Abstract
To investigate the clinical efficacy of adoptive immunotherapy using dendritic cells (DC) and cytokine-induced killer (CIK) cells combined with chemotherapy in multiple myeloma. The immunomodulatory effect of the therapy was discussed by detecting the levels of peripheral blood T cell subsets and CD4+CD25+ regulatory cells (Treg). Fifty MM patients were randomly divided into two groups: 24 cases in the simple chemotherapy group and 26 cases in the combined therapy group (chemotherapy plus DC/CIK immunotherapy). The therapeutic efficacy and the proportions of peripheral blood T cell subsets and Treg cells were compared between the two groups. The cellular immunity indicators were also compared, including IL-2, IFN-γ, IL-4, IL-10, AgNORs ratio and TGF-β. After 3 weeks of treatment, the life quality and clinical efficacy of the combined therapy group were superior to those of the simple chemotherapy group (P<0.05). CD3+CD8+ ratio, CD4+CD25+ ratio, CD4+CD25+/CD4+ ratio, CD4+CD25+FoxP3+/CD4+CD25+ ratio, IL-4, IL-10 and TGF-β levels of the combined therapy group were obviously lower than those of the simple chemotherapy group (P<0.05). The CD3+CD4+/CD3+CD8+ ratio, AgNOR ratio, IL-2 and IFN-γ level and positive rate of NKG2D in the combined therapy group were significantly higher than those of the simple chemotherapy group (P<0.05). These results indicated better immunomodulatory effect of the combined therapy. DC/CIK immunotherapy combined with chemotherapy has a good clinical efficacy and prospect for MM, reversing the Th1 to Th2 shift and increasing the anti-tumor capacity of the immune system.
Keywords: Multiple myeloma (MM), immunotherapy, dendritic cells (DCs), cytokine-induced killer cells (CIK cells), immunomodulatory
Introduction
Multiple myeloma (MM) is a plasmacyte proliferative disease that cannot be completely cured so far and the incidence of MM is rising every year. As the main treatment against MM, chemotherapy causes great harm to the human body and the high relapse rate is high. In light of the immune defects in MM, adoptive immunotherapy may be an alternative choice [1]. Dendritic cells (DCs) are the most powerful antigen-presenting cells and trigger organism-specific anti-tumor response. CIK cells constitute a heterogeneous group of cells presenting a high tumor killing activity [2]. A new anti-tumor therapy after surgery, chemotherapy and radiotherapy, CIK cells and DC cells in co-culture exhibit a strong synergistic anti-tumor effect [3,4]. Adoptive immunotherapy based on DC and CIK cells has been successfully applied to treat solid cancers and also in hematologic malignancy with certain efficacy [5,6]. We reported the comparison of the efficacy of simple chemotherapy and DC/CIK immunotherapy combined with chemotherapy in MM and the influence on peripheral blood T cell subsets and cellular immunity.
Subjects and methods
Subjects
Fifty MM patients treated at Shengli Oilfield Central Hospital from March 2012 to February 2015 who satisfied the following criteria were included: Confirmed MM according to the standards in literature [3]; Having indications for chemotherapy and immunotherapy and the willingness to receive the treatment; Good general conditions with expected survival longer than 3 months. The protocol was approved by Ethics Committee in the hospital, and all cases signed the informed consent and consent for treatment.
Grouping and treatment
The 24 cases in the simple chemotherapy group received BD chemotherapy consisting of bortezomib (bortezomib 1.0-1.3 mg/m2 d1, d4, d8 and d11; dexamethasone 20 mg/d, IV, 1-2 d, 4-5 d, 8-9 d and 11-12 d during each cycle that lasted 28 days. On the basis of the above chemotherapy, 26 cases in the combined therapy group received DC/CIK adoptive immunotherapy. At 2 h before chemotherapy for the first time, peripheral blood mononuclear cells (PBMNCs) were collected and cultured in vitro. DCs and CIK cells were subjected to 1:10 mixed culture at 7 d. These cells were transfused back at 15-20 d of chemotherapy for a total of 6 times and once daily. The amount of cells transfused each time was 2.0-5.0×109. Detections for endotoxins and bacteria were based on 2005 Edition of the Chinese Pharmacopeia. Before each transfusion, the cells were centrifuged, washed, resuspended in 100 ml of normal saline and intravenously injected within 2 h. All cases in the combined therapy group received immunotherapy for over 3 cycles. The baseline data of the cases in the two groups did not differ significantly (P>0.05) (Table 1).
Table 1.
Comparison of general data between two groups before treatment in patients with multiple myeloma (cases)
| Group | Cases | Age | Gender | Immune classification | International staging system (ISS) | History of chemotherapy | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| Male | female | IgG | IgA | IgD | Light chain | I | II | III | initial treatment | retreatment | |||
| Chemotherapy group | 24 | 56.33±10.64 | 13 | 11 | 14 | 6 | 2 | 2 | 5 | 7 | 2 | 16 | 8 |
| Joint group | 26 | 57.14±11.78 | 14 | 12 | 16 | 5 | 3 | 2 | 4 | 8 | 14 | 18 | 26 |
Note: Joint group: DC/CIK for MDT effector cells of the immune therapy.
