Abstract
Gastric cancer pathogenesis is a multi-factor, multi-step, complicated process that related to gene abnormal expression. This study intended to explore the miR-340 effect on human gastric cancer cell line SGC-7901 and BGG823 proliferation and apoptosis, as to provide theoretical basis and experimental evidence for potential clinical application. Array was used to screen gastric cancer related abnormal genes. Q-PCR was applied to detect the screened genes expression in tissue and gastric cancer cells. MTT and colony formation assay were performed to evaluate miR-340 impact on gastric cancer proliferation. Flow cytometry was used to determine cell cycle and cell apoptosis. Q-PCR showed that miR-340 overexpressed in gastric cancer tissue significantly compared with normal control (P < 0.01). MiR-340 overexpression can promote SGC-7901 and BGC823 cells proliferation with 50% proliferation rate. Soft agar colony formation assay also showed that miR-340 overexpression can facilitate gastric cancer cell proliferation. Cell cycle analysis revealed that miR-340 overexpression can reduce cell apoptosis. Annexin V/PI staining demonstrated that miR-340 transfection can decrease cell apoptotic rate (4.58%, 1.98%, 2.11%). MiR-340 can promote tumor cell growth and reduce cell apoptosis effectively.
Keywords: miR-340, gastric cancer, proliferation, apoptosis
Introduction
Gastric cancer is considered to be one of the most common malignant tumors with high mortality, especially in East Asia and South Africa. Its incidence and mortality are also significantly higher in developing countries than that in developed countries. Gastric cancer pathogenesis is a multi-factor, multi-step, complicated process that related to gene abnormal expression [1,2]. But its potential mechanism has not been clarified. Many gastric cancer patients died quickly, which is caused by tumor cells’ rapid growth. Surgical resection is the potential treatment options with best prognosis. However, about 80% of the patients have gastritis, while only 10-15% of these gastritis patients can receive surgery [3,4]. The left unresectable tumors are mainly due to the tumor size and underlying stomach disease. More importantly, numerous patients appeared recurrence after successful surgery. A study showed that high AFP expression is a risk factor of gastric cancer recurrence [5,6]. The imperious demand now is identifying potential pathogenesis of gastric cancer.
RNA interference is used for target gene overexpression or lower expression. MicroRNA is a type of small non-coding RNA that can regulate RNA transcription and further promote or inhibit tumor gene expression. Up to now, more than 1000 human miRNAs have been identified and reported in the database, and some have been treated as a molecular biomarker for diagnosis, prognosis and treatment [7,8]. In gastric cancer patients, for example, miRNA overexpression or low expression has been investigated in tissue samples. It was reported that miR18 and miR224 overexpressed in gastric cancer, while miR195 and miR200a expression decreased [9,10]. However, there is still lack of report about miR-340 impact on gastric cancer, thus we intended to explain its effect on gastric cancer occurrence and development in this study.
Materials and methods
Clinical information
20 cases of gastric cancer tissue samples and adjacent tissue between July 2014 and December 2014 in the first affiliated hospital of Harbin medical university were collected. There were 12 cases with lymph node metastases and 8 cases without lymphatic metastasis. The tumor was staged according to 2009 international union against cancer TNM staging standard, including 14 cases in stage I-II and 6 cases in stage III-IV. Furthermore, 13 cases were squamous cell carcinoma and 7 cases were adenocarcinoma. No patients received radiotherapy preoperatively, and all cases were diagnosed by pathology. This study has been approved by the ethical committee of the first affiliated hospital of Harbin medical university and has obtained written consents from all participants.
Q-PCR
Reverse transcription kit was bought from TaKaRa. Trizol was added to tissue and serum. After centrifuged at 12,000 r/min at 4°C for 10 min, the supernatant was moved to a new EP tube. And then 200 μl chloroform was added and vibrated for 15 s, the sample was centrifuged at 12,000 r/min at 4°C for 15 min. The top layer was moved to a new EP tube and added with equal volume of isopropanol. After deposited at room temperature for 10 min, the sample was centrifuged at 12,000 r/min at 4°C for 10 min and the supernatant was removed. 75% ethanol was added for washing and the sample was then centrifuged at 12,000 r/min at 4°C for 5 min. At last, the RNA was dissolved in 20 μl DEPC water. Real time PCR was used to detect miR-340 expression in gastric cancer. Primer sequences as follows: miR-340 forward, GCGAGCTACATTGTCTGCTGGGTT; reverse, GTCGAGGGTCCGAGGTATTCCG. U6 forward, CGGCGGTAGCTTATCAGACTGATG; reverse, CCAGTCGAGGGTCCGAGGTATT. PCR reaction contained 95°C for 10 min, followed by 40 cycles including 95°C for 30 s, 55°C for 30 s and 72°C for 30 s. Melting curve was verified for analysis.
