Abstract
The aim of this study was to investigate the relationship among MTA2, Ki-67 and HCC patient prognosis. Tissue microarray and immunohistochemistry were used to detect the expression of MTA2 and Ki-67 in HCC samples and corresponding adjacent samples. We found MTA2 and Ki-67 were both increased in HCC tissues than those in adjacent tissues and nuclear MTA2 was associated with Ki-67 (P = 0.019). Moreover, nuclear MTA2 was a risk factor of distant metastasis in patients with HCC andKi-67 showed a negative correlation with histological grade (P < 0.05, P < 0.01, respectively). Multivariate Cox model analysis revealed that Ki-67 expression was an independent prognosis factor in HCC patients (P = 0.020). These results indicated there might be a tight correlation among MTA2, Ki-67 and HCC prognosis. MTA2 combined with Ki-67 might be used to predict HCC patient prognosis.
Keywords: MTA2, Ki-67, HCC, liver cancer
Introduction
Liver cancer is one of the most common malignancies, and is responsible for more than 300,000 deaths per annum in China. The majority of diagnosed liver cancer cases are hepatocellular carcinoma. A growing number of studies have found many important proteins, enzymes and genes in liver cancer [1-3].
It’s well known that Metastasis is the ultimate cause of death for most cancer patients. MTA2 is one of the members of Metastasis-associated tumor gene family (MTA). It’s reported that MTA2 played an important role in distant metastasis of various cancer. In breast cancer, MTA2 enhances metastasis by regulating the Rho signaling pathway [4]. MTA2 also affect prognosis by promoting cell invasion in gastric cancer [5]. Recently, a study found that MTA2 is overexpressed in HCC and its level is associated with tumor size and differentiation in the South Korean population. Additionally, they found patients with high MTA2 expression had a poorer survival than patients with low MTA2 expression [6]. However, related molecular network was still unclear. Several studies had revealed the relationship between MTA2 and Ki-67 in malignant tumors [5,7]. However, little attention was focused on their relationship in HCC.
Ki-67 is a nuclear antigen present only in proliferating cells. It’s one of the most widely used proliferation-associated markers in cancer cells. Numerous studies also found Ki-67 had a close connection with metastasis. Researchers found Ki-67 score was an independent prognostic molecular marker to predict distant metastasis in soft tissue sarcoma [8]. Multivariate regression analysis showed that Ki-67 labeling index in the mucosa adjacent to cancer was the most significant marker for colorectal distant metastasis [9].
Since both MTA2 and Ki-67 had a close connection with metastasis and prognosis, we suspected that MTA2 and Ki-67 might operate together with each other, collaborating in the control of HCC.
In our study, tissue microarray and immunohistochemical were performed to determine the expressions of MTA2 and Ki-67 in Chinese HCC patients, and analyzed whether any relationships exist among the level of MTA2, Ki-67, pathological variables and prognosis of tumor.
Materials and methods
Clinical sample collection
HCC tissues and adjacent tissues were obtained from the Biobank of National Engineering Center for Biochip at Shanghai. Tissues were quickly fixed with 4% formaldehyde and embedded in paraffin wax. Two clinical pathologists examine tissue samples and full clinical data were obtained. This study was approved by the Ethics Committees of National Engineering Center for Biochip in Shanghai. Informed consent forms were obtained before the operation. We calculated the survival time from the surgery date to the last date of follow-up.
Tissue microarray construction
Tissue microarray was performed according to previous study [10]. Briefly, holes with a 0.6 mm diameters were made to preserve the HCC tissue. Pathologist selected tumor and its adjacent tissues on the paraffin blocks. 0.66 μm serial sections were cut from the arrayed paraffin block and placed into glass slides. At last, the tissue microarray was validated by using HE staining.
Immunohistochemistry
Tissue microarray was removed from the refrigerator and placed in the glass slide for rewarming. Then we heated the tissue microarray at 65°C for about 1 hour to melt away the seal wax. Then, we soaked the slide twice in xylene for 10 minutes, and placed it in absolute alcohol for 8 minutes followed by 95% ethanol for another 5 minutes. Finally, slides were soaked at 70% ethanol for 10 minutes. Antigen retrieval was performed in citrate buffer (pH 6.0) for 30 minutes at room temperature to increase immunoreactivity. Slides were incubated with primary antibody MTA2 (Santa Cruz, sc-55566), Ki-67 (DAKO, IR626) followed incubated with HRP labeled secondary antibody. The intensity of MTA2 and Ki-67 staining was scored as 1 (weak), or 2 (strong). Percentage scores for MTA2 were assigned as 1 (≤ 95%) and 2 (> 95%). Percentage scores for Ki-67 were assigned as 1 (≤ 15%) and 2 (> 15%). A consensus was reached by joint review in case of disagreement.
