Abstract
Objective: To investigate the expression and clinical significance of TRAP1 (tumor necrosis factor receptor-associated protein 1) in kidney cancer. Methods: TRAP1 expression was detected in kidney cancer and normal kidney tissues by qRT-PCR and immunohistochemistry (IHC), respectively. Then, the correlation of TRAP1 expression with clinicopathological characters and patients’ prognosis was evaluated in kidney cancer. Results: IHC results revealed that the high-expression rates of TRAP1 in kidney cancer tissues and normal kidney tissues were 51.3% (41/80), 23.3% (7/30), and the difference was statistically significant (P=0.01). Also, TRAP1 mRNA level in kidney cancer was found to be significantly greater compared with those in normal kidney by qRT-PCR. In addition, TRAP1 expression in kidney cancer significantly correlated with lymph node metastasis and clinical stage (P<0.05). Kaplan-Meier survival analysis indicated that the mean survival time of patients with TRAP1 low-expression was significantly higher (56 months) than those patients with TRAP1 high-expression (47 months). Meanwhile, Kaplan-Meier and Cox survival analysis indicated that TRAP1, lymph node metastasis and clinical stage were correlated with patients’ prognosis. Conclusion: TRAP1 is highly expressed in kidney cancer and correlates with patients prognosis, which may be served as a potential marker for the diagnosis and treatment of kidney cancer.
Keywords: Kidney cancer, TRAP1, histological grade, lymph node metastasis, clinical stage, prognosis
Introduction
TRAP1, a member of heat shock protein 90 (HSP90) family, is reported to be involved in stress protection and apoptosis [1,2]. Primary studies demonstrated that TRAP1 was mainly located in mitochondria and abundantly presented in the matrix [3,4], which might play important roles in regulating mitochondrial protein homeostasis [5,6]. Recently, TRAP1 is found to be correlated with Parkinson’s disease by protecting against mitochondrial dysfunction [7]. Moreover, TRAP1 mRNA is variably expressed in normal human tissues, such as pancreas, breast, colon and kidney tissues [8]. TRAP1 is selectively upregulated in some malignancies and implicated in tumor occurrence and progression [9-12]. However, the relationship between TRAP1 expression and kidney cancer has never been reported.
In this study, we investigated the expression of TRAP1 in normal kidney and kidney cancer tissues, and evaluated the correlation of TRAP1 expression with clinicopathological characters and patients’ prognosis in kidney cancer.
Materials and methods
Patients
Eighty cases of kidney cancer tissues and thirty cases of normal kidney tissues were obtained from the Department of Urology, Guangdong No. 2 Provincial People’s Hospital, during the year of 2012-2014. All patients aged from 46 to 72 years (Mean: 58 years) did not underwent radiotherapy or chemotherapy before surgery resection. Clinicopathological characters including histological type, lymph node metastasis, tumor size, tumor location, smoke history and clinical stage were evaluated in this study. Histological diagnosis was confirmed independently by three pathologists in a double-blinded manner. The complete data about 80 cases of kidney cancer was obtained by hospitalized records and telephone inquiries. Postoperative survival time ranged from 10 to 65 months (mean 41 months). All patients signed informed consent and permitted to use samples. This study was supported by the Ethnics Committee of Guangdong No. 2 Provincial People’s Hospital.
Immunohistochemical staining
All samples were fixed with 10% formalin, embedded in paraffin, and cut consecutively at 3 μm. Sections were dewaxed in xylene and graded ethanols. Antigen retrieval was performed by 0.01 M sodium citrate buffer (pH 6.0). Non-specific binding was blocked by 3% hydrogen peroxide and 4% bovine serum. Monoclonal antibody-TRAP1 (dilution 1:100; Santa Cruz, CA) was incubated for 2 h at room temperature. Negative controls were performed by fetal bovine serum (FBS) to substitute the primary antibody. Positive controls were performed by the sections with confirmed positive expression of TRAP1. Secondary biotinylated antibody (Jingqiao, Beijing, China) was incubated for 30 min at room temperature. Then, sections were developed by diaminobenzidine (Jingqiao, Beijing, China), and stained with haematoxylin.