Micro-array
RNA was extracted according to the abovementioned method and received concentration detection (> 100 ng) and purity detection (260/280 OD value between 1.8 and 2.0). RNA was then mixed with system according to the manual. The reaction contained 95°C for 10 min, followed by 40 cycles including 95°C for 30 s, 56°C for 30 s and 72°C for 30 s. The data was further analyzed.
miR-340 transfection
SGC-7901 and BGC823 cells (ATCC) in logarithmic phase were seeded in six-well plate at 3~8 × 105 cells/well. 5 μl lipo2000 in 100 μl medium and 12 μl miR-296 plasmid (RiboBio, China) in 100 μl medium were mixed and added to the cells to reach 2000 μl/well. After 48 h incubation, RNA was extracted for detection.
MTT assay
The cells were seeded in the 96-well plate at 1000-10000/well. The cells were cultured for 4 h and added with 20 μl 5 mg/ml MTT (pH = 7.4). 150 μl DMSO was added to each well after 4 hours’ incubation. The well was tested at 490 nm for calculation.
Colony formation assay
100 cells were seeded in dish and cultured for macroscopic clone. The cells were washed by PBS and fixed by 4% paraformaldehyde for 15 min. And then the cells were stained by crystal violet for 10-30 min and washed by water.
Cell cycle analysis
The cells were digested by enzyme and centrifuged at 800 rpm for 10 min. Then the cells were washed with 0.5 ml PBS and blocked in 5 ml 70% ethanol at 4°C overnight. After that, the cells were collected after centrifuged at 1000 rpm for 10 min, and added with PI containing RNase for detection.
Flow cytometry
The cells in logarithmic phase were digested by enzyme and centrifuged at 800 rpm for 10 min. Then the cells were fixed at -20°C overnight and stained with 100 μl Annexin-V-Fluos. At last, the cells were incubated in 150 μl buffer containing 10 μl PI and 10 μl Annexin at room temperature for 7 min in dark and detected by flow cytometry.
Statistical analysis
All statistical analyses were performed using SPSS11.0 software (Chicago, IL). Differences between multiple groups were analyzed by t test. P < 0.05 was considered as significant difference.
Results
MiR-340 expression in the tissue
To confirm the regulatory effect of miRNA in gastric cancer, we applied micro-array detection. It was found that miR-340 expressed lower in gastric cancer tissues compared with normal control (Figure 1A). To further certify our results, we performed Q-PCR and found that miR-340 expressed significantly higher level in gastric cancer tissues than in normal tissues (P < 0.01, Figure 1B).
Figure 1.
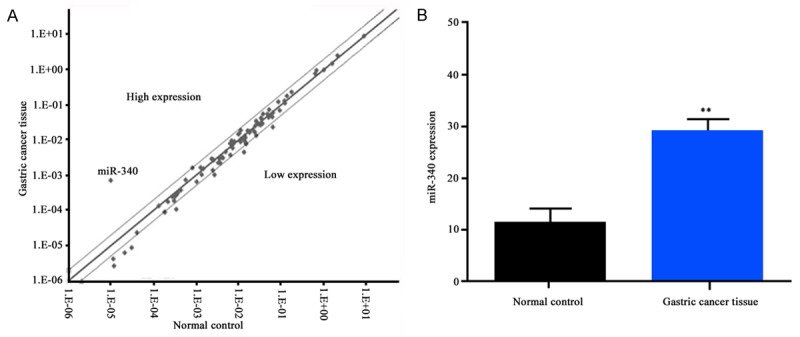
A. Screened miR-340 overexpressed in normal tissue; B. Q-PCR verification confirmed that miR-340 expressed high in normal tissue. **P < 0.01.
miR-340 overexpression affected gastric cancer cell proliferation
To investigate miR-340 activity in tumor growth, we transfected miR-340 plasmid to SGC-7901 and BGC823 cells. We also performed Q-PCR to confirm the transfection efficiency and found miR-340 overexpressed significantly after transfection (P < 0.05) (Figure 2A). Cell proliferation assay showed that miR-340 overexpression promote cell proliferation obviously (P < 0.05) (Figure 2B). Colony formation assay was used to test single cell proliferation ability. It was found that miR-340 overexpression can increase single cell’s proliferative ability markedly (P < 0.05) (Figure 2C).
