Abstract
Solitary fibrous tumors (SFTs)/hemangiopericytomas (HPCs) are uncommon mesenchymal neoplasms of fibroblastic type that can arise anywhere in the body. Recently, NGFI-A binding protein 2 (NAB2)-signal transducer and the activator of transcription 6 (STAT6) fusion gene were discovered as a hallmark of SFTs/HPCs by using whole-exome, and transcriptome sequencing; consequently, the fusion gene can be rapidly detected by STAT6 immunohistochemistry. In this study, 53 formalin-fixed, paraffin-embedded (FFPE) tissues were performed using immunohistochemistry with antibodies against STAT6, CD34, CD99 and Bcl-2. Nuclear STAT6 positive staining was present in 51 cases (51/53, sensitivity 96.2%), which were usually diffuse (4+ in 14 cases; 3+ in 13 cases; 2+ in 9 cases; 1+ in 15 cases) and intense (strong in 17 cases; moderate in 22 cases; and weak in 12 cases) staining. CD34 was positive in 47 cases (47/53, sensitivity 88.7%), CD99 was positive in 50 cases (50/53, sensitivity 94.3%) and Bcl-2 was positive in 51 cases (51/53, sensitivity 96.2%). There is no difference among categories such as age, sex, location, tumor size, or estimated dignity in immunohostochemical staining of STAT6, CD34, CD99 and Bcl-2. The nuclear STAT6 being positive is a helpful and highly sensitive marker in diagnosis of SFTs/HPCs. Considering immunohistochemical STAT6, CD34, CD99 and Bcl-2 findings together can provide more supportive diagnostic information.
Keywords: Solitary fibrous tumor, hemangiopericytoma, immunohistochemistry
Introduction
In 1942, a kind of vascular tumor had been described by Stout and Murray, characterized by the formation of endothelial tubes and sprouts with a surrounding sheath of rounded and sometimes elongated cells [1]. They believed that these cells derive from the capillary pericytes and these tumors should be distinguished by a specific descriptive name such as hemangiopericytomas (HPCs). Also in 1942, Stout and Murray published a study of a solitary malignant tumor in the lung and pleura [2]. This tumor is now known as solitary fibrous tumor (SFT), a soft tissue tumor characterized by cells surrounding thick-walled, branching “staghorn” blood vessels. As long ago as 1931 Klemperer and Rabin published an exhaustive study of pleural neoplasms that were both diffuse and solitary. The report contained at least three solitary cases, which conformed to the appearance and characteristics of the tumors reported below [3]. In 1951, Stout described the tumors were either encapsulated or sharply circumscribed and composed chiefly of spindle-shaped cells and connective tissue fibers with a variable arrangement. Later, in another study, he renamed them as solitary fibrous tumors (SFTs) [4].
However, HPC-like vascular pattern was also seen in other soft tissue sarcomas, and whether the contention in HPC represented a distinct pathologic entity or a non-specific vascular pattern were debated for a long time among pathologists [5]. In the 2013, the fourth edition of the World Health Organization classification of tumors of soft tissue and bone has made the term of “hemangiopericytoma” obsolete and the new classification no longer distinguishes between SFTs and HPCs. Emerging together, these tumors are all categorized as SFTs under the classification of fibroblastic/myofibroblastic tumors [6]. In addition, pleural and extrapleural SFTs are distinguished by their anatomical locations and they can emerge in any extrapleural sites in fact. Although most SFTs/HPCs are benign, approximately 13-23% of tumors have been reported to be malignant [7,8]. Increased mitotic index (> 4 mitoses per 10 high-power fields), high cellularity, cellular pleomorphic, and necrosis are regarded as criteria of malignancy according to England et al [7].
Previously, CD34, CD99 and B cell lymphoma 2 (Bcl-2) were the most useful positive immunohistochemical markers for diagnosing SFTs/HPCs. Nevertheless, their expressions are non-specific and also common in other tumors that may mimic SFTs/HPCs. Until recently, in 2013, three groups have discovered that NGFI-A binding protein 2 (NAB2)-signal transducer and activator of transcription 6 (STAT6) fusion gene as a hallmark of SFTs/HPCs by using whole-exome and transcriptome sequencing [9-11]. Schweizer et al recently demonstrated NAB2-STAT6 fusion gene could be rapidly detected by STAT6 immunohistochemistry, which exhibited strong nuclear STAT6 expression [12]. Some other studies also demonstrated that STAT6 is a highly sensitive and specific marker of SFTs/HPCs and can be helpful when conventional methods are inconclusive [13-15]. In this study, we investigated STAT6, CD34, CD99 and Bcl-2 protein expressions by immunohistochemistry in SFTs/HPCs.
Materials and methods
Case selection
After receiving approval from the institutional review board, 53 formalin-fixed, paraffin-embedded (FFPE) tissues were obtained from the archives between February 2008 and February 2015 of the Department of Pathology, the first affiliated hospital of China Medical University, in China. All specimens were previously diagnosed according to the WHO classification in effect at the time of initial diagnosis. The final diagnosis of all cases was confirmed by morphologic examination and conventional immunohistochemistry. This study was carried out in accordance with the Helsinki Declaration.