Statistical analysis
Fisher’s exact test and X2 test were used to compare the variables between groups. Kaplan-Meier was used to compare the survival rates among groups. The Cox proportional hazard model was used for multivariate survival analysis. P < 0.05 was regarded as statistically significant. All analyses were carried out by using SPSS 13.0 software.
Results
MTA2 and Ki-67 was highly expressed in HCC tissues
The immunohistochemistry staining results showed that, MTA2 protein was located in the nucleus and cytoplasm. The expression of MTA2 was higher in cancer tissue than that in paracancerous tissues. Nuclear Ki-67 expression was primarily increased in cancer tissues than in paracancerous tissues (P = 0.000) (Table 1; Figure 1).
Table 1.
The expression of MTA2 and Ki-67 in liver tissue
| Cancer tissue | Paracancerous tissue | P value | |
|---|---|---|---|
| MTA2 (Nucleus) | 1.178±0.384 | 1.000±0.000 | 0.000 |
| MTA2 (Cytoplasm) | 1.278±0.450 | 1.111±0.316 | 0.005 |
| Ki-67 (Nucleus) | 1.330±0.473 | 1.000±0.000 | 0.000 |
Figure 1.
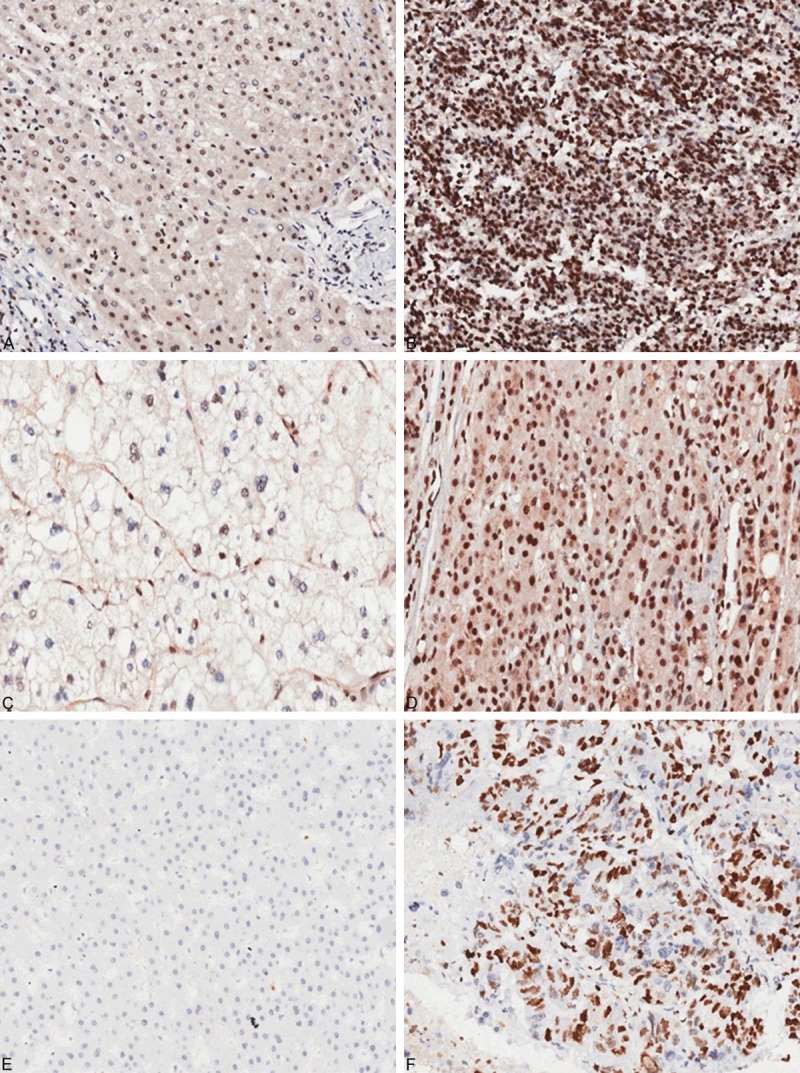
Immunohistochemical analysis of MTA2 and Ki-67 expression in HCC (200×). A. Low expression of nuclear MTA2 in paracancerous tissue. B. High expression of nuclear MTA2 in tumor tissue. C. Low expression of cytoplasmic MTA2 in paracancerous tissue. D. High expression of cytoplasmic MTA2 in tumor tissue. E. Negative expression of nuclear Ki-67 in paracancerous tissue. F. Positive expression of Nuclear Ki-67 in tumor tissue.