Figure 2.
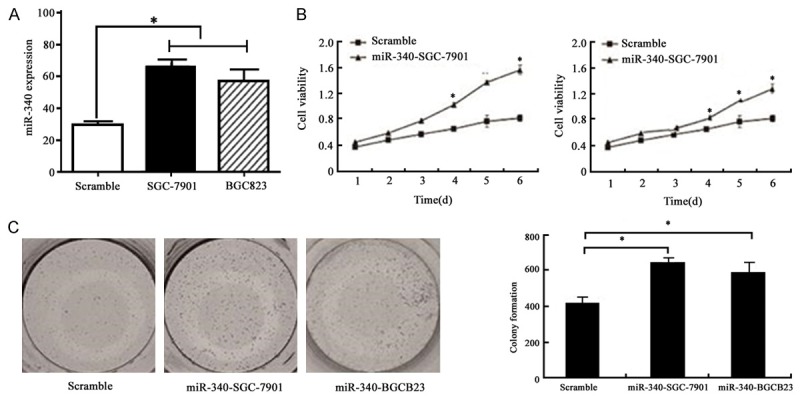
A. Q-PCR detection of cells transfected with miR-340 plasmid. B. MTT detection of cells with miR-340 overexpression, P < 0.05. C. Gastric cancer single cell proliferation ability after miR-340 overexpression detected by colony formation, P < 0.05.
MiR-340 overexpression facilitated gastric cancer cell growth and decreased cell apoptosis
For we have realized that miR-340 overexpression can promote cell proliferation, we detected its impact on cell cycle. It was found that miR-340 overexpression can intensify SGC-7901 and BGC823 cell growth significantly. As shown in Figure 3A, cell rate in S phase elevated from 18.29% to 30.21% and 32.48%. At the same time, cell apoptotic rate decreased from 4.58% to 1.98% and 2.11%, respectively (P < 0.05) (Figure 3B).
Figure 3.