Induction of DCs and CIK cells and phenotypic analysis
PBMNCs in the amount of 1.0-5.0×1010 were collected in the combined therapy group and subjected to adherent culture at 37°C in a saturated 5% CO2 incubator. The suspended cells were removed, and the adherent cells were added with GM-CSF and IL-4 (Diclone, France) to induce DCs. Into the suspended cells, RPMI1640 medium (Hyclone, USA) containing IFN-γ and anti-CD3 monoclonal antibodies, IL-12 and IL-1 were added to induce CIK. Mononuclear cells and DCs at 7 d of culture were added with 10 μL FITC-labeled CD83 and CD86 monoclonal antibodies and 10 μL PE-labeled HLAII monoclonal antibodies (Diclone, France). Determination was done with a flow cytometer (BD, USA). A small amount of PBMNCs and CIK cells at 7 d of culture were harvested and added with 10 μL of FITC-labeled CD3, CD8 and CD56 monoclonal antibodies, respectively. Detection was carried out with a flow cytometer.
Comparison of short-term clinical indicators
After 3 weeks of treatment, the cases in the two groups were compared in the following indicators: life quality (performance status (PS) scores), percentage of MM cells, serum level of β2-microglobin (β2-MG), serum level of M protein, 24 h urinary light chain content and serum creatinine level (Cr).
Using specific antibody labeling and flow cytometry, the changes of peripheral blood T cell subsets before treatment and after 3 weeks of treatment were compared in the two groups
50 μL of anticoagulated whole blood was collected before and after treatment and added with 20 μL of antibodies (CD3+/CD4+/CD8+ monoclonal antibodies, Diclone, France). After incubation at room temperature in the dark for 15 min, 1.5 ml of red blood cell lysis buffer was added and mixed well. The cells were incubated at room temperature in the dark for 15 min, and then centrifuged (1500 r/min, 5 min) with supernatant discarded. After that, 1.5 ml of PBS was added, mixed well and centrifuged again (1500 r/min, 5 min) with supernatant discarded. Detection was performed with a flow cytometer.
By labeling with specific antibodies and flow cytometery, the changes of Treg cells and peripheral blood NKG2D expression were compared before treatment and after 3 weeks of treatment for the two groups
Using EDTA anticoagulant tubes, 2 ml of peripheral blood was collected before and after treatment. Mononuclear cells (white layer) were isolated using lymphocyte separation fluid and washed twice with PBS. Centrifugation was performed with supernatant discarded, and single-cell suspension was prepared. The cell concentration was adjusted. Then 100 μL of the cell solution was added with FCM buffer in tube 1 and 2, followed by the addition of 20 μL CD4-FITC and 5 μL CD25-APC (Modiatech, USA). The cells were incubated at room temperature in the dark for 15 min, fixed and subjected to membrane disruption and intracellular staining. PBS buffer containing 1% FBS and 0.1% sodium azide was used to wash the cells twice. Centrifugation was performed with supernatant discarded. The cells were resuspended in 300 ml of PBS containing 1% paraformaldehyde and preserved in the dark at 4°C before detection.
PBMNCs used were labeled with CD314 (PE) antibodies and lymphocytes were shot. Cells falling into UL and UR quadrant were positive for NKG2D. The percentage of positive cells was calculated.
Detection of cellular immunity indicators before treatment and 3 weeks after treatment for the two groups
PBMNCs were isolated and subjected to silver stain (silver stain reagent was purchased from Beijing Jianerkang Biotechnology Development Co., Ltd.). KL analysis system (Beijing Jianerkang Biotechnology Development Co., Ltd.) was used to calculate the ratio of area of silver-stained nucleolar organizer regions (AgNORs) to nuclear area (IS %). The serum levels of IL-2, IFN-γ, IL-4, IL-10 and TGF-β were detected using quantitative ELISA kit (Shenzhen Jingmei Biotech Co., Ltd) with reference to the standard curve provided in the kit. As determined from pre-experiment and instruction of the kit, the maximum concentration detected using the IL-2, IL-4, IL-10, IFN-γ and TGF-β standards started from 250 ng/L, 500 ng/L, 1000 ng/L, 2000 ng/L and 500 ng/L, respectively. All procedures were implemented according to the manufacturer’s instruction, with replicate wells for standards and the blank control, respectively.
Adverse reactions
The changes of body temperature, pulse, breath rate and blood pressure during treatment were monitored. Adverse events were recorded according to Common Terminology Criteria for Adverse Events (3.0 Version) by National Cancer Institute (NCI-CTCAE).
Statistical analysis
Statistical analysis was performed using SPSS17.0 software. Data were expressed as mean ± standard deviation (x̅±s). Means were compared using one-way ANOVA, and pairwise comparison of multiple groups was performed using q test. Percentages were compared with chi-square test on a four-fold table, and P < 0.05 indicated significant differences.
Results
Phenotypes of DCs and CIK cells
After induction, DCs in the combined therapy group expressed CD83, the maturity marker. The positive rates of CD86 co-stimulatory molecule and HLA-II were increased compared with PBMNCs (P<0.05) (Table 2). After induction, CIK cells in the combined therapy group proliferated, with cell volume enlargement and some cells presenting a spindle shape. The positive rates of CD3, CD8 and CD56 on the surface of CIK cells increased compared with PBMNCs (P<0.05) (Table 3).
Table 2.