Immunostaining evaluation
The sections were assessed by two observers independently in a double-blinded manner. TRAP1 expression was analyzed by semi-quantitative method. Briefly, the percentage of positive cells was scored as follows: 0, <5%; 1, 6%-25%; 2, 26%-50%; 3, >50%. The intensity of positive cells was recorded as 0 (no staining), 1 (weak staining), 2 (moderate staining), and 3 (strong staining). The sections with intensity score of 2 and >50% of positive cells were regarded as high-expression (or over-expression), and others were regarded as low-expression. The discrepancies would be reevaluated together and reached a consensus.
Quantitative reverse transcription PCR (qRT-PCR)
Total RNA was obtained from frozen tissues by Trizol reagent (TAKARA, Japan) and reversely transcribed into cDNA by using Reverse Transcription System (TAKARA, Japanese). The PCR analysis of TRAP1 gene expression was performed by using the SYBR Green RT-PCR Kit (TAKARA, Japanese). The primer sequences of TRAP1 were (F) 5’-GACGCACCGCTCAACAT-3’ and (R): 5’-CACATCAAACATGGACGGTTT-3’. GAPDH was used as internal control and its primer sequences of were (F) 5’-AGGTCGGTGTGAACGGATTTG-3’ and (R) 5’-TGTAGACCATGTAGTTGAGGTCA-3’. All primers were purified and synthesized by the Huada company (HuaDa, Shenzhen, China). Real-time PCR cycle conditions were one cycles of 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 34 s.
Statistical analysis
All data were analyzed by SPSS 19.0 (SPSS, Chicago, IL, USA). The difference of TRAP1 mRNA expression in normal and tumor tissues was analyzed by independent samples t test. The correlation between TRAP1 expression and clinicopathological characters was performed by Chi-square test (χ2-Test). Survival analysis was performed using the Kaplan-Meier method and Cox’s proportional hazards model. Statistical significance was defined as a P-value of <0.05 in two-sided test.
Results
Expression of TRAP1 in normal kidney and kidney cancer tissues
TRAP1 expression in normal kidney and kidney cancer tissues was detected by immunochemistry (IHC). IHC results showed that positive TRAP1 expression was located in the cytoplasm as orange or brown staining, as showed in Figure 1. The high-expression rates of TRAP1 in normal kidney tissues and kidney cancer tissues were 51.3% (41/80) and 23.3% (7/30), respectively, and the difference was statistically significant (Table 1, P=0.01). In addition, in order to validate the expression of TRAP1, we measured TRAP1 mRNA level in five cases of kidney cancer and matched normal kidney tissues by qRT-PCR. The results revealed that TRAP1 mRNA levels in kidney cancer tissues were significantly greater compared with those in normal kidney tissues (Figure 2, P<0.05).
Figure 1.