Immunohistochemistry
4 µm thickness sections from FFPE blocks were prepared and stained with hematoxylin-eosin. Immunohistochemical staining was performed for STAT6 (S-20, sc-621, dilution 1:150, Santa Cruz Biotechnology, USA), CD34 (MAB-0034, QBEnd/10, Fuzhou Maixin Biotech, China), CD99 (MAB-0059, O13, Fuzhou Maixin Biotech, China) and Bcl-2 (RMA-0660, SP66, Fuzhou Maixin Biotech, China). Immunohistochemistry (IHC) was performed using the avidin-biotin-peroxidase complex method (UltrasensitiveTM, Fuzhou Maixin Biotech, China) as follows: after xylene deparaffinization and a graded concentrations of ethanol dehydration, the sections were boiled in 0.01 M citrate buffer (pH 6.0) for 2 minutes with an autoclave. Then hydrogen peroxide (0.3%) was applied to block endogenous peroxide activity and the sections were incubated with normal goat serum to reduce non-specific binding. Sections were then incubated with the primary antibody at 4°C overnight. Next day, biotinylated goat anti-mouse serum IgG was used as a secondary antibody. After washing, the slides were incubated with the streptavidin-peroxidase complex and then they were visualized using 3,3’-diaminobenzidine tetrahydrochloride. Counterstaining with hematoxylin was performed, and the sections were dehydrated in ethanol before mounting.
Immunohistochemical evaluation
Two independent blinded investigators randomly evaluated all the slides. STAT6 scored using dichotomized according to Schweizer et al [12] into either nuclear expression or cytoplasmic expression. The extent of STAT6 immunoreactivity was graded according to the percentage of positive tumor cells (0, <5%; 1+, 5-25%; 2+, 26-50%; 3+, 51-75%; and 4+, 76-100%), and the intensity of staining was graded as weak, moderate, or strong. The nuclear staining ≥5% was deemed positive. CD34, CD99 and Bcl-2 were deemed as positive when at least 10% of tumor cells showed strong staining. Negative controls were stained similarly without the primary antibody.
Statistical analysis
The statistical analyses were carried out using SPSS 20.0 statistical software package. The numerical data was presented as mean and standard deviation. The Pearson Chi squared test or Fisher exact probability test was used to assess the association between categorical variables. Student t-test or one-way analysis of variance (ANOVA) was used to compare the difference with the means among two or more groups, respectively. P values less than 0.05 were considered to be significant.
Results
Clinical features of 53 cases of SFTs/HPCs and the results of IHC for STAT6, CD34, CD99 and Bcl-2 are summarized in Table 1.
Table 1.
Clinical information and immunohistochemical findings in 53 cases of SFTs/HPCs
| Case | Sex | Age (years) | Location | Tumor size (cm) | Malignant [7] | STAT6 | CD34 | CD99 | Bcl-2 | |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Nuclear | Cytoplasm | |||||||||
| 1 | M | 67 | Lung, rt | 10×9×9 | 4 | - | + | + | + | |
| 2 | F | 50 | Breast, lt | 2.5×2.2 | 3 | + | + | + | + | |
| 3 | M | 48 | Abdominal cavity | 5 | 1 | - | + | + | + | |
| 4 | M | 65 | Spermatic cord, rt | 4 | 1 | - | + | - | - | |
| 5 | M | 39 | Thoracic cavity | 20×17×6 | 2 | - | + | + | + | |
| 6 | M | 56 | Pleura, lt | 4 | 4 | - | + | + | + | |
| 7 | F | 20 | Abdominal wall, lt | 11×9×8 | 1 | - | + | + | + | |
| 8 | F | 59 | Lung, rt | 19×18×13 | + | 3 | - | + | + | + |
| 9 | M | 41 | Axilla, rt | 8×7×2.5 | + | 3 | - | - | + | + |
| 10 | F | 38 | Neck, rt | 4.5×3 | 1 | - | + | + | + | |
| 11 | M | 43 | Lung, rt | 13×9×5 | + | 4 | - | + | + | + |
| 12 | M | 40 | External auditory canal, rt | 2×1.5 | 3 | - | + | + | + | |