Relationship among MTA2, Ki-67 and clinic pathological features in HCC
To investigate the relationship between MTA2 and Ki-67, we examined their expression in nucleus and cytoplasm respectively. Nuclear MTA2 expression in cancer tissues was associated with Ki-67 (r = 0.261, P = 0.019) (Table 2).
Table 2.
The relationship between MTA2 and Ki-67
| Nucleus | Cytoplasm | Positive rate of Ki-67 (Cancer) | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| MTA2 (Cancer) | MTA2 (Paracancerous) | MTA2 (Cancer) | MTA2 (Paracancerous) | |||
| Nuclear MTA2 (Cancer) | Correlation | 1.000 | . | .014 | -.011 | .388** |
| P value | . | . | .906 | .921 | 0.001 | |
| N | 78 | 77 | 77 | 77 | 74 | |
| Nuclear MTA2 (Paracancerous) | Correlation | . | . | . | . | . |
| P value | . | . | . | . | . | |
| N | 77 | 77 | 77 | 77 | 73 | |
| Cytoplasmic MTA2 (Cancer) | Correlation | .014 | . | 1.000 | .148 | -.066 |
| P value | .906 | . | . | .198 | .577 | |
| N | 77 | 77 | 77 | 77 | 73 | |
| Cytoplasmic MTA2 (Paracanerous) | Correlation | -.011 | . | .148 | 1.000 | -.096 |
| P value | .921 | . | .198 | . | .421 | |
| N | 77 | 77 | 77 | 77 | 73 | |
| Positive rate of Ki-67 | Correlation | .388** | . | -.066 | -.096 | 1.000 |
| P value | .001 | . | .577 | .421 | . | |
| N | 74 | 73 | 73 | 73 | 84 | |
The results suggested that there were no significant differences between MTA2 expression and sex, age, pathological grade, tumor size, depth of invasion and presence of regional lymph node metastasis. However, there was a correlation between the nuclear MTA2 expression and presence of distant metastasis (P < 0.05). Further analysis showed that there was a close relationship between the positive rate of Ki-67 and pathological grade (P < 0.01). However, no significant difference was found between Ki-67 and other clinic pathological features (Table 3).
Table 3.
The relationship among MTA2, Ki-67 and pathological features
| Age | Pathological grade | Tumor size | T stage | LMN | Metastasis | TNM stage | Sex | ||
|---|---|---|---|---|---|---|---|---|---|
| Nuclear MTA2 (Cancer) | Correlation | .223 | .202 | .101 | .119 | -.083 | .242* | .110 | .049 |
| P value | .051 | .076 | .379 | .320 | .488 | .039 | .358 | .667 | |
| N | 77 | 78 | 78 | 72 | 72 | 73 | 72 | 78 | |
| Nuclear MTA2 (Paracancerous) | Correlation | . | . | . | . | . | . | . | . |
| P value | . | . | . | . | . | . | . | . | |
| N | 76 | 77 | 77 | 71 | 71 | 72 | 71 | 77 | |
| Cytoplasmic MTA2 (Cancer) | Correlation | -.126 | -.190 | .133 | -.006 | -.099 | . | -.006 | .078 |