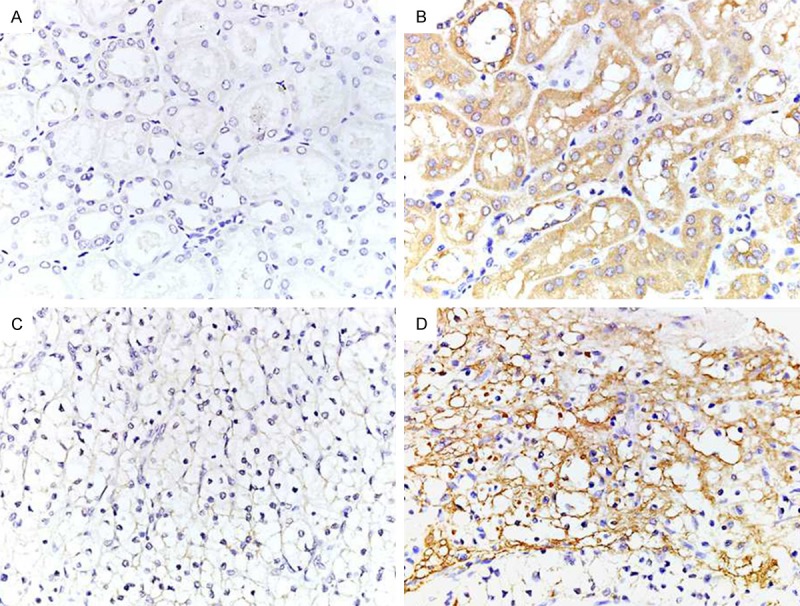
The expression of TRAP1 in normal kidney and kidney cancer tissues (40×). A: Low-expression of TRAP1 in normal kidney tissues. B: High-expression of TRAP1 in normal kidney tissues. C: Low-expression of TRAP1 in kidney cancer tissues. D: High-expression of TRAP1 in kidney cancer tissues.
Table 1.
The expression of TRAP1 in normal kidney and kidney cancer tissues
| Types | N | Low-expression | High-expression | P value |
|---|---|---|---|---|
| Normal kidney tissue | 30 | 23 | 7 | 0.01 |
| Kidney cancer tissue | 80 | 39 | 41 |
Figure 2.
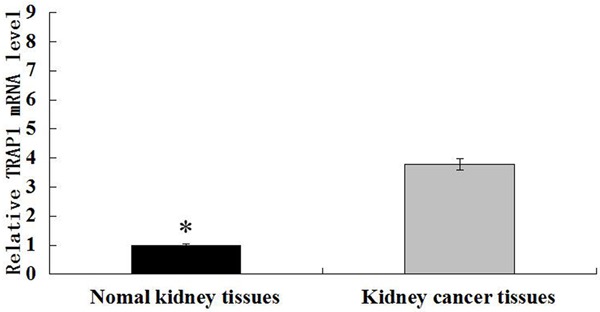
TRAP1 expression in normal kidney and kidney cancer tissues was detected by qRT-PCR. *P<0.05.
Association of TRAP1 expression with clinicopathological characters and patients’ prognosis in kidney cancer
Then, we evaluated the correlation between TRAP1 expression and clinicopathological characters in kidney cancer (Table 2). The results exhibited that TRAP1 expression was significantly associated with lymph node metastasis and clinical stage (P<0.05). TRAP1 expression was significantly elevated in patients with lymph node metastasis in comparison with those without lymph node metastasis (P=0.016). Also, significant high-expression of TRAP1 was found in patients with histological grade stage II-III and clinical stages III-IV (P=0.001). But no significant association between TRAP1 expression and age, histological type, tumor size, tumor location and smoke history was found (P>0.05).
Table 2.
Correlation between TRAP1 expression and clinicopathological characters in Kidney cancer
| Clinicopathological characters | N | Low-expression | High-expression | P value |
|---|---|---|---|---|
| Age (Years) | ||||
| <58 | 46 | 23 | 23 | 0.824 |
| ≥58 | 34 | 16 | 18 | |
| Gender | ||||
| Male | 54 | 30 | 24 | 0.098 |
| Female | 26 | 9 | 17 | |
| Smoke history | ||||
| Positive | 35 | 20 | 15 | 0.083 |
| Negative | 45 | 19 | 26 | |
| Histological type | 0.864 | |||
| Clear cell type | 68 | 34 | 34 | |
| Papillary cell type | 5 | 2 | 3 | |
| Granule cell type | 7 | 3 | 4 | |
| Tumor location | ||||
| Left | 44 | 25 | 19 | 0.123 |
| Right | 36 | 14 | 22 | |
| Tumor size (cm) | ||||
| ≤5 | 52 | 28 | 24 | 0.247 |
| >5 | 28 | 11 | 17 | |
| Lymph node metastasis | ||||
| Positive | 25 | 7 | 18 | 0.016 |
| Negative | 55 | 32 | 23 | |
| Clinical stage | ||||
| I-II | 37 | 26 | 11 | 0.001 |
| III-IV | 43 | 13 | 30 |
In addition, Kaplan-Meier survival analysis showed that patients with TRAP1 high-expression had poorer prognosis compared with those with TRAP1 low-expression (Figure 3). The median survival time of patients with TRAP1 high-expression was 47 months, which was significantly lower in comparison with that those with TRAP1 low-expression (56 months). Meanwhile, Kaplan-Meier survival analysis showed that patients’ prognosis was correlated with lymph node metastasis and clinical stage, but not with age, histological type, tumor size, tumor location and smoke history (Table 3). Cox proportional hazards regression analysis indicated TRAP1 expression, lymph node metastasis and clinical stage could be served as independent prognostic factors in kidney cancer (Table 4).