Changes of Dendritic cells (DC) phenotype after induction in patients with multiple myeloma (%, x̅±s)
| Group | Cases | CD83 | CD86 | HLA-II |
|---|---|---|---|---|
| PBMNC Group | 24 | 4.96±2.66 | 36.79±6.36 | 48.28±7.88 |
| DC Group | 26 | 40.44±6.03a | 69.22±8.09a | 79.35±12.30a |
Note: Compared with the PBMNC group;
P<0.05.
Table 3.
Changes of cytokine induced killer cells (CIK) phenotype after induction in patients with multiple myeloma (%, x̅±s)
| Group | Cases | CD3 | CD8 | CD56 |
|---|---|---|---|---|
| PBMNC Group | 24 | 44.22±7.89 | 16.34±7.98 | 4.78±3.24 |
| CIK Group | 26 | 74.89±10.67a | 36.78±9.56a | 20.78±5.93a |
Note: Compared with the PBMNC group;
P<0.05.
Comparison of clinical indicators
All indicators including PS scores, percentage of MM cells, β2-MG, serum M protein, 24 h urinary light chain content and Sc4 in both groups were improved after 3 weeks of treatment compared with those before treatment (P<0.05). In the combined therapy group, PS scores, percentage of MM cells, β2-MG, serum M protein, 24 h urinary light chain content and Scr were higher than those of the simple chemotherapy group (P<0.05) (Table 4).
Table 4.
Comparisons of the quality of life (PS score) and laboratory indexes between two groups of patients before and after treatment (x̅±s)
| Group | Testing time | Cases | PS score | Tumor cell percentage | β2-MG (mg/L) | Serum M protein(g/L) | Urine light chain (mg/24 h) | Scr (μmol/L) |
|---|---|---|---|---|---|---|---|---|
| Chemotherapy group | A1 | 24 | 3.63±0.92 | 59.46±15.60 | 27.04±4.97 | 41.56±15.89 | 19.98±5.56 | 320.20±41.45 |
| Group A | A2 | 24 | 2.59±0.56a | 37.55±11.54a | 17.36±3.98a | 33.26±10.62a | 14.96±4.22a | 200.19±37.32a |
| Joint group | B1 | 26 | 3.62±0.67 | 58.23±16.09 | 26.88±5.02 | 41.65±15.58 | 20.15±6.02 | 330.26±32.88 |
| Group B | B2 | 26 | 2.09±0.52b,c | 23.55±6.64b,c | 10.21±3.27b,c | 23.11±6.34b,c | 8.52±3.88b,c | 136.65±27.54b,c |
Note: A1: Chemotherapy group before treat; A2: Chemotherapy group after treat; B1: Joint group before treat; B2: Joint group after treat. Compared with A1;
P<0.05.
Compared with B1;
P<0.05.
Compared with A2;
P<0.05.
Comparison of peripheral blood T cell subsets before and after treatment in the two groups
After 3 weeks of treatment, either of the two groups did not show significant differences in percentage of peripheral blood total T cells (CD3+) and CD3+CD4+ ratio compared with those before the treatment (P>0.05). The CD3+CD8+ ratio decreased obviously (P<0.05), and the CD3+CD8+ ratio of the combined therapy group was significantly lower than that of the simple chemotherapy group (P<0.05); CD3+CD4+/CD3+CD8+ ratio increased compared with that before treatment (P<0.05) for the two groups; however, the CD3+CD4+/CD3+CD8+ ratio in the combined therapy group after treatment was much higher than that of the simple chemotherapy group (P<0.05) (Table 5; Figure 1).
Table 5.
Comparisons of T cell subsets in peripheral blood between two groups of patients before and after treatment (%, x̅±s)
| Group | Testing time | Cases | CD3+ | CD3+CD4+ | CD3+CD8+ | CD3+CD4+/CD3+CD8+ |
|---|---|---|---|---|---|---|
| Chemotherapy group | A1 | 24 | 62.68±3.87 | 35.55±2.62 | 29.56±5.77 | 1.30±0.23 |
| Group A | A2 | 24 | 63.46±3.78 | 36.04±2.55 | 26.56±6.52a | 1.37±0.29a |
| Joint group | B1 | 26 | 62.25±4.00 | 34.99±3.03 | 29.88±5.09 | 1.29±0.26 |
| Group B | B2 | 26 | 64. 52±3.99 | 37.11±2.95 | 22.67±7.21b,c | 1.49±0.33b,c |
Note: A1: Chemotherapy group before treat; A2: Chemotherapy group after treat; B1: Joint group before treat; B2: Joint group after treat. Compared with A1;
P<0.05.
Compared with B1;
P<0.05.
Compared with A2;
P<0.05.
Figure 1.