| P value | .279 | .097 | .247 | .960 | .410 | . | .960 | .499 | |
| N | 76 | 77 | 77 | 71 | 71 | 72 | 71 | 77 | |
| Cytoplasmic MTA2 (Paracanerous) | Correlation | -.158 | -.133 | .161 | .108 | -.052 | . | .108 | -.099 |
| P value | .173 | .249 | .162 | .372 | .668 | . | .372 | .392 | |
| N | 76 | 77 | 77 | 71 | 71 | 72 | 71 | 77 | |
| Positive rate of Ki-67 | Correlation | .066 | .307** | .099 | .155 | -.108 | -.076 | .129 | .046 |
| P value | .554 | .004 | .372 | .170 | .340 | .504 | .255 | .678 | |
| N | 83 | 84 | 83 | 80 | 80 | 80 | 80 | 84 |
Survival analysis
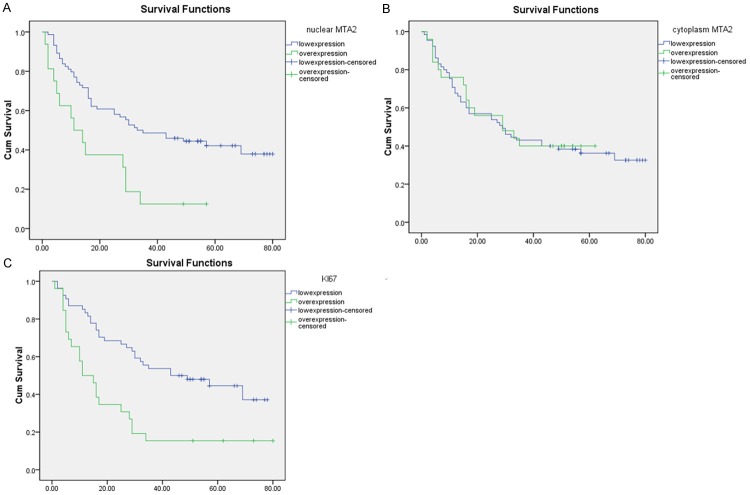
As the results showed MTA2 was closely related to tumor metastasis and Ki-67, we further examine the role of MTA2 and Ki-67 in overall survival. The Kaplan-Meier survival analysis revealed that survival time for patients with high MTA2 expression in the nucleus was obviously shorter than those with low MTA2 expression (P = 0.002). However, cytoplasmic MTA2 expression level had no correlation with survival (P = 0.851). Then we analyzed the relationship between Ki-67 and survival time. The positive rate of Ki-67 had a negative correlation with survival rate (P = 0.001) (Figure 2). We hypothesized that there was a closely connection between these two protein. We divided patients into three groups (Group 1: Ki-67 positive and MTA2 positive, Group 2: Ki-67 negative and MTA2 negative, Group 3: Ki-67 positive/MTA2 negative and Ki-67 negative/MTA2 positive). Group 1 has the best survival rate. Group 2 has the worst survival rate. Group 3 was medium (P = 0.000) (Figure 3).
Figure 2.
The relationship among MTA2, Ki-67 and survival time. A. Kaplan-Meier estimates of overall survival among HCC patients according to levels of nuclear MTA2 expression. B. Kaplan-Meier estimates of overall survival among HCC patients according to levels of cytoplasmic MTA2 expression. C. Kaplan-Meier estimates of overall survival among HCC patients according to levels of Ki-67.
Figure 3.
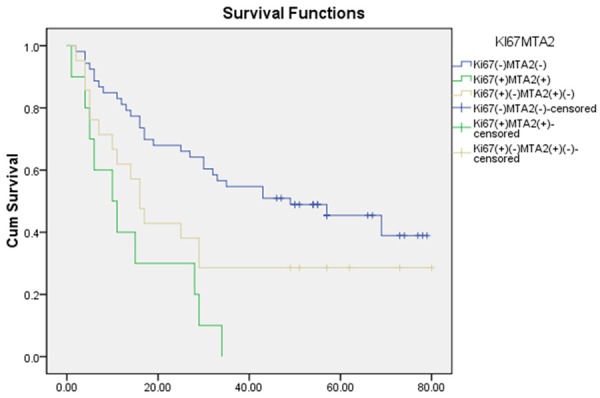
Kaplan-Meier estimates of overall survival among HCC patients according to levels of nuclear MTA2 and Ki-67 expression.
Single factor analysis indicated that MTA2 expression, Ki-67 expression, tumor size, TNM classification, tumor status and presence of distant metastasis were the primary factors influencing the prognosis of HCC patients. Multivariate Cox model analysis revealed that only Ki-67 expression was an independent prognosis factor in HCC patients (hazard ratio = 2.224, 95% confidence interval 1.135-4.357, P = 0.020) (Table 4).