Figure 3.
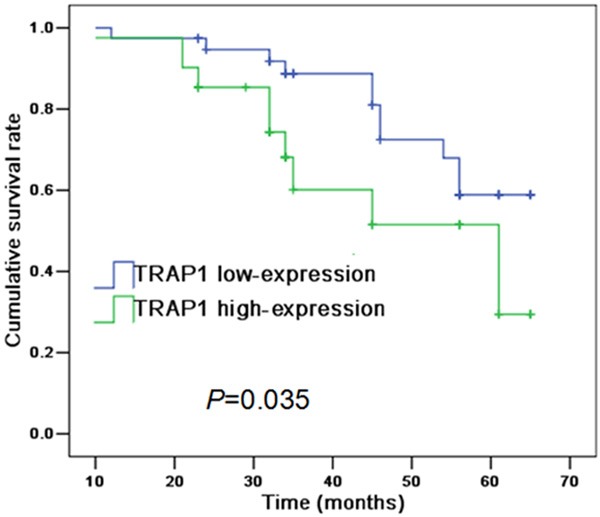
Kaplan-Meier analysis indicated TRAP1 expression correlates with patients’ prognosis in kidney cancer.
Table 3.
Kaplan-Meier analysis of prognostic factors in Kidney cancer
| Variables | N | Median survival time (95% CI) (months) | P value |
|---|---|---|---|
| TRAP1 expression | |||
| Low-expression | 39 | 56 (52-60) | 0.035 |
| High-expression | 41 | 47 (41-53) | |
| Age (Years) | |||
| <58 | 46 | 53 (48-58) | 0.276 |
| ≥58 | 34 | 51 (46-56) | |
| Gender | |||
| Male | 54 | 53 (49-57) | 0.25 |
| Female | 26 | 48 (41-55) | |
| Smoke history | |||
| Positive | 35 | 55 (51-59) | 0.107 |
| Negative | 45 | 48 (42-54) | |
| Histological type | |||
| Clear cell type | 68 | 52 (48-56) | 0.326 |
| Papillary cell type | 5 | 55 (44-66) | |
| Granule cell type | 7 | 44 (33-55) | |
| Tumor location | |||
| Left | 44 | 54 (49-59) | 0.141 |
| Right | 36 | 49 (43-55) | |
| Tumor size (cm) | 51 (47-55) | 0.94 | |
| ≤5 | 52 | 52 (46-58) | |
| >5 | 28 | ||
| Lymph node metastasis | 57 (53-61) | 0.000 | |
| Positive | 25 | 41 (34-48) | |
| Negative | 55 | ||
| Clinical stage | |||
| I-II | 37 | 58 (51-65) | 0.013 |
| III-IV | 43 | 48 (42-54) |
Table 4.
Cox regression analysis of prognostic factors in kidney cancer
| Variables | Hazard radio | 95% CI | P value |
|---|---|---|---|
| TRAP1 expression | 4.039 | 0.941-10.477 | 0.044 |
| Lymph node metastasis | 6.611 | 1.872-105.038 | 0.04 |
| Clinical stage | 2.564 | 0.755-16.374 | 0.049 |
Discussion
TRAP1 is originally described as a protein responsible for maintaining mitochondrial integrity and suppressing cell apoptosis [13]. Recent observations demonstrate that TRAP1 expression may be correlated with tumor occurrence and progression. Costantino et al. reported that TRAP1 expression was contributed to multi-drug resistance and suppressing apoptosis in human colorectal carcinoma cells [14]. Chen et al. reported that TRAP1 expression was elevated in ulcerative colitis associated colorectal cancer [15]. Han et al. reported that patients with positive TRAP1 expression had poorer prognosis [16]. In addition, Agorreta et al. reported that TRAP1 expression in lung cancer tissues was increased in comparison with those in normal lung tissues and predicted poorer outcome [17]. Li et al. Reported that TRAP1 expression in glioma tissue was increased compared with those in normal brain tissue, and also associated with patients’ prognosis [18]. However, whether TRAP1 expression correlates with kidney cancer remains unclear.
In present study, it is the first to evaluate the expression of TRAP1 in kidney cancer. The IHC results exhibited that positive TRAP1 expression was not only observed in tumor tissues but also in normal tissues. However, the high-expression rate of TRAP1 in kidney cancer tissues was markedly higher than those in normal kidney tissues. Meanwhile, significant increase of TRAP1 mRNA level was recorded in kidney cancer tissues compared with those in normal kidney tissues. Thus, these observations indicated that TRAP1 high-expression might be contributed to the occurrence of kidney cancer. Moreover, TRAP1 mutations were found to be highly linked to congenital abnormalities of the kidney and urinary tract, and might be related to Leigh syndrome [19,20]. TRAP1 up-regulation was implicated in the progression of lupus nephritis [21].
In addition, we analyzed the correlation of TRAP1 expression with clinicopathological characters and patients’ prognosis in kidney cancer. The results revealed that TRAP1 expression was significantly correlated with lymph node metastasis, which was consistent with the observation in esophageal squamous cell cancer [22]. Also, it is reported that TRAP1 was involved in cell adhesion, motility, invasion and metastasis [23]. Meanwhile, our results also demonstrated that TRAP1 expression was significantly connected with clinical stage and histological grade in kidney cancer. Moreover, Kaplan-Meier and Cox survival analysis indicated that TRAP1 was associated with the prognosis. Patients with TRAP1 high-expression presented poorer prognosis compared with those TRAP1 low-expression. Thus, these observations suggested that TRAP1 played an important role in the progression of kidney cancer, which further supported the notion that TRAP1 could be a molecular predictive marker for prognosis in malignancies [16-18].
In conclusions, this study demonstrates that TRAP1 is highly expressed in kidney cancer and correlates with clinical pathological characters and patients’ prognosis, which may be a useful molecular target for the diagnosis and immunotherapy. Of course, further studies are necessary to validate our findings.
Acknowledgements
This study was supported by National Natural Science Foundation of China (Grant No. 81472776) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Disclosure of conflict of interest
None.