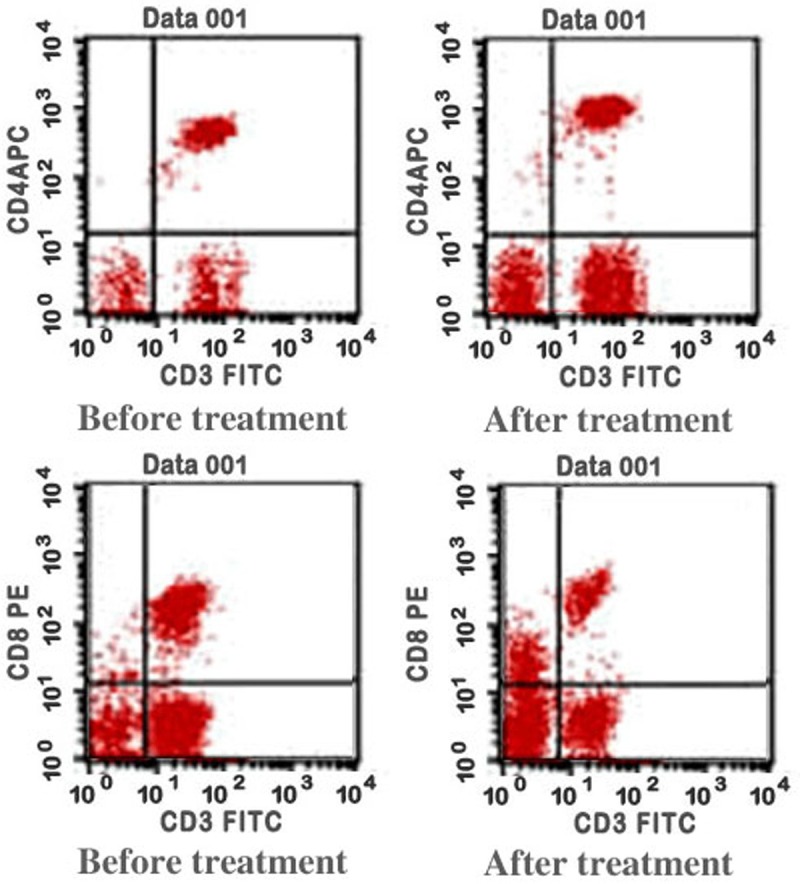
The expression of CD3+CD4+, CD3+CD8+ T cell before and after the combined treatment.
Comparison of Tregs cell before and after treatment in the two groups
The CD4+CD25+/CD4+ and CD4+CD25+FoxP3+/CD4+CD25+ ratios in the peripheral blood in both groups decreased after 3 weeks of treatment (P<0.05). These two ratios in the combined therapy group were much lower than those of the simple chemotherapy group after treatment (P<0.05). The positive rate of NKG2D increased after treatment in both two groups (P<0.05), and the positive rate in the combined therapy group was significantly increased compared with that in the simple chemotherapy group (P<0.05) (Table 6; Figure 2).
Table 6.
Comparisons of Treg cell subsets and the positive rate of NKG2D in peripheral blood between two groups of patients before and after treatment (%, x̅±s)
| Group | Testing time | Cases | CD4+CD25+ | CD4+CD25+/CD4+ | CD4+CD25+FoxP3+/CD4+CD25+ | NKG2D |
|---|---|---|---|---|---|---|
| Chemotherapy group | A1 | 24 | 1.65±0.35 | 5.66±2.56 | 41.46±6.79 | 8.78±9.11 |
| Group A | A2 | 24 | 1.45±0.40a | 4.67±2.77a | 41.46±6.79 | 18.55±7.66a |
| Joint group | B1 | 26 | 1.62±0.41 | 5.78±3.03 | 27.56±5.59a | 9.35±8.69 |
| Group B | B2 | 26 | 1.22±0.26b,c | 3.58±1.69b,c | 42.08±7.06 | 26.92±7.27b,c |
Note: A1: Chemotherapy group before treat; A2: Chemotherapy group after treat; B1: Joint group before treat; B2: Joint group after treat. Compared with A1;
P<0.05.
Compared with B1;
P<0.05.
Compared with A2;
P<0.05.
Figure 2.
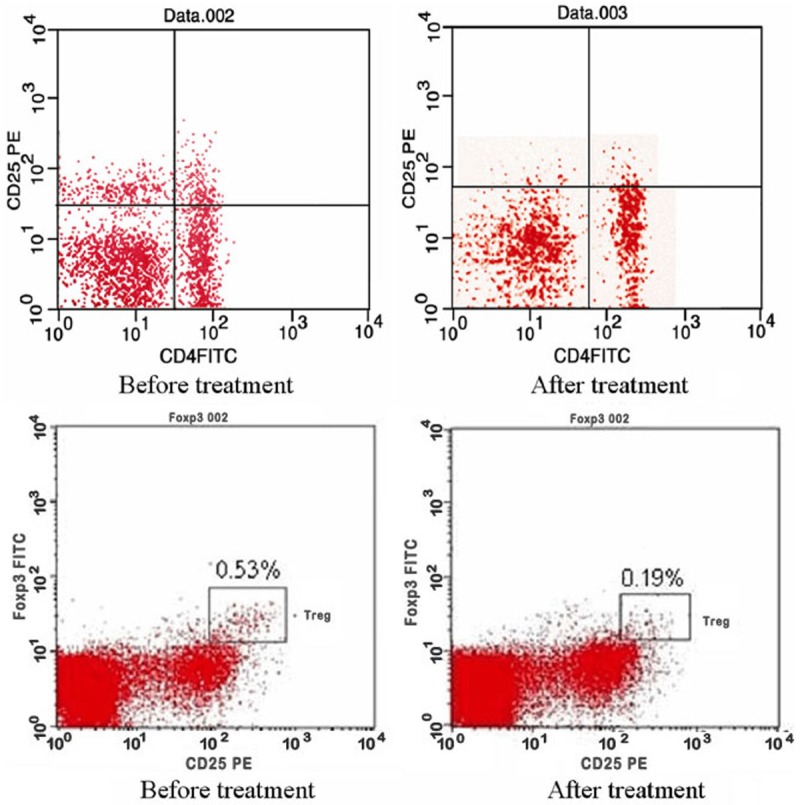
The expression of CD4+CD25+ and CD4+CD25+FoxP3+ before and after the combined treatment.