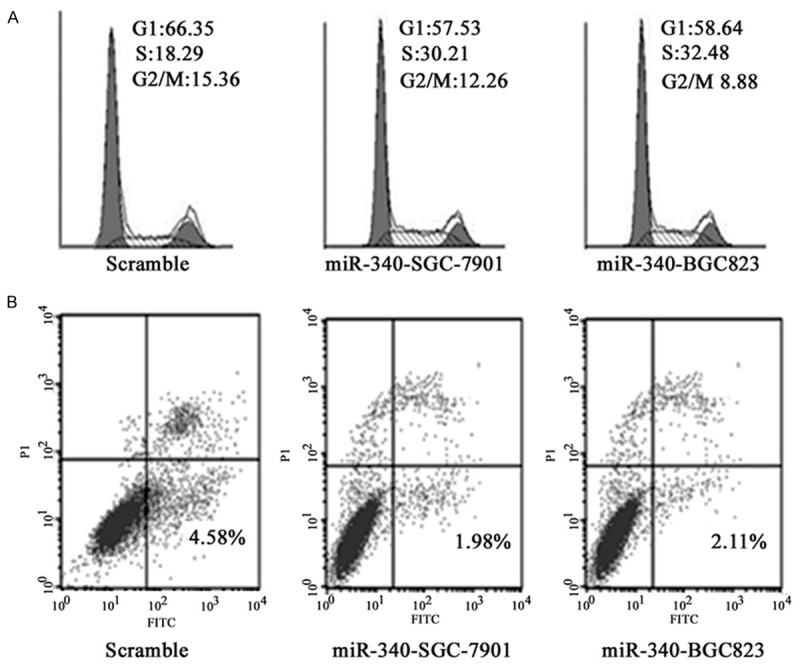
A. miR-340 overexpression promoted cell cycle. B. miR-340 overexpression decreased cell apoptosis.
Discussion
Gastric cancer is a highly malignant tumor with complicated pathogenesis. More and more researches focused on miRNA in gastric cancer. Numerous miRNAs were screened out in the early gastric cancer, and many had been considered as tumor suppressor genes, while some were recognized as carcinogenic gene. Gastric cancer development is a typical multiple factors, multi-step process that involves abnormal cell proliferation, apoptosis, invasion and metastasis. MiRNAs can regulate gene post-transcriptional level by binding with 3’-UTR of the sequence [11,12]. Previous studies reported that miRNAs were considered to promote tumor or inhibit tumor mainly through regulating cell proliferation, apoptosis, metabolism, and signal transduction [13]. Many researchers reported that miRNAs participated in gastric cancer occurrence and development. More miRNAs had been used as tumor marker in clinic, such as miR-21. Some researchers also reported AFP can be used in early gastric cancer screening and diabetes diagnosis. Infinite proliferation can make tumor continue division and proliferation, leading to anabolism stronger than catabolism and even capture normal cells protein metabolite. It makes the body in cachexia, resulting in further deterioration. Therefore, inducing tumor cell apoptosis becomes an effective way to restrain tumor unlimited proliferation.
Recent study demonstrated that miR-340 expression was suppressed in malignant tissue group compared with normal, and miR-340 deficiency may contribute to tumorigenesis and tumor progress [14,15]. For example, Zhou et al. found in mice xenograft model that miR-340 overexpression can significantly aggravate osteosarcoma cell lines proliferation, migration, invasion, tumor growth and metastasis [16]. Wu et al. reported miR-340 expression deficiency was related to lymph node metastasis, high tumor pathological grade, clinical stage and breast cancer patients’ overall survival [17,18]. Sun et al. indicated that miR-340 expression intensified colorectal cancer cells growth, and was associated with colorectal cancer prognosis [19,20].
However, there is still lack of investigation about miR-340 in gastric cancer. Our research illustrated that miR-340 expressed high in gastric cancer tissue. In this case, we transfected miR-340 to the gastric cancer cell line SGC-7901 and BGC823, and we performed Q-PCR for verification. We detected cell proliferation after miR-340 overexpression and found it can strengthen both cell proliferate ability and single cell proliferation. How to regulate such phenomenon still needs further research. For cell proliferation is an accelerated growth process, we wonder whether it act through regulating the cell cycle or apoptosis. In fact, cell cycle arrest is one of the premise conditions of cell apoptosis. MiR-340 overexpression in gastric cell can increase cell apoptosis, suggesting that miR-340 can promote oncogenesis by inducing cell growth.
This study focused on explaining miR-340 in inducing cell apoptosis, and further illustrated that it mainly acts through aggravating cell cycle. Our study first revealed the role of miR-340 in gastric cancer, providing basis for the following investigation.
Disclosure of conflict of interest
None.
References
- 1.Rugge M, Meggio A, Pennelli G, Piscioli F, Giacomelli L, De Pretis G, Graham DY. Gastritis staging in clinical practice: the OLGA staging system. Gut. 2007;56:631–636. doi: 10.1136/gut.2006.106666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rugge M, Genta RM, Group O. Staging gastritis: an international proposal. Gastroenterology. 2005;129:1807–1808. doi: 10.1053/j.gastro.2005.09.056. [DOI] [PubMed] [Google Scholar]
- 3.Miao J, Wu S, Peng Z, Tania M, Zhang C. MicroRNAs in osteosarcoma: diagnostic and therapeutic aspects. Tumour Biol. 2013;34:2093–2098. doi: 10.1007/s13277-013-0940-7. [DOI] [PubMed] [Google Scholar]