References
- 1.Matassa DS, Amoroso MR, Maddalena F, Landriscina M, Esposito F. New insights into TRAP1 pathway. Am J Cancer Res. 2012;2:235–248. [PMC free article] [PubMed] [Google Scholar]
- 2.Matassa DS, Amoroso MR, Agliarulo I, Maddalena F, Sisinni L, Paladino S, Romano S, Romano MF, Sagar V, Loreni F, Landriscina M, Esposito F. Translational control in the stress adaptive response of cancer cells: a novel role for the heat shock protein TRAP1. Cell Death Dis. 2013;4:e851. doi: 10.1038/cddis.2013.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Felts SJ, Owen BA, Nguyen P, Trepel J, Donner DB, Toft DO. The hsp90-related protein TRAP1 is a mitochondrial protein with distinct functional properties. J Biol Chem. 2000;275:3305–3312. doi: 10.1074/jbc.275.5.3305. [DOI] [PubMed] [Google Scholar]
- 4.Cechetto JD, Gupta RS. Immunoelectron microscopy provides evidence that tumor necrosis factor receptor-associated protein 1 (TRAP-1) is a mitochondrial protein which also localizes at specific extramitochondrial sites. Exp Cell Res. 2000;260:30–39. doi: 10.1006/excr.2000.4983. [DOI] [PubMed] [Google Scholar]
- 5.Amoroso MR, Matassa DS, Laudiero G, Egorova AV, Polishchuk RS, Maddalena F, Piscazzi A, Paladino S, Sarnataro D, Garbi C, Landriscina M, Esposito F. TRAP1 and the proteasome regulatory particle TBP7/Rpt3 interact in the endoplasmic reticulum and control cellular ubiquitination of specific mitochondrial proteins. Cell Death Differ. 2012;19:592–604. doi: 10.1038/cdd.2011.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takamura H, Koyama Y, Matsuzaki S, Yamada K, Hattori T, Miyata S, Takemoto K, Tohyama M, Katayama T. TRAP1 controls mitochondrial fusion/fission balance through Drp1 and Mff expression. PLoS One. 2012;7:e51912. doi: 10.1371/journal.pone.0051912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costa AC, Loh SH, Martins LM. Drosophila Trap1 protects against mitochondrial dysfunction in a PINK1/parkin model of Parkinson’s disease. Cell Death Dis. 2013;4:e467. doi: 10.1038/cddis.2012.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song HY, Dunbar JD, Zhang YX, Guo D, Donner DB. Identification of a protein with homology to hsp90 that binds the type 1 tumor necrosis factor receptor. J Biol Chem. 1995;270:3574–3581. [PubMed] [Google Scholar]
- 9.Costantino E, Maddalena F, Calise S, Piscazzi A, Tirino V, Fersini A, Ambrosi A, Neri V, Esposito F, Landriscina M. TRAP1, a novel mitochondrial chaperone responsible for multi-drug resistance and protection from apoptosis in human colorectal carcinoma cells. Cancer Lett. 2009;279:39–46. doi: 10.1016/j.canlet.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 10.Liu D, Hu J, Agorreta J, Cesario A, Zhang Y, Harris AL, Gatter K, Pezzella F. Tumor necrosis factor receptor-associated protein 1 (TRAP1) regulates genes involved in cell cycle and metastases. Cancer Lett. 2010;296:194–205. doi: 10.1016/j.canlet.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 11.Sciacovelli M, Guzzo G, Morello V, Frezza C, Zheng L, Nannini N, Calabrese F, Laudiero G, Esposito F, Landriscina M, Defilippi P, Bernardi P, Rasola A. The mitochondrial chaperone TRAP1 promotes neoplastic growth by inhibiting succinate dehydrogenase. Cell Metab. 2013;17:988–999. doi: 10.1016/j.cmet.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Condelli V, Piscazzi A, Sisinni L, Matassa DS, Maddalena F, Lettini G, Simeon V, Palladino G, Amoroso MR, Trino S, Esposito F, Landriscina M. TRAP1 is involved in BRAF regulation and downstream attenuation of ERK phosphorylation and cell-cycle progression: a novel target for BRAF-mutated colorectal tumors. Cancer Res. 2014;74:6693–6704. doi: 10.1158/0008-5472.CAN-14-1331. [DOI] [PubMed] [Google Scholar]