| 13 | M | 39 | Pleura, rt | 7.08×4.92 | 4 | - | + | + | + | |
| 14 | F | 60 | Peritoneum, lt | 10×7×7 | + | 3 | + | + | + | + |
| 15 | F | 43 | Chest wall, rt | 11×7×6 | 4 | - | + | + | + | |
| 16 | F | 46 | Neck, rt | 5.8×2.8 | 3 | - | + | + | + | |
| 17 | M | 60 | Lung, lt | 11×9×7 | 2 | - | + | + | + | |
| 18 | F | 51 | Neck | 4×3 | + | 1 | - | + | + | + |
| 19 | F | 55 | Head | 5.3×5.2×4 | + | 4 | - | + | + | + |
| 20 | F | 54 | Pleura, lt | 6×5.5×4 | 2 | - | + | + | + | |
| 21 | F | 53 | Nasal cavity, lt | 2×1 | 2 | - | + | + | + | |
| 22 | F | 81 | Neck, rt | 6×5 | + | 3 | - | + | + | + |
| 23 | M | 61 | Peritoneum, rt | 20×17 | + | 1 | - | + | - | + |
| 24 | F | 49 | Pleura, rt | 6.18×2.77 | 4 | - | + | + | + | |
| 25 | F | 46 | Chest wall, rt | 2.5 | 1 | - | + | + | + | |
| 26 | F | 37 | Nasal cavity, lt | 2×1×1 | + | 4 | - | - | + | - |
| 27 | F | 28 | Kidney, rt | 5.88×8.17 | 3 | - | - | - | + | |
| 28 | F | 46 | Pleura, lt | 4×3×3 | 1 | - | + | + | + | |
| 29 | M | 43 | Lung, rt | 6×5×4 | 2 | - | + | + | + | |
| 30 | F | 53 | Abdominal wall, rt | 4×4×3 | + | 0 | + | + | + | + |
| 31 | M | 41 | Pleura, lt | 7×5×3 | 4 | - | + | + | + | |
| 32 | F | 39 | Lung, rt | 6.7×6.7 | 4 | - | + | + | + | |
| 33 | F | 67 | Abdominal cavity | 5.5×5×4 | 1 | - | + | + | + | |
| 34 | M | 70 | Buttock, lt | 7.11×4.83 | 1 | - | + | + | + | |
| 35 | F | 27 | Nasal cavity, lt | 5×4×3 | 1 | - | - | + | + | |
| 36 | M | 34 | Mediastinum | 11×9×6 | + | 2 | + | + | + | + |
| 37 | M | 59 | Mediastinum | 15×9×8 | + | 1 | - | + | + | + |
| 38 | F | 57 | Pleura, rt | 5×4×3 | 3 | - | + | + | + | |
| 39 | F | 48 | Lung, rt | 3×2.5×2.5 | 4 | - | + | + | + | |
| 40 | M | 55 | Lung, rt | 2.5×2 | 1 | - | - | + | + | |
| 41 | F | 59 | Adrenal gland, rt | 11×8.5×8 | 4 | - | + | + | + | |
| 42 | F | 44 | Pharynx, rt | 4×2 | + | 2 | - | + | + | + |
| 43 | F | 68 | Thoracic cavity, rt | 14.39×8.5×3 | + | 0 | + | + | + | + |
| 44 | F | 43 | Pleura, rt | 3×3×1.5 | 3 | - | + | + | + | |
| 45 | M | 41 | Chest wall, lt | 8×5×4 | 3 | - | + | + | + | |
| 46 | F | 54 | Thoracic cavity, lt | 8×6×5 | + | 2 | - | + | + | + |
| 47 | F | 50 | Parotid gland, lt | 2×1 | 3 | + | - | + | + | |
| 48 | F | 39 | Chest wall | 6.7×6.7 | 1 | + | + | + | + | |
| 49 | M | 63 | Lung, rt | 1.9×1.2 | 1 | - | + | + | + | |
| 50 | F | 16 | Lung, lt | 5×4×3 | 4 | - | + | + | + | |
| 51 | F | 59 | Lung, lt | 1.5×1×1 | 3 | - | + | + | + | |
| 52 | M | 57 | Nasal cavity, lt | 3×2×2 | + | 4 | - | + | + | + |
| 53 | F | 63 | Pleura, rt | 2.5×1.5×0.5 | 2 | - | + | + | + | |
Abbreviation: F, female; M, male; lt, left; rt, right; STAT6 nucleus: 0, <5%; 1+, 5-25%; 2+, 26-50%; 3+, 51-75%; and 4+, 76-100%.
Clinical data
53 cases were studied from 20 males and 33 females, with a mean age of 50 years (range, 16-81 years). The mean tumor size was 6.85 cm (range, 2-20 cm). These SFTs/HPCs arose over a variety of anatomical sites including the thorax (29 cases, including 10 pleural, 11 pulmonary, 2 mediastinal, 3 chest wall, 3 cheat cavity); abdominal (8 cases, including 2 peritoneum, 2 abdominal wall, 2 abdominal cavity, 1 kidney, 1 adrenal gland); head and neck (12 cases; including 4 neck, 4 nasal cavity, 1 head, 1 pharynx, 1 external auditory canal, 1 parotid gland); and 1 in breast, 1 in axilla, 1 in spermatic cord, 1 in buttock. Extrapleura SFTs/HPCs were 43 (81.1%) cases. 49 (92.5%) cases were SFTs, 4 (7.5%) cases were HPCs. In our 53 cases, 16 (30.2%) were malignant by the England et al definition [7] and 37 (69.8%) were benign SFTs/HPCs. There were no significant differences of age (P=0.120) and sex (P=1.000) between patients with malignant and benign SFTs/HPCs.