Cellular immunity before and after treatment
The levels of cytokines IL-2 and IFN-γ secreted by Th1 in both two groups increased after 3 weeks of treatment (P<0.05). The IL-2 and IFN-γ levels in the combined therapy group were obviously higher than those of the simple chemotherapy group (P<0.05). The levels of IL-4 and IL-10 secreted by Th2 in the two groups decreased after treatment (P<0.05), and the levels in the combined therapy group were obviously lower than those of the simple chemotherapy group (P<0.05). Serum AgNORs was considerably higher after treatment (P<0.05), and AgNORs was much higher in the combined therapy group than in the simple chemotherapy group (P<0.05). Serum TGF-β content decreased significantly after treatment (P<0.05); TGF-β content of the combined therapy group was obviously lower than that of the simple chemotherapy group (P<0.05) (Table 7).
Table 7.
Comparisons of the index of Immunology in peripheral blood between two groups of patients before and after treatment (x̅±s)
| Group | Testing time | Cases | IL-2 | IL-4 | IL-10 | IFN-γ | AgNORs (IS%) | TGF-β (ng/mL) |
|---|---|---|---|---|---|---|---|---|
| Chemotherapy group | A1 | 24 | 23.56±3.35 | 19.69±3.56 | 41.46±3.79 | 56.23±6.81 | 3.65±0.74 | 1.67±0.42 |
| Group A | A2 | 24 | 30.46±4.68a | 14.35±2.69a | 37.56±2.59a | 77.56±4.07a | 4.37±0.67a | 1.49±0.59a |
| Joint group | B1 | 26 | 22.98±4.05 | 19.56±4.01 | 41.78±4.06 | 55.77±5.68 | 3.72±0.78 | 1.65±0.45 |
| Group B | B2 | 26 | 37.52±4.67b,c | 11.22±1.99b,c | 30.68±3.38b,c | 89.68±6.21b,c | 5.06±0.69b,c | 1.32±0.39b,c |
Note: A1: Chemotherapy group before treat; A2: Chemotherapy group after treat; B1: Joint group before treat; B2: Joint group after treat. Compared with A1;
P<0.05.
Compared with B1;
P<0.05.
Compared with A2;
P<0.05.
Adverse reaction
Five cases in the simple chemotherapy group reported the symptoms of nerve terminal injury such as mild numbness of the four limbs. Two cases in the combined therapy group reported transient chills and fever 2 h-3 h after blood cell transfusion. The highest body temperature reached 38.2°C, and the symptoms disappeared after symptomatic treatment and the symptoms did not recur. No cases died during the research period.
Discussion
The incidence of MM in China is 2-4 per ten thousand every year, and the middle-aged and the senior make up the most vulnerable population. MM is only second to non-Hodgkin’s lymphoma in terms of incidence among all hematologic cancers. The remission rate of MM is improved due to some new drugs (eg. bortezomib and lenalidomide) and hematopoietic stem cell transplantation, and allogeneic hematopoietic stem cell transplantation is considered the best treatment option. However, this therapy is not practical for many patients because of lack of allogeneic donor, elder age of onset and high transplantation-related mortality. Bortezomib and lenalidomide have successfully prolonged the survival of MM patients and even achieved complete remission in some recurrent and refractory patients. A few patients die because of primary drug resistance and rapid disease progression or relapse [7,8]. Therefore, cellular immunotherapy is promising for the treatment of MM.
From an immunological perspective, MM has some properties of an immunodeficiency disease, in which low immunity is not only related to the humoral immune defect mediated by B cells and plasmacytes, but also to the cellular immune defect mediated by T cells. MM is associated with Th1/Th2 imbalance, and Th1/Th2 ratio plays an important role in cancer immunity [9,10]. Th1 and Th2 cells are balanced in a healthy immune system, and shift in Th1/Th2 balance occurs under abnormal conditions. There is a shift from a Th1 towards a Th2 cytokine profile in hematologic cancers, including acute and chronic lymphocytic leukemia, non-Hodgkin’s lymphoma and MM. As Th1 response is weakened, it becomes easier for the tumor cells to escape the immune monitoring [11]. Thus the reversal of Th1 to Th2 shift is a new aspect of immunotherapy [12]. It has been proved that some new drugs such as lenalidomide functions through regulating the T cells [13,14].
Treg cells are T lymphocyte subsets, whose immunomodulatory effects play an important role in negative regulatory of immune response and autoimmune tolerance. According to some studies, peripheral blood Treg cells increased in MM patients receiving first treatment [15]. Sylvia Feyler et al. [16] performed in vitro co-culture of CD4+CD25-FoxP3- T cells and MM cells and found that CD4+CD25+FoxP3+ T cells (Tregs) were induced by MM cells. More importantly, these Treg cells were not different from the naturally occurring Treg cells in human in terms of immunosuppressive activity. Karthick Raja et al. [17] showed that the conditions of MM patients with an increase of Treg cells were deteriorated and the progression was rapid. It was apparent that Treg cells also affected the clinical outcome and prognosis and the level of Treg cells was predicative of the tumor load and prognosis in MM.
NK cells are derived from hematopoietic stem cells and directly kill the tumor cells and the virus-infected cells. The killing activity of NK cells is realized by the binding of receptors on cell surface to the ligand on the surface of the target cells. A member of c type lectin superfamly, NKG2D is the important active receptor mediating the killing activity of NK cells. The activation of NKG2D alone can stimulate the activation of NK cells and overcome the strong signals from the inhibitory receptors. NKG2D also provides the target for immune editing of NK cells by tumor cells [18]. Studies showed [19] that the reduction of NK cell activity in MM patients was related to clinical staging and progression, LDH, amount and severity of plasmacytes infiltrating the bone marrow and also to β2-MG. Therefore, MM patients can be treated by inducing the upregulation of NKG2D to restore the killing activity of NK cells.
DCs can trigger and enhance T cell response, while activate the antitumor effect of NK cells and macrophages. CD3+CD56+ CIK cells possess the specific killing activity of T cells and the non-MHC restricted activity of NK cells. These CIK cells can accurately kill the tumor cells (including drug-resistant cells) without damaging the normal cells. CD3+CD56+ CIK cells are considered the most powerful killer cells, and the killing activity is about 50 times of that of LAK cells [20]. DC-CIK therapy can repeatedly kill and clear the occult tumor cells, reducing relapse and metastasis. The therapy has high safety and causes nearly no side effects to normal tissues [21]. Quach H et al. recommend thalidomide combined with DC-CIK therapy for recurrent and refractory MM, which can promote tumor-bearing survival and the patients’ life quality [22].
Chinese scholars have already noticed the effectiveness of DC-CIK immunotherapy combined with chemotherapy for MM. Most of the existing studies focus on the clinical outcome and nursing of MM [23-26], but few of them deal with the immunomodulatory effects of DC-CIK therapy combined with chemotherapy in MM. We observed the changes of peripheral blood T cell subsets and cellular immunity indicator before and after treatment and discussed the immunomodulatory effects of DC-CIK immunotherapy in MM.
We performed a retrospective analysis on MM patients who were treated by DC-CIK immunotherapy combined with chemotherapy. In the combined therapy group, CD3 and CD56 expressions on the surface of CIK cells increased, rendering a higher tumor cell killing activity. Percentage of MM cells, β2-MG, serum M protein, 24 h urinary light chain content and Scr were closely related to disease progression and efficacy. According to the results, PS scores and the above indicators in the combined therapy group were obviously higher than those of the simple chemotherapy group after 3 weeks of treatment (P<0.05). It was indicated that DC-CIK cells further killed the tumor cells while lowered the tumor load, thereby improving the patients’ life quality.
One study [27] showed that the level of peripheral blood CD3+CD8+ T cells in MM patients upon first diagnosis was significantly higher compared with that in healthy population. This led to CD4/CD8 imbalance, indicating the relationship between T-cell subset imbalance and occurrence and progression of MM. After 3 weeks of treatment, CD3+CD8+ ratio decreased in the two groups (P<0.05), and the CD3+CD8+ ratio of the combined therapy group was much lower than that of simple chemotherapy group (P<0.05). CD3+CD4+/CD3+CD8+ ratio increased after treatment in the two groups (P<0.05), and CD3+CD4+/CD3+CD8+ ratio in the combined therapy group was obviously higher than that of the simple chemotherapy group (P<0.05). Simple chemotherapy or combined therapy may inhibit the immunosuppressive activity of CD8+ T cells and therefore achieve the anti-tumor effect. This effect is enhanced by cellular immunotherapy.
We compared the levels of peripheral blood Treg subsets before and after treatment for the two groups. After 3 weeks of treatment, peripheral blood CD4+CD25+/CD4+ ratio and CD4+CD25+FoxP3+ cell/CD4+CD25+ cell ratio in both two groups decreased considerably; these two indicators in the combined therapy group were much lower than those of the simple chemotherapy group (P<0.05). The positive rate of NKG2D was improved significantly after treatment (P<0.05), and the positive rate of NKG2D in the combined therapy group was obviously higher than that of the simple chemotherapy group (P<0.05). It was thus indicated that either chemotherapy alone or combined therapy reduced the activation of CD4+CD25+ Treg cells, upregulated the expression of NKG2D, thereby reducing the immune escape of MM Cells and restoring the killing activity of NK cells. The anti-tumor effect of the therapy was improved, and MRD was effectively cleared.
The levels of IL-2, IL-4, IL-10 and IFN-γ in the peripheral blood were detected before and after treatment so as to investigate the action mechanism of DC-CIK immunotherapy combined with chemotherapy. After 3 weeks of treatment, the levels of IL-2 and IFN-γ secreted by Th1 were increased in both two groups, and they were obviously higher in the combined therapy group than in the simple chemotherapy group. Obviously, combined therapy promoted the secretion of IL-2 and IFN-γ by Th1. The levels of IL-4 and IL-10 secreted by Th2 decreased after treatment in the two groups, and they were much lower in the combined therapy group than in the simple chemotherapy group. It was inferred that the immunomodulatory effects of DC-CIK immunotherapy combined with chemotherapy were associated with the reversal of Th1 to Th2 shift.
We also detected the following indicators before and after treatment to characterize the cellular immunity: AgNORs is the subunit of RNA polymerase and a measure of the transcriptional efficiency of rDNA, reflecting the lymphocyte activity and cellular immune status; TGF-β is the immunosuppressive cytokine secreted by both tumor cells and Treg cells and represents the degree of immunosuppression by tumor cells. Both two groups had an increase of AgNORs after 3 weeks of treatment (P<0.05), and AgNORs of the combined therapy group was much higher than that of the simple chemotherapy group (P<0.05). This suggested the reversal of Th1 to Th2 shift by inhibiting the secretion of TGF-β by tumor cells and Treg cells using chemotherapy alone or combined therapy. The microenvironment where the tumor cells grew was improved, and the cellular immunity disorder corrected. Chemotherapy with cellular immunotherapy had higher immunomodulatory effects due to synergism.
To conclude, chemotherapy combined with DC-CIK immunotherapy is a very promising treatment for MM. The two therapies can synergistically reduce the immunosuppressive activity of CD8+ T cells and inhibit the secretion of TGF-β by tumor cells and Treg cells. Through the reduction of the activation of CD4+CD25+ Treg cells and the upregulation of NKG2D, the Th1/Th2 balance can be restored at a higher effectiveness in patients with MM. Considering that the immunomodulatory mechanism is complex and multi-faceted, studies with a larger sample size are required for confirmations.
Disclosure of conflict of interest
None.
References
- 1.Mahindra A, Laubach J, Raje N, Munshi N, Richardson PG, Anderson K. Latest advances and current challenges in the treatment of multiple myeloma. Nat Rev Clin Oncol. 2012;9:135–143. doi: 10.1038/nrclinonc.2012.15. [DOI] [PubMed] [Google Scholar]
- 2.Su X, Zhang L, Jin L, Ye J, Guan Z, Chen R. Coculturing dendritic cells with zoledronate acid efficiently enhance the anti-tumor effects of cytokine-induced killer cells. J Clin Immunol. 2010;30:766–774. doi: 10.1007/s10875-010-9434-1. [DOI] [PubMed] [Google Scholar]
- 3.Tan G, Zhang X, Feng H, Luo H, Wang Z. The therapeutic effect of cytokine-induced killer cells on pancreatic cancer enhanced by dendritic cells pulsed with K-ras mutant peptide. Clin Dev Immunol. 2011;2011:649359. doi: 10.1155/2011/649359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen R, Deng X, Wu H, Peng P, Wen B, Li F, Li F. Combined immunotherapy with dendritic cells and cytokine-induced killer cells for malignant tumors: A systematic review and meta-analysis. Int Immunopharmacol. 2014;22:451–464. doi: 10.1016/j.intimp.2014.07.019. [DOI] [PubMed] [Google Scholar]
- 5.Pan Y, Tao Q, Wang H, Xiong S, Zhang R, Chen T, Tao L, Zhai Z. Dendritic cells decreased the concomitant expanded Tregs and Tregs related IL-35 in cytokine-induced killer cells and increased their cytotoxicity against leukemia cells. PLoS One. 2014;9:e93591. doi: 10.1371/journal.pone.0093591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Introna M, Golay J, Rambaldi A. Cytokine Induced Killer (CIK) cells for the treatment of haematological neoplasm. Immunol Lett. 2013;155:27–30. doi: 10.1016/j.imlet.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Rosiñol L, Oriol A, Teruel AI, Hernández D, López-Jiménez J, de la Rubia J, Granell M, Besalduch J, Palomera L, González Y, Etxebeste MA, Díaz-Mediavilla J, Hernández MT, de Arriba F, Gutiérrez NC, Martín-Ramos ML, Cibeira MT, Mateos MV, Martínez J, Alegre A, Lahuerta JJ, San Miguel J, Bladé J. Superiority of bortezomib, thalidomide, and dexamethasone (VTD) as induction pretransplantation therapy in multiple myeloma: a randomized phase 3 PETHEMA/GEM study. Blood. 2012;120:1589–1596. doi: 10.1182/blood-2012-02-408922. [DOI] [PubMed] [Google Scholar]
- 8.Murray ME, Gavile CM, Nair JR, Koorella C, Carlson LM, Buac D, Utley A, Chesi M, Bergsagel PL, Boise LH, Lee KP. CD28-mediated pro-survival signaling induces chemotherapeutic resistance in multiple myeloma. Blood. 2014;123:3770–3779. doi: 10.1182/blood-2013-10-530964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ostad M, Andersson M, Gruber A, Sundblad A. Expansion of immunoglobulin autoreactive T-helper cells in multiple myeloma. Blood. 2008;111:2725–2732. doi: 10.1182/blood-2006-11-056242. [DOI] [PubMed] [Google Scholar]
- 10.Hong S, Qian J, Li H, Yang J, Lu Y, Zheng Y, Yi Q. CpG or IFN-alpha are more potent adjuvants than GM-CSF to promote anti-tumor immunity following idiotype vaccine in multiple myeloma. Cancer Immunol Immunother. 2012;61:561–571. doi: 10.1007/s00262-011-1123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng P, Yan R, Dai X, Xie X, Wen H, Yang S. The alteration and clinical significance of th1/th2/th17/treg cells in patients with multiple myeloma. Inflammation. 2015;38:705–709. doi: 10.1007/s10753-014-9980-4. [DOI] [PubMed] [Google Scholar]
- 12.Guo JR, Xu F, Jin XJ, Shen HC, Liu Y, Zhang YW, Shao Y. Impact of allogenic and autologous transfusion on immune function in patients with tumors. Asian Pac J Cancer Prev. 2014;15:467–474. doi: 10.7314/apjcp.2014.15.1.467. [DOI] [PubMed] [Google Scholar]
- 13.Luptakova K, Rosenblatt J, Glotzbecker B, Mills H, Stroopinsky D, Kufe T, Vasir B, Arnason J, Tzachanis D, Zwicker JI, Joyce RM, Levine JD, Anderson KC, Kufe D, Avigan D. Lenalidomide enhances anti-myeloma cellular immunity. Cancer Immunol Immunother. 2013;62:39–49. doi: 10.1007/s00262-012-1308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harada T, Ozaki S, Oda A, Fujii S, Nakamura S, Miki H, Kagawa K, Takeuchi K, Matsumoto T, Abe M. Association of Th1 and Th2 cytokines with transient inflammatory reaction during lenalidomide plus dexamethasone therapy in multiple myeloma. Inter J Hematol. 2013;97:743–748. doi: 10.1007/s12185-013-1321-0. [DOI] [PubMed] [Google Scholar]
- 15.Brown R, Suen H, Favaloro J, Yang S, Ho PJ, Gibson J, Joshua D. Trogocytosis generates acquired regulatory T cells adding further complexity to the dysfunctional immune response in multiple myeloma. OncoImmunology. 2012;1:1658–1660. doi: 10.4161/onci.22032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feyler S, Scott GB, Parrish C, Jarmin S, Evans P, Short M, McKinley K, Selby PJ, Cook G. Tumour cell generation of inducible regulatory T-cells in multiple myeloma is contact-dependent and antigen-presenting cell-independent. PLoS One. 2012;7:e35981. doi: 10.1371/journal.pone.0035981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muthu Raja KR, Rihova L, Zahradova L, Klincova M, Penka M, Hajek R. Increased T regulatory cells are associated with adverse clinical features and predict progression in multiple myeloma. PLoS One. 2012;7:e47077. doi: 10.1371/journal.pone.0047077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiemann K, Mittrücker HW, Feger U, Welte SA, Yokoyama WM, Spies T, Rammensee HG, Steinle A. Systemic NKG2D down regulation impairs NK and CD8 T cell responses vivo. J Immunol. 2005;175:720–729. doi: 10.4049/jimmunol.175.2.720. [DOI] [PubMed] [Google Scholar]
- 19.Smyth MJ, Swann J, Cretney E, Zerafa N, Yokoyama WM, Hayakawa Y. NKG2D function protects the host from tumor initiation. J Exp Med. 2005;202:583–588. doi: 10.1084/jem.20050994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang D, Zhang B, Gao H, Ding G, Wu Q, Zhang J, Liao L, Chen H. Clinical research of genetically modified dendritic cells in combination with cytokine-induced killer cell treatment in advanced renal cancer. BMC Cancer. 2014;14:251. doi: 10.1186/1471-2407-14-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wongkajornsilp A, Sangsuriyong S, Hongeng S, Waikakul S, Asavamongkolkul A, Huabprasert S. Effective osteosarcoma cytolysis using cytokine-induced killer cells pre-inoculated with tumor RNA-pulsed dendritic cells. J Orthop Res. 2005;23:1460–1466. doi: 10.1016/j.orthres.2005.03.009.1100230632. [DOI] [PubMed] [Google Scholar]
- 22.Quach H, Ritchie D, Stewart AK, Neeson P, Harrison S, Smyth MJ, Prince HM. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia. 2010;24:22–32. doi: 10.1038/leu.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xie J, Xu XM, Zhu JP, Ding L, Guo HF, Zhou X. Bortezomib allogeneic CIK cell treatment of multiple myeloma effect and nursing. Chinese Journal of Modern Drug Application. 2014;8:182–183. [Google Scholar]
- 24.Ding L, Zhu JP, Shao LZ, Xu M. Clinical observations between cytokine-induced killer cell immunotherapy and bortezomib plus dexamethasone regimen in elderly patients with relapsed/refractory multiple myelom. Chinese Medicinal Biotechnology. 2013;8:324–328. [Google Scholar]
- 25.Sun Y, Kuang H, Zhang XY, Zhang C, Hu YH, Chen J, Li S, Zhong GC. DC-CIK adoptive immunotherapy combined with thalidomide retrospective study relapsed and refractory multiple myeloma. J Immunol. 2012;28:324–328. [Google Scholar]
- 26.Zhong GC, Yan B, Sun Y, Zhang XY, Chen J, Su Y, Sun HP, Long HX, Zhu B. Clinical efficacy of immunotherapy of dendritic cell and cytokine-induced killer cell combined with chemotherapy for treatment of multiple myeloma. Chinese Journal of Hematology. 2012;33:1000–1003. [PubMed] [Google Scholar]
- 27.Yan B, Wei J, Chen J, Zhong GC, Kuang H, Yue LL, Wang K, Liao H, Sun Y. Study on T lymphocyte subsets and the cellular immune functions in peripheral blood from the patients with multiple myeloma. Chongqing Medicine. 2013;42:1212–1212. [Google Scholar]