- 4.Nana-Sinkam SP, Croce CM. Clinical applications for microRNAs in cancer. Clin Pharmacol Ther. 2013;93:98–104. doi: 10.1038/clpt.2012.192. [DOI] [PubMed] [Google Scholar]
- 5.Shen J, Stass SA, Jiang F. MicroRNAs as potential biomarkers in human solid tumors. Cancer Lett. 2013;329:125–136. doi: 10.1016/j.canlet.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Osman A. MicroRNAs in health and disease--basic science and clinical applications. Clin Lab. 2012;58:393–402. [PubMed] [Google Scholar]
- 7.Amiel J, de Pontual L, Henrion-Caude A. miRNA, development and disease. Adv Genet. 2012;80:1–36. doi: 10.1016/B978-0-12-404742-6.00001-6. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez S, Risolino M, Mandia N, Talotta F, Soini Y, Incoronato M, Condorelli G, Banfi S, Verde P. miR-340 inhibits tumor cell proliferation and induces apoptosis by targeting multiple negative regulators of p27 in non-small cell lung cancer. Oncogene. 2015;34:3240–3250. doi: 10.1038/onc.2014.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babae N, Bourajjaj M, Liu Y, Van Beijnum JR, Cerisoli F, Scaria PV, Verheul M, Van Berkel MP, Pieters EH, Van Haastert RJ, Yousefi A, Mastrobattista E, Storm G, Berezikov E, Cuppen E, Woodle M, Schaapveld RQ, Prevost GP, Griffioen AW, Van Noort PI, Schiffelers RM. Systemic miRNA-7 delivery inhibits tumor angiogenesis and growth in murine xenograft glioblastoma. Oncotarget. 2014;5:6687–6700. doi: 10.18632/oncotarget.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang HW, Mendell JT. MicroRNAs in cell proliferation, cell death, and tumorigenesis. Br J Cancer. 2007;96(Suppl):R40–44. [PubMed] [Google Scholar]
- 11.Frankel LB, Wen J, Lees M, Hoyer-Hansen M, Farkas T, Krogh A, Jaattela M, Lund AH. microRNA-101 is a potent inhibitor of autophagy. EMBO J. 2011;30:4628–4641. doi: 10.1038/emboj.2011.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Li Y, Lai M. The microRNA network and tumor metastasis. Oncogene. 2010;29:937–948. doi: 10.1038/onc.2009.406. [DOI] [PubMed] [Google Scholar]
- 13.Dong H, Luo L, Hong S, Siu H, Xiao Y, Jin L, Chen R, Xiong M. Integrated analysis of mutations, miRNA and mRNA expression in glioblastoma. BMC Syst Biol. 2010;4:163. doi: 10.1186/1752-0509-4-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han S, Roman J. Peroxisome proliferator-activated receptor gamma: a novel target for cancer therapeutics? Anticancer Drugs. 2007;18:237–244. doi: 10.1097/CAD.0b013e328011e67d. [DOI] [PubMed] [Google Scholar]
- 15.Theocharis S, Margeli A, Kouraklis G. Peroxisome proliferator activated receptor-gamma ligands as potent antineoplastic agents. Curr Med Chem Anticancer Agents. 2003;3:239–251. doi: 10.2174/1568011033482431. [DOI] [PubMed] [Google Scholar]
- 16.Zhou X, Wei M, Wang W. MicroRNA-340 suppresses osteosarcoma tumor growth and metastasis by directly targeting ROCK1. Biochem Biophys Res Commun. 2013;437:653–658. doi: 10.1016/j.bbrc.2013.07.033. [DOI] [PubMed] [Google Scholar]
- 17.Wu ZS, Wu Q, Wang CQ, Wang XN, Huang J, Zhao JJ, Mao SS, Zhang GH, Xu XC, Zhang N. miR-340 inhibition of breast cancer cell migration and invasion through targeting of oncoprotein c-Met. Cancer. 2011;117:2842–2852. doi: 10.1002/cncr.25860. [DOI] [PubMed] [Google Scholar]
- 18.Yao Y, Suo AL, Li ZF, Liu LY, Tian T, Ni L, Zhang WG, Nan KJ, Song TS, Huang C. MicroRNA profiling of human gastric cancer. Mol Med Rep. 2009;2:963–970. doi: 10.3892/mmr_00000199. [DOI] [PubMed] [Google Scholar]
- 19.Sun Y, Zhao X, Zhou Y, Hu Y. miR-124, miR-137 and miR-340 regulate colorectal cancer growth via inhibition of the Warburg effect. Oncol Rep. 2012;28:1346–1352. doi: 10.3892/or.2012.1958. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda M, Shiota S, Matsunari O, Watada M, Murakami K, Fujioka T, Yamaoka Y. Prevalence of two homologous genes encoding glycosyltransferases of Helicobacter pylori in the United States and Japan. J Gastroenterol Hepatol. 2011;26:1451–1456. doi: 10.1111/j.1440-1746.2011.06779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