Immunohistochemical findings
Nuclear STAT6 positive staining was present in 51 of cases (51/53, sensitivity 96.2%), staining was usually diffuse (4+ in 14 cases; 3+ in 13 cases; 2+ in 9 cases; 1+ in 15 cases) and intense was most moderate and strong (strong in 17 cases; moderate in 22 cases; weak in 12 cases) (Figure 1).
Figure 1.
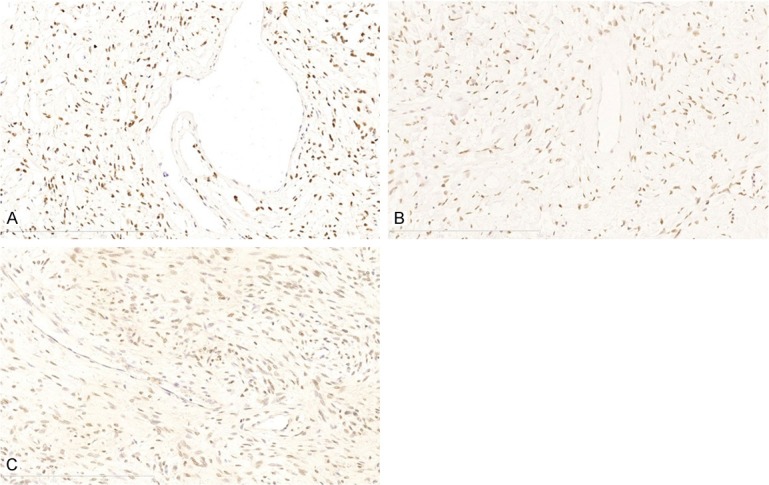
Immunohistochemical staining of STAT6 showing nuclear positivity in 51/53 cases. A. Case #1, strong staining and diffuse positive (4+) for STAT6, 200×; B. Case #24, moderate staining and diffuse positive (4+) for STAT6, 200×; C. Case #38, weak staining diffuse positive (3+) for STAT6, 200×.
As expected, CD34, CD99 and Bcl-2 were positive in most of our cases. CD34 was positive in 47 cases (47/53, sensitivity 88.7%), and 6 cases were negative in CD34, while 2 were malignant. These cases had histological manifestations of SFTs/HPCs on H&E staining and except for the loss of CD34 expression they had typical immunohistochemical features, such as positive STAT6 staining. CD99 was positive in 50 cases (50/53, sensitivity 94.3%) and Bcl-2 was positive in 51 cases (51/53, sensitivity 96.2%) (Figure 2). There were no significant differences among age, sex, location, tumor size, or estimated dignity in immunohostochemical staining of STAT6, CD34, CD99 and Bcl-2 (Table 2). SFTs/HPCs had significant differences between CD34-positive and CD34-negative immunohistochemical staining (P=0.003).
Figure 2.
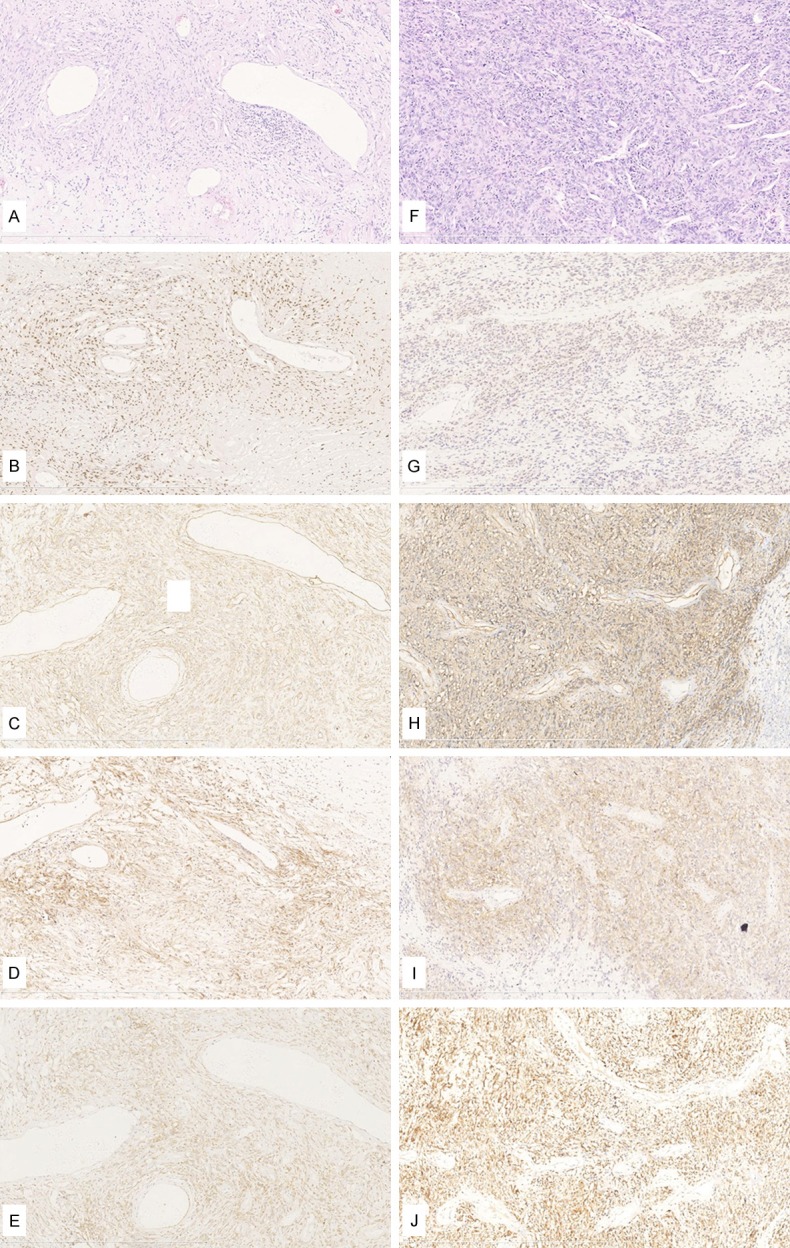
H&E and immunohistochemical studies of SFTs/HPCs. A-E: Case #1, benign SFT. F-J: Case #43, malignant SFT. A: SFT component was composed of spindle or oval-shaped tumor cells intervening with different thickness collagen, some were broad bands of hyalinized collagen. Tumor cells arranged sparse and showed hemangiopericytoma-like growth pattern. The cellular pleomorphism was invisible; H&E, 100×; B: Strong staining and diffuse nuclear positive (4+) for STAT6, 100×; C: Positive for CD34, 100×; D: Positive for CD99, 100×; E: Positive for Bcl-2, 100×; F: SFT hypercellular tumor cells arranged in hemangiopericytoma-like growth pattern. Tumor lesions showed increased mitoses (10 mitoses per 10 HPF), variable cytological atypia and dark stained nuclei; H&E, 100×; G: Cytoplasm positive for STAT6, 100×; H: Positive for CD34, 100×; I: Positive for CD99, 100×; J: Positive for Bcl-2, 100×.