Table 4.
Cox regression model for prediction of survival of HCC patients
| Features | Multivariable analysis | |
|---|---|---|
|
| ||
| HR (95% CI) | P value | |
| Nuclear MTA2 expression | 1.899 (0.834-4.321) | 0.126 |
| Ki-67 expression | 2.224 (1.135-4.357) | 0.020 |
| Tumor size | 0.899 (0.410-1.970) | 0.790 |
| T stage | 1.423 (0.432-4.692) | 0.562 |
| M stage | 14.472 (0.753-278.205) | 0.076 |
| TNM stage | 1.686 (0.454-6.267) | 0.435 |
Discussion
As a newly discovered tumor metastasis-related protein family, MTA family is involved in metastasis and prognosis in various cancers. For example, it was reported that MTA2 was negatively associated with HCC prognosis. Nevertheless, this research failed to find relevant genes through which MTA2 affect patient prognosis [11-13]. Some studies have shown MTA2 influence on patient prognosis by interacting with other genes, among which Ki-67 was an important gene involved in cancer cell proliferation and distant metastasis [5,7]. However, nobody has conducted the relationship between MTA2 and Ki-67 in HCC and joint contributions on HCC.
In the present study, the results showed that both MTA2 and Ki-67 were increased in tumor tissues and nuclear MTA2 expression was positive related to Ki-67 expression (0.019). Then, we found nuclear MTA2 in cancer tissue was related to distant metastasis and Ki-67 was positively correlated with pathological grades. Further Kaplan-Meier survival analysis showed that both MTA2 and Ki-67 had a negative relationship with patient prognosis and combined analysis of positive-MTA2 and positive Ki-67 correlated more highly with overall survival than either positive-MTA2 or positive- Ki-67 alone. Multivariate Cox model analysis revealed that only Ki-67 expression was an independent prognosis factor in HCC patients. We considered that Ki-67 played a critical role in HCC prognosis. Overexpression of Ki-67 might result in poor overall survival of HCC by affecting pathological grades. More probably, overexpression of Ki-67 might lead to poor overall survival of HCC by regulating MTA2 level. We hypothesized thatKi-67 was the most important factor in this regulatory process. Patient with high level of Ki-67 had a higher grade malignancy and worse prognosis. However, whether the regulation mechanism of Ki-67 is dependent of MTA2 or not remains unknown. Recently, researchers had found that Knockdown of MTA2 could significantly reduce the Ki-67 expression and attenuated xenograft growth and lung metastasis [5]. Researchers also found that nuclear MTA2 in lung cancer was associated with Ki-67 expression (r = 0.538, P = 0.000). Ki-67 proliferation index was much higher in nuclear MTA2-positive tumors than in nuclear MTA2-negative tumors. Furthermore, nuclear MTA2 staining had identical distribution to Ki-67 [7].
Proliferation is closely linked to distant metastasis in the development of cancer. The proliferative cancer cells were more likely to attach to vascular endothelium and initiate to metastasis. Ki-67 is primarily expressed in cells at G1, D and G2-M phases but not in the Go phase of the cell cycle. Since our results showed that the relationship between MTA2 and Ki-67, we hypothesized that MTA2 might play an important role in cancer cell proliferation. To further validate our hypothesis, more cell experiments were needed in the next phase of research. MTA2 and Ki-67 were needed to knockdown respectively in liver cancer cell, and then the mobility and proliferation of cancer cell were needed to examine.
In summary, our study showed that there was a closely relationship among MTA2, Ki-67, and prognosis. NuclearKi-67 expression was associated with the prognosis of HCC patients. Ki-67 is an independent factor to predict the prognosis of HCC patients. Considering our sample size is limited, more cases are required to validate our finding. Moreover, the mechanism of translocation and regulation of MTA2 and Ki-67 in HCC need to be studied.
Acknowledgements
This work was supported by grants from the National Science Foundation of Shanghai Municipal Health Bureau Project (Grant No. 20134361). The 12th Five-Year Plan Key Project of Science and Technology, China (Grant No. 2013ZX10002007).
Disclosure of conflict of interest
None.