- 13.Jeong H, Kang BH, Lee C. Crystallization and preliminary X-ray diffraction analysis of Trap1 complexed with Hsp90 inhibitors. Acta Crystallogr F Struct Biol Commun. 2014;70:1683–1687. doi: 10.1107/S2053230X14024959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costantino E, Maddalena F, Calise S, Piscazzi A, Tirino V, Fersini A, Ambrosi A, Neri V, Esposito F, Landriscina M. TRAP1, a novel mitochondrial chaperone responsible for multi-drug resistance and protection from apoptosis in human colorectal carcinoma cells. Cancer Lett. 2009;279:39–46. doi: 10.1016/j.canlet.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 15.Chen R, Pan S, Lai K, Lai LA, Crispin DA, Bronner MP, Brentnall TA. Up-regulation of mitochondrial chaperone TRAP1 in ulcerative colitis associated colorectal cancer. World J Gastroenterol. 2014;20:17037–17048. doi: 10.3748/wjg.v20.i45.17037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han JJ, Baek SK, Lee JJ, Kim GY, Kim SY, Lee SH. Combination of TRAP1 and ERCC1 Expression Predicts Clinical Outcomes in Metastatic Colorectal Cancer Treated with Oxaliplatin/5-Fluorouracil. Cancer Res Treat. 2014;46:55–64. doi: 10.4143/crt.2014.46.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agorreta J, Hu J, Liu D, Delia D, Turley H, Ferguson DJ, Iborra F, Pajares MJ, Larrayoz M, Zudaire I, Pio R, Montuenga LM, Harris AL, Gatter K, Pezzella F. TRAP1 regulates proliferation, mitochondrial function, and has prognostic significance in NSCLC. Mol Cancer Res. 2014;12:660–669. doi: 10.1158/1541-7786.MCR-13-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li S, Lv Q, Sun H, Xue Y, Wang P, Liu L, Li Z, Tian X, Liu YH. Expression of TRAP1 predicts poor survival of malignant glioma patients. J Mol Neurosci. 2015;55:62–68. doi: 10.1007/s12031-014-0413-5. [DOI] [PubMed] [Google Scholar]
- 19.Saisawat P, Kohl S, Hilger AC, Hwang DY, Yung Gee H, Dworschak GC, Tasic V, Pennimpede T, Natarajan S, Sperry E, Matassa DS, Stajic N, Bogdanovic R, de Blaauw I, Marcelis CL, Wijers CH, Bartels E, Schmiedeke E, Schmidt D, Marzheuser S, Grasshoff-Derr S, Holland-Cunz S, Ludwig M, Nothen MM, Draaken M, Brosens E, Heij H, Tibboel D, Herrmann BG, Solomon BD, de Klein A, van Rooij IA, Esposito F, Reutter HM, Hildebrandt F. Whole-exome resequencing reveals recessive mutations in TRAP1 in individuals with CAKUT and VACTERL association. Kidney Int. 2014;85:1310–1317. doi: 10.1038/ki.2013.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skinner SJ, Doonanco KR, Boles RG, Chan AK. Homozygous TRAP1 sequence variant in a child with Leigh syndrome and normal kidneys. Kidney Int. 2014;86:860. doi: 10.1038/ki.2014.208. [DOI] [PubMed] [Google Scholar]
- 21.Fismen S, Thiyagarajan D, Seredkina N, Nielsen H, Jacobsen S, Elung-Jensen T, Kamper AL, Johansen SD, Mortensen ES, Rekvig OP. Impact of the tumor necrosis factor receptor-associated protein 1 (Trap1) on renal DNaseI shutdown and on progression of murine and human lupus nephritis. Am J Pathol. 2013;182:688–700. doi: 10.1016/j.ajpath.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Ou Y, Liu L, Xue L, Zhou W, Zhao Z, Xu B, Song Y, Zhan Q. TRAP1 Shows Clinical Significance and Promotes Cellular Migration and Invasion through STAT3/MMP2 Pathway in Human Esophageal Squamous Cell Cancer. J Genet Genomics. 2014;41:529–537. doi: 10.1016/j.jgg.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Neckers L, Kern A, Tsutsumi S. Hsp90 inhibitors disrupt mitochondrial homeostasis in cancer cells. Chem Biol. 2007;14:1204–1206. doi: 10.1016/j.chembiol.2007.11.002. [DOI] [PubMed] [Google Scholar]