Table 2.
Correlation between clinicopathological factors and IHC of STAT6, CD34, CD99 and Bcl-2 in 53 cases of SFTs/HPCs
| STAT6 | CD34 | CD99 | Bcl-2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||||
| Parameters | N/53 | + | - | P-value | + | - | P-value | + | - | P-value | + | - | P-value |
| Sex | 53/53 | 0.521 | 1.000 | 0.549 | 1.000 | ||||||||
| Male | 20 | 0 | 18 | 2 | 18 | 2 | 19 | 1 | |||||
| Female | 31 | 2 | 29 | 4 | 32 | 1 | 32 | 1 | |||||
| Age (years) | 53/53 | 0.88 | 0.050 | 0.805 | 0.784 | ||||||||
| Means (±SD) | 49.12 (±12.680) | 60.5 (±10.607) | 50.77 (±12.462) | 40 (±11.341) | 49.44 (±12.426) | 51.33 (±20.306) | 49.45 (±12.689) | 52 (±18.385) | |||||
| Location | 53/53 | 1.000 | 0.581 | 1.000 | 1.000 | ||||||||
| Pleura | 10 | 0 | 10 | 0 | 10 | 0 | 10 | 0 | |||||
| Extrapleura | 41 | 2 | 37 | 6 | 40 | 3 | 41 | 2 | |||||
| Size (cm) | 53/53 | 0.434 | 0.317 | 1.000 | 1.000 | ||||||||
| <10 cm | 39 | 1 | 34 | 6 | 38 | 2 | 38 | 2 | |||||
| ≥10 cm | 12 | 1 | 13 | 0 | 12 | 1 | 13 | 0 | |||||
| Estimated dignity | 53/53 | 0.87 | 1.000 | 1.000 | 0.517 | ||||||||
| Benign | 37 | 0 | 33 | 4 | 35 | 2 | 36 | 1 | |||||
| Malignant | 14 | 2 | 14 | 2 | 15 | 1 | 15 | 1 | |||||
| Classification | 53/53 | 1.000 | 0.003 | 1.000 | 0.147 | ||||||||
| SFTs | 47 | 2 | 46 | 3 | 46 | 3 | 48 | 1 | |||||
| HPCs | 4 | 0 | 1 | 3 | 4 | 0 | 3 | 1 | |||||
There are two cases (#30, #43) that were negative for STAT6 nuclear staining but were positive in cytoplasm by immunohistochemistry (Figure 2G). They were positive for CD34 (Figure 2H), CD99 (Figure 2I), Bcl-2 (Figure 2J), and the H&E staining (Figure 2F) showed typical histological features, which were spindle tumor cells hyperplasia within collagenous stroma. Vessels separated the patternless architecture, which combines of hypocellular and hypercellular areas.
Positive nuclear expression of STAT6 was also observed in six CD34 negative cases, four of them were positive for CD99 and Bcl-2.