References
- 1.Chen X, Cheung ST, So S, Fan ST, Barry C, Higgins J, Lai KM, Ji J, Dudoit S, Ng IO, Van De Rijn M, Botstein D, Brown PO. Gene expression patterns in human liver cancers. Mol Biol Cell. 2002;13:1929–1939. doi: 10.1091/mbc.02-02-0023.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi JK, Choi JY, Kim DG, Choi DW, Kim BY, Lee KH, Yeom YI, Yoo HS, Yoo OJ, Kim S. Integrative analysis of multiple gene expression profiles applied to liver cancer study. FEBS Lett. 2004;565:93–100. doi: 10.1016/j.febslet.2004.03.081. [DOI] [PubMed] [Google Scholar]
- 3.Liu L, Zhou J, Wang X. Gene apoptosis expression profiles in liver cancer and their comparison to normal peri-cancerous liver tissues. Zhonghua Zhong Liu Za Zhi. 2001;23:273–277. [PubMed] [Google Scholar]
- 4.Covington KR, Tsimelzon A, Fuqua SAW. MTA2 Enhances Breast Cancer Metastasis by Regulating the Rho Signaling Pathway. Cancer Res. 2009;69:870s–871s. [Google Scholar]
- 5.Zhou C, Ji J, Cai Q, Shi M, Chen X, Yu Y, Liu B, Zhu Z, Zhang J. MTA2 promotes gastric cancer cells invasion and is transcriptionally regulated by Sp1. Mol Cancer. 2013;12:102. doi: 10.1186/1476-4598-12-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee H, Ryu SH, Hong SS, Seo DD, Min HJ, Jang MK, Kwon HJ, Yu E, Chung YH, Kim KW. Over-expression of metastasis-associated protein 2 is associated with hepatocellular carcinoma size and differentiation. J Gastroenterol Hepatol. 2009;24:1445–1450. doi: 10.1111/j.1440-1746.2009.05965.x. [DOI] [PubMed] [Google Scholar]
- 7.Liu SL, Han Y, Zhang Y, Xie CY, Wang EH, Miao Y, Li HY, Xu HT, Dai SD. Expression of metastasis-associated protein 2 (MTA2) might predict proliferation in non-small cell lung cancer. Target Oncol. 2012;7:135–143. doi: 10.1007/s11523-012-0215-z. [DOI] [PubMed] [Google Scholar]
- 8.Heslin MJ, Cordon-Cardo C, Lewis JJ, Woodruff JM, Brennan MF. Ki-67 detected by MIB-1 predicts distant metastasis and tumor mortality in primary, high grade extremity soft tissue sarcoma. Cancer. 1998;83:490–497. doi: 10.1002/(sici)1097-0142(19980801)83:3<490::aid-cncr18>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 9.Kuniyasu H, Oue N, Shigeishi H, Ito R, Kato Y, Yokozaki H, Yasui W. Prospective study of Ki-67 labeling index in the mucosa adjacent to cancer as a marker for colorectal cancer metastasis. J Exp Clin Cancer Res. 2001;20:543–548. [PubMed] [Google Scholar]
- 10.Zhang X, He C, He C, Chen B, Liu Y, Kong M, Wang C, Lin L, Dong Y, Sheng H. Nuclear PKM2 expression predicts poor prognosis in patients with esophageal squamous cell carcinoma. Pathol Res Pract. 2013;209:510–515. doi: 10.1016/j.prp.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Hamatsu T, Rikimaru T, Yamashita Y, Aishima S, Tanaka S, Shirabe K, Shimada M, Toh Y, Sugimachi K. The role of MTA1 gene expression in human hepatocellular carcinoma. Oncol Rep. 2003;10:599–604. [PubMed] [Google Scholar]
- 12.Moon WS, Chang K, Tarnawski AS. Overexpression of metastatic tumor antigen 1 in hepatocellular carcinoma: Relationship to vascular invasion and estrogen receptor-alpha. Hum Pathol. 2004;35:424–429. doi: 10.1016/j.humpath.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Ryu SH, Chung YH, Lee H, Kim JA, Shin HD, Min HJ, Seo DD, Jang MK, Yu E, Kim KW. Metastatic tumor antigen 1 is closely associated with frequent postoperative recurrence and poor survival in patients with hepatocellular carcinoma. Hepatology. 2008;47:929–936. doi: 10.1002/hep.22124. [DOI] [PubMed] [Google Scholar]