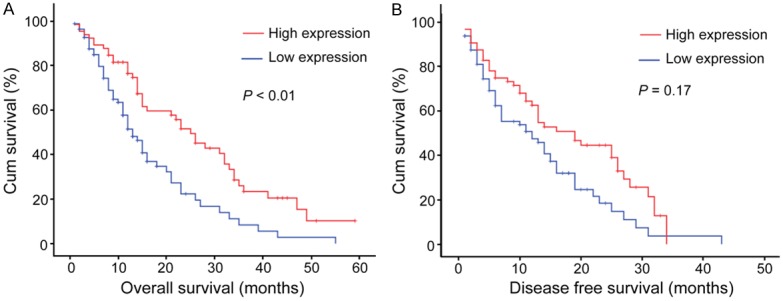
Relationship between Bad expression and SCLC prognosis. Bad protein level showed prognostic role in overall survival (A), disease-free survival (B) a, as indicated by Kaplan-Meier analysis. Statistical significance was assessed with the log-rank test. (P < 0.05; n = 147).
Univariate and multivariate analyses of prognostic variables in CSLC Patients
We next evaluated the expression of Bad and other clinicopathologic parameters on prognosis of SCLC with univariate analysis. According to the results, Bad, as well as serum NSE level, tumor size and tumor recurrence was responsible for efficacy of surgical treatment in SCLC patient, by showing that Bad expression was significantly associated with overall survival (P < 0.0001) (Table 2). Furthermore, Bad expression and those clinic pathologic variables which made significant in univariate analysis were further calculated in multivariate analysis (Table 2 and Table 3). Results suggested that Bad remained to be an independent predictor for overall survival (hazard ration = 0.620, 95% confidence interval: 0.389-0.987, P < 0.001) of SCLC patients (Table 2).
Table 2.
Univariate and multivariate analysis of clinic pathological and Bad for overall survival in SCLC (n = 147)
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
|
||||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Overall survival | ||||
| Age (< 49 vs. ≥ 49 years) | 1.010 (0.660-1.543) | 0.557 | ||
| Gender (female vs. male) | 0.882 (0.580-1.341) | 0.903 | ||
| Lymph node metastasis (yes vs. no) | 0.590 (0.226-1.540) | 0.281 | ||
| Tumor size (< 5 vs. ≥ 5 cm) | 2.312 (1.259-4.246) | 0.007 | 2.059 (1.711-3.892) | 0.000 |
| LDH (U/L) | 0.656 (0.256-1.682) | 0.380 | ||
| NSE (μg/L) | 0.837 (0.731-1.902) | 0.059 | ||
| Tumor recurrence (yes vs. no) | 0.451 (0.244-0.831) | 0.011 | 0.444 (0.245-0.803) | 0.007 |
| Tumor location | 0.995 (0.617-1.606) | 0.984 | ||
| Stage (I-II vs. III-IV) | 1.391 (0.834-2.319) | 0.206 | ||
| Bad expression (low vs. high) | 0.620 (0.389-0.987) | 0.044 | 0.530 (0.350-0.803) | 0.003 |
Table 3.
Univariate and multivariate analysis of clinicopathological and Bad for disease-free survival in SCLC (n = 147)
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
|
||||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Disease-free survival | ||||
| Age (< 49 vs. ≥ 49 years) | 1.035 (0.654-1.638) | 0.884 | ||
| Gender (female vs. male) | 1.111 (0.701-1.761) | 0.654 | ||
| Lymph node metastasis (yes vs. no) | 0.723 (0.237-2.208) | 0.569 | ||
| Tumor size (< 5 vs. ≥ 5 cm) | 1.224 (0.812-1.417) | 0.623 | ||
| LDH (U/L) | 0.770 (0.488-1.215) | 0.261 | ||
| NSE (μg/L) | 0.786 (0.455-1.315) | 0.169 | ||
| Tumor recurrence (yes vs. no) | 1.385 (0.824-1.778) | 0.000 | 0.301 (0.167-0.544) | 0.000 |
| Tumor location | 0.689 (0.442-1.076) | 0.102 | ||
| Stage (I-II vs. III-IV) | 0.964 (0.580-1.602) | 0.887 | ||
| Bad expression (low vs. high) | 0.703 (0.428-1.154) | 0.030 | 0.577 (0.386-0.864) | 0.008 |
Discussion
In the present study, we first investigate the relationship between Bad expression and clinic pathological variables as well as survival outcome in SCLC patients. Our data showed that both mRNA and protein levels of Bad was decreased from SCLC tissues to normal tissues. This result was consistent with the reports that expression of Bad is down regulated in human breast carcinoma and gastric cancer [23,24]. The Bad expression was significantly associated with tumor size indicating that the loss of Bad may facilitate SCLC growth. However, we did not observe a significant relationship between Bad expression and lymph node metastasis in paraffin SCLC samples. In addition, the absence of Bad expression was significantly associated with worse tumor stage and also indicated a significant relationship with NSE level and the tumor recurrence. These results were agreeable with the previous findings that the Bad may play a crucial rule in cell proliferation and apoptosis [25]. In our study, we found that low expression of Bad in SCLC patients was significantly associated with several malignant tumor characteristics, such as advanced stage and high NSE level which indicated that Bad might be served as a hallmark of advanced stage tumors.
In the results of Kaplan-Meier survival analysis, we found that SCLC patients with high Bad expression possess longer overall survival time. We next found that Bad may serve as an independent prognostic factor for overall survival by Cox regression analysis. The results of univariate analysis showed that high Bad expression was a statistically significant indicator of longer overall survival and disease-free survival of SCLC patients. The results were agreeable with the reports of Albazz et al. and Sinicrope et al. in breast cancer and colon cancer which demonstrated that the loss of Bad protein was an independent poor prognostic marker for disease free survival and was associated with increased mortality and poor prognosis [16,23]. The down regulation of BAD expression was related to a worse survival in SCLC patients and this could be explained by the fact that Bad is a death regulator and its loss could make the tumor more aggressive and therefore indicated a poor prognosis. Korsmeyer SJ et al. established Bad-deficient mice model and the mice eventually showed an increased incidence of hematopoietic malignancy principally attributable to diffuse large B cell lymphoma (DLBCL) which demonstrated that Bad plays a crucial rule in tumor progression [26]. Our data demonstrated that evaluation of Bad expression could be a promising prognostic marker in SCLC. SCLC showed a shorter doubling time and higher growth rate than non-small cell lung cancer and the mechanism related to the biological behavior is not yet resolved, but it is postulated that decreased amounts of Bad may inactivate a signal for tumor cell death. Our findings add new data to the emerging picture of the association between Bad expression and longer survival of SCLC patients.
Furthermore, other studies also showed that the loss of Bad was related to worse DFS in patients with malignant glioma [27]. In the present study, our data showed that Bad may be involved in SCLC metastasis and cell progression. However, the detailed mechanism by which Bad is involved in regulating the SCLC progression requires future investigation. Overall, we found that Bad protein level was decreased in SCLC patients and was associated with poor prognosis. According to the results, Bad could act as an independent prognostic biomarker in SCLC. To detect the expression of Bad in SCLC patients may improve new therapeutic strategies.
Acknowledgements
This work was supported by Educational Commission of Jiangxi Province of China (No. GJJ13036).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Planchard D, Le Pechoux C. Small cell lung cancer: new clinical recommendations and current status of biomarker assessment. Eur J Cancer. 2011;47(Suppl 3):S272–283. doi: 10.1016/S0959-8049(11)70173-3. [DOI] [PubMed] [Google Scholar]
- 3.Rossi A, Di Maio M, Chiodini P, Rudd RM, Okamoto H, Skarlos DV, Fruh M, Qian W, Tamura T, Samantas E, Shibata T, Perrone F, Gallo C, Gridelli C, Martelli O, Lee SM. Carboplatin- or cisplatin-based chemotherapy in first-line treatment of small-cell lung cancer: the COCIS meta-analysis of individual patient data. J. Clin. Oncol. 2012;30:1692–1698. doi: 10.1200/JCO.2011.40.4905. [DOI] [PubMed] [Google Scholar]
- 4.Fruh M, De Ruysscher D, Popat S, Crino L, Peters S, Felip E. Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi99–105. doi: 10.1093/annonc/mdt178. [DOI] [PubMed] [Google Scholar]
- 5.Sher T, Dy GK, Adjei AA. Small cell lung cancer. Mayo Clin Proc. 2008;83:355–367. doi: 10.4065/83.3.355. [DOI] [PubMed] [Google Scholar]
- 6.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 7.Hassen S, Ali N, Chowdhury P. Molecular signaling mechanisms of apoptosis in hereditary non-polyposis colorectal cancer. World J Gastrointest Pathophysiol. 2012;3:71–79. doi: 10.4291/wjgp.v3.i3.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galluzzi L, Morselli E, Kepp O, Vitale I, Rigoni A, Vacchelli E, Michaud M, Zischka H, Castedo M, Kroemer G. Mitochondrial gateways to cancer. Mol Aspects Med. 2010;31:1–20. doi: 10.1016/j.mam.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Kim YH, Girard L, Giacomini CP, Wang P, Hernandez-Boussard T, Tibshirani R, Minna JD, Pollack JR. Combined microarray analysis of small cell lung cancer reveals altered apoptotic balance and distinct expression signatures of MYC family gene amplification. Oncogene. 2006;25:130–138. doi: 10.1038/sj.onc.1208997. [DOI] [PubMed] [Google Scholar]
- 10.Kaiser U, Schilli M, Haag U, Neumann K, Kreipe H, Kogan E, Havemann K. Expression of bcl-2-protein in small cell lung cancer. Lung Cancer. 1996;15:31–40. doi: 10.1016/0169-5002(96)00568-5. [DOI] [PubMed] [Google Scholar]
- 11.Sartorius UA, Krammer PH. Upregulation of Bcl-2 is involved in the mediation of chemotherapy resistance in human small cell lung cancer cell lines. Int J Cancer. 2002;97:584–592. doi: 10.1002/ijc.10096. [DOI] [PubMed] [Google Scholar]
- 12.Ilievska Poposka B, Smickova S, Jovanovska Crvenkovska S, Zafirovska Ivanovska B, Stefanovski T, Petrusevska G. Bcl-2 as a prognostic factor for survival in small-cell lung cancer. Prilozi. 2008;29:281–293. [PubMed] [Google Scholar]
- 13.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 14.Sastry KS, Smith AJ, Karpova Y, Datta SR, Kulik G. Diverse antiapoptotic signaling pathways activated by vasoactive intestinal polypeptide, epidermal growth factor, and phosphatidylinositol 3-kinase in prostate cancer cells converge on BAD. J Biol Chem. 2006;281:20891–20901. doi: 10.1074/jbc.M602928200. [DOI] [PubMed] [Google Scholar]
- 15.Al-Bazz YO, Underwood JC, Brown BL, Dobson PR. Prognostic significance of Akt, phospho-Akt and BAD expression in primary breast cancer. Eur J Cancer. 2009;45:694–704. doi: 10.1016/j.ejca.2008.11.044. [DOI] [PubMed] [Google Scholar]
- 16.Sinicrope FA, Rego RL, Foster NR, Thibodeau SN, Alberts SR, Windschitl HE, Sargent DJ. Proapoptotic Bad and Bid protein expression predict survival in stages II and III colon cancers. Clin Cancer Res. 2008;14:4128–4133. doi: 10.1158/1078-0432.CCR-07-5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zha J, Harada H, Yang E, Jockel J, Korsmeyer SJ. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L) Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 18.Huang Y, Liu D, Chen B, Zeng J, Wang L, Zhang S, Mo X, Li W. Loss of Bad expression confers poor prognosis in non-small cell lung cancer. Med Oncol. 2012;29:1648–1655. doi: 10.1007/s12032-011-0060-4. [DOI] [PubMed] [Google Scholar]
- 19.Dai C, Ma S, Wang F, Zhao H, Wu X, Huang Z, Chen Z, To K, Fu L. Lapatinib promotes the incidence of hepatotoxicity by increasing chemotherapeutic agent accumulation in hepatocytes. Oncotarget. 2015;6:17738–52. doi: 10.18632/oncotarget.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamada H, Sano Y. The biotinylation of the rabbit serotonin antibody and its application to immunohistochemical studies using the two-step ABC method. Histochemistry. 1985;83:285–289. doi: 10.1007/BF00684372. [DOI] [PubMed] [Google Scholar]
- 21.Zhu H, Zhang H, Jin F, Fang M, Huang M, Yang CS, Chen T, Fu L, Pan Z. Elevated Orai1 expression mediates tumor-promoting intracellular Ca2+ oscillations in human esophageal squamous cell carcinoma. Oncotarget. 2014;5:3455–3471. doi: 10.18632/oncotarget.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laskin WB, Miettinen M. Epithelioid sarcoma: new insights based on an extended immunohistochemical analysis. Arch Pathol Lab Med. 2003;127:1161–1168. doi: 10.5858/2003-127-1161-ESNIBO. [DOI] [PubMed] [Google Scholar]
- 23.Yu B, Sun X, Shen HY, Gao F, Fan YM, Sun ZJ. Expression of the apoptosis-related genes BCL-2 and BAD in human breast carcinoma and their associated relationship with chemosensitivity. J Exp Clin Cancer Res. 2010;29:107. doi: 10.1186/1756-9966-29-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith L, Berrieman HK, O’Kane SL, Campbell A, Maraveyas A, Cawkwell L. Immunohistochemical detection of apoptotic markers in gastric cancer. Oncol Res. 2006;15:441–444. doi: 10.3727/096504005776568246. [DOI] [PubMed] [Google Scholar]
- 25.Cekanova M, Fernando RI, Siriwardhana N, Sukhthankar M, De la Parra C, Woraratphoka J, Malone C, Strom A, Baek SJ, Wade PA, Saxton AM, Donnell RM, Pestell RG, Dharmawardhane S, Wimalasena J. BCL-2 family protein, BAD is down-regulated in breast cancer and inhibits cell invasion. Exp Cell Res. 2015;331:1–10. doi: 10.1016/j.yexcr.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ranger AM, Zha J, Harada H, Datta SR, Danial NN, Gilmore AP, Kutok JL, Le Beau MM, Greenberg ME, Korsmeyer SJ. Bad-deficient mice develop diffuse large B cell lymphoma. Proc Natl Acad Sci U S A. 2003;100:9324–9329. doi: 10.1073/pnas.1533446100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cartron PF, Loussouarn D, Campone M, Martin SA, Vallette FM. Prognostic impact of the expression/phosphorylation of the BH3-only proteins of the BCL-2 family in glioblastoma multiforme. Cell Death Dis. 2012;3:e421. doi: 10.1038/cddis.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]