Discussion
SFTs/HPCs are uncommon mesenchymal neoplasms of fibroblastic type which can arise anywhere in the body. SFTs/HPCs have recurrently displayed a paracentric inversion in chromosome 12q13, forming a fusion of two neighboring and partly overlapping genes: NAB2, which is transcribed from centromere to telomere, and STAT6, which is transcribed from telomere to centromere.
NAB2 is a transcriptional repressor involved in cellular differentiation and proliferation through the early growth response (EGR) family of zinc finger transcription factors, including EGR1, EGR2 and EGR3 [16]. STAT6 is a member of the signal transducers and activators of transcription (STAT) family that mediates signal transduction and has an important role in the immune function and cellular proliferation. STAT6 is normally located in the cytoplasm in resting cells as an inactive homodimer enters the nucleus stimulated by cytokines interleukin-4 (IL-4) and IL-13 and subsequent tyrosine phosphorylation [17].
Recently, researchers have identified this novel NAB2-STAT6 fusion gene that represents the first molecular feature unique to SFTs/HPCs of any anatomical sites and regardless of benign or malignant [9-11]. In the context of SFTs/HPCs, the tumor proliferating effect of the NAB2-STAT6 fusion gene is thought to result from the conversion of the transcriptional repressor NAB2 into a transcriptional activator by inheriting an activation domain from the signaling molecule STAT6 [9].
The detection of the NAB2-STAT6 fusion gene can aid in diagnosing in SFTs/HPCs, however, the relevant molecular assays require special experts and expensive cost, so they are not used extensively in many laboratories or pathological departments. Schweizer et al recently proposed the utility of STAT6 immunohistochemistry as a surrogate for detection of the NAB2-STAT6 fusion gene [12].
Previous findings of the aggregate sensitivity of nuclear STAT6 immunohistochemistry for SFTs/HPCs is 98%, with 202 positive staining in 206 reported cases [12-14,18]. In the Demicco et al recent research, they performed immunohistochemical studies against the C-terminus of STAT6 in tissue microarrays, among their cases, they found an overall sensitivity of nuclear STAT6 for SFTs/HPCs was 87%, which was lower than previous results. However, further analysis revealed that among cases resected within the past 5 years, STAT6 immunohistochemistry displayed 97% sensitivity for SFTs/HPCs, which was equivalent to the findings previously [19]. Thus, to reevaluate STAT6 staining in retrospective analysis, the risk of antigenicity losing should be kept in mind.
In our study, the sensitivity of STAT6 immunohistochemistry is 96.2%, which is 1%-2% less than previous studies. The difference may relate to the different study population and the histology of the cases, the specimen types, fixation methods, the antibodies used and the interpretation of immunostaining. The other two STAT6 nuclear negative cases were both positive in cytoplasm for STAT6. Thus, STAT6 positive in cytoplasm may have significance in diagnosis.
Previously, CD34, Bcl-2 and CD99 had been defined as sensitive markers of SFTs/HPCs, used in combination with an appropriate immunohistochemical panel to separate SFTs/HPCs from other histologically similar tumors [20-22]. The most useful marker is CD34, which is a single-chain transmembrane glycoprotein expressed on the surface of the human hematopoietic stem and progenitor cells, which is also present in endothelial cells, and embryonic fibroblasts [23]. Previous studies had considered the SFTs supposed origin from CD34-positive dendritic interstitial cells [24] and had the ability to differentiate to fibroblastic cells. Thus, CD34 was considered a specific marker for SFTs. CD34 positive in immunohistochemistry has been reportedly revealed to be diffusely and strongly expressed in many cases of SFTs/HPCs. However, approximately 5-10% of SFTs/HPCs can be negative in CD34 [6]. The lost expression of CD34 can be found in high-grade foci or a recurrent tumor, and the conversion of a CD34-positive SFTs/HPCs to a CD34-negative lesion is related to malignant transformation [25]. Although CD34 is a useful marker in the diagnosis of SFTs/HPCs, one should keep in mind that its expression can be lost in high-grade tumors and absence of CD34 does not rule out SFTs/HPCs and these three are less specific [15].
Moreover, recent studies showed CD34 is not entirely specific for SFTs/HPCs, because it can express in a variety of mesenchymal tumors, including dermatofibrosarcoma protuberans [26], hemangioendothelioma [27], gastrointestinal stromal tumor (GIST) [28], pleomorphic hyalinizing angiectatic tumor of soft parts (PGAT) [29], acral myxoinflammatory fibroblastic sarcoma (AMIFS) [30].
Establishment of SFTs/HPCs IHC diagnosis can be challenging as immunihistochemical markers such as CD34, CD99 and Bcl-2, because they are widely expressed in a variety of mesenchymal tumors that are neither sensitive nor specific. Therefore, a more specific marker needs to be found in clinical diagnosis.
CD34, bcl-2, and CD99 have recently been defined as sensitive markers of SFT, used in conjunction with an appropriate immunohistochemical panel in order to separate SFT from other histologically similar neoplasms.
In conclusion, we found the nuclear STAT6 positive staining was present in 51 of 53 cases (51/53, 96.2% sensitivity). STAT6 is a highly sensitive marker for the diagnosing SFTs/HPCs. Together considered as immunohistochemical STAT6, CD34, CD99 and Bcl-2 findings could provide more supportive diagnostic information.
Acknowledgements
This work was supported by grants from National Health and Family Planning Commission of The People’s Republic of China (No. 2015SQ00159) and Natural Science Foundation of Liaoning Province of China (No. L2015598).
Disclosure of conflict of interest
None.
References
- 1.Stout AP, Murray MR. Hemangiopericytoma: a vascular tumor featuring zimmermann’s pericytes. Ann Surg. 1942;116:26–33. doi: 10.1097/00000658-194207000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stout AP, Murray MR. Localized pleural mesothelioma. Arch Pathol. 1942;34:951–964. [Google Scholar]
- 3.Klemperer P, CB R. Primary neoplasms of the pleura: a report of five cases. Arch Pathol. 1931;11:385–412. doi: 10.1002/ajim.4700220103. [DOI] [PubMed] [Google Scholar]
- 4.Stout AP, Himadi GM. Solitary (localized) mesothelioma of the pleura. Ann Surg. 1951;133:50–64. doi: 10.1097/00000658-195101000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nappi O, Ritter JH, Pettinato G, Wick MR. Hemangiopericytoma: histopathological pattern or clinicopathologic entity? Semin Diagn Pathol. 1995;12:221–232. [PubMed] [Google Scholar]
- 6.Fletcher CDM, Bridge JA, JC L. Extrapleural solitary fibrous tumour. In: Fletcher CDM, Bridge JA, Hogendoorn PCW, Mertens F, editors. World Health Organisation Classification of Tumours of Soft Tissue and Bone. 4th edition. Lyon: IARC Press; 2013. pp. 80–82. [Google Scholar]
- 7.England DM, Hochholzer L, McCarthy MJ. Localized benign and malignant fibrous tumors of the pleura. A clinicopathologic review of 223 cases. Am J Surg Pathol. 1989;13:640–658. doi: 10.1097/00000478-198908000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Hanau CA, Miettinen M. Solitary fibrous tumor: histological and immunohistochemical spectrum of benign and malignant variants presenting at different sites. Hum Pathol. 1995;26:440–449. doi: 10.1016/0046-8177(95)90147-7. [DOI] [PubMed] [Google Scholar]
- 9.Robinson DR, Wu YM, Kalyana-Sundaram S, Cao X, Lonigro RJ, Sung YS, Chen CL, Zhang L, Wang R, Su F, Iyer MK, Roychowdhury S, Siddiqui J, Pienta KJ, Kunju LP, Talpaz M, Mosquera JM, Singer S, Schuetze SM, Antonescu CR, Chinnaiyan AM. Identification of recurrent NAB2-STAT6 gene fusions in solitary fibrous tumor by integrative sequencing. Nat Genet. 2013;45:180–185. doi: 10.1038/ng.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chmielecki J, Crago AM, Rosenberg M, O’Connor R, Walker SR, Ambrogio L, Auclair D, McKenna A, Heinrich MC, Frank DA, Meyerson M. Whole-exome sequencing identifies a recurrent NAB2-STAT6 fusion in solitary fibrous tumors. Nat Genet. 2013;45:131–132. doi: 10.1038/ng.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohajeri A, Tayebwa J, Collin A, Nilsson J, Magnusson L, von Steyern FV, Brosjo O, Domanski HA, Larsson O, Sciot R, Debiec-Rychter M, Hornick JL, Mandahl N, Nord KH, Mertens F. Comprehensive genetic analysis identifies a pathognomonic NAB2/STAT6 fusion gene, nonrandom secondary genomic imbalances, and a characteristic gene expression profile in solitary fibrous tumor. Genes Chromosomes Cancer. 2013;52:873–886. doi: 10.1002/gcc.22083. [DOI] [PubMed] [Google Scholar]
- 12.Schweizer L, Koelsche C, Sahm F, Piro RM, Capper D, Reuss DE, Pusch S, Habel A, Meyer J, Gock T, Jones DT, Mawrin C, Schittenhelm J, Becker A, Heim S, Simon M, Herold-Mende C, Mechtersheimer G, Paulus W, Konig R, Wiestler OD, Pfister SM, von Deimling A. Meningeal hemangiopericytoma and solitary fibrous tumors carry the NAB2-STAT6 fusion and can be diagnosed by nuclear expression of STAT6 protein. Acta Neuropathol. 2013;125:651–658. doi: 10.1007/s00401-013-1117-6. [DOI] [PubMed] [Google Scholar]
- 13.Doyle LA, Vivero M, Fletcher CD, Mertens F, Hornick JL. Nuclear expression of STAT6 distinguishes solitary fibrous tumor from histologic mimics. Mod Pathol. 2014;27:390–395. doi: 10.1038/modpathol.2013.164. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida A, Tsuta K, Ohno M, Yoshida M, Narita Y, Kawai A, Asamura H, Kushima R. STAT6 immunohistochemistry is helpful in the diagnosis of solitary fibrous tumors. Am J Surg Pathol. 2014;38:552–559. doi: 10.1097/PAS.0000000000000137. [DOI] [PubMed] [Google Scholar]
- 15.Vogels RJ, Vlenterie M, Versleijen-Jonkers YM, Ruijter E, Bekers EM, Verdijk MA, Link MM, Bonenkamp JJ, van der Graaf WT, Slootweg PJ, Suurmeijer AJ, Groenen PJ, Flucke U. Solitary fibrous tumor-clinicopathologic, immunohistochemical and molecular analysis of 28 cases. Diagn Pathol. 2014;9:224. doi: 10.1186/s13000-014-0224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumbrink J, Kirsch KH, Johnson JP. EGR1, EGR2, and EGR3 activate the expression of their coregulator NAB2 establishing a negative feedback loop in cells of neuroectodermal and epithelial origin. J Cell Biochem. 2010;111:207–217. doi: 10.1002/jcb.22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wurster AL, Tanaka T, Grusby MJ. The biology of Stat4 and Stat6. Oncogene. 2000;19:2577–2584. doi: 10.1038/sj.onc.1203485. [DOI] [PubMed] [Google Scholar]
- 18.Koelsche C, Schweizer L, Renner M, Warth A, Jones DT, Sahm F, Reuss DE, Capper D, Knosel T, Schulz B, Petersen I, Ulrich A, Renker EK, Lehner B, Pfister SM, Schirmacher P, von Deimling A, Mechtersheimer G. Nuclear relocation of STAT6 reliably predicts NAB2-STAT6 fusion for the diagnosis of solitary fibrous tumour. Histopathology. 2014;65:613–622. doi: 10.1111/his.12431. [DOI] [PubMed] [Google Scholar]
- 19.Demicco EG, Harms PW, Patel RM, Smith SC, Ingram D, Torres K, Carskadon SL, Camelo-Piragua S, McHugh JB, Siddiqui J, Palanisamy N, Lucas DR, Lazar AJ, Wang WL. Extensive survey of STAT6 expression in a large series of mesenchymal tumors. Am J Clin Pathol. 2015;143:672–682. doi: 10.1309/AJCPN25NJTOUNPNF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Westra WH, Gerald WL, Rosai J. Solitary fibrous tumor. Consistent CD34 immunoreactivity and occurrence in the orbit. Am J Surg Pathol. 1994;18:992–998. doi: 10.1097/00000478-199410000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Chilosi M, Facchettti F, Dei Tos AP, Lestani M, Morassi ML, Martignoni G, Sorio C, Benedetti A, Morelli L, Doglioni C, Barberis M, Menestrina F, Viale G. bcl-2 expression in pleural and extrapleural solitary fibrous tumours. J Pathol. 1997;181:362–367. doi: 10.1002/(SICI)1096-9896(199704)181:4<362::AID-PATH764>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 22.Renshaw AA. 013 (CD99) in spindle cell tumors: reactivity with hemangiopericytoma, solitary fibrous tumor, synovial sarcoma, and meningioma but rarely with sarcomatoid mesothelioma. Appl Immunohistochem. 1995;3:250–256. [Google Scholar]
- 23.Krause DS, Ito T, Fackler MJ, Smith OM, Collector MI, Sharkis SJ, May WS. Characterization of murine CD34, a marker for hematopoietic progenitor and stem cells. Blood. 1994;84:691–701. [PubMed] [Google Scholar]
- 24.Chan JK. Solitary fibrous tumour--everywhere, and a diagnosis in vogue. Histopathology. 1997;31:568–576. doi: 10.1046/j.1365-2559.1997.2400897.x. [DOI] [PubMed] [Google Scholar]
- 25.Yokoi T, Tsuzuki T, Yatabe Y, Suzuki M, Kurumaya H, Koshikawa T, Kuhara H, Kuroda M, Nakamura N, Nakatani Y, Kakudo K. Solitary fibrous tumour: significance of p53 and CD34 immunoreactivity in its malignant transformation. Histopathology. 1998;32:423–432. doi: 10.1046/j.1365-2559.1998.00412.x. [DOI] [PubMed] [Google Scholar]
- 26.Karanian M, Perot G, Coindre JM, Chibon F, Pedeutour F, Neuville A. Fluorescence in situ hybridization analysis is a helpful test for the diagnosis of dermatofibrosarcoma protuberans. Mod Pathol. 2015;28:230–237. doi: 10.1038/modpathol.2014.97. [DOI] [PubMed] [Google Scholar]
- 27.Shao J, Zhang J. Clinicopathological characteristics of pulmonary epithelioid hemangioendothelioma: A report of four cases and review of the literature. Oncol Lett. 2014;8:2517–2522. doi: 10.3892/ol.2014.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rege TA, Wagner AJ, Corless CL, Heinrich MC, Hornick JL. “Pediatric-type” gastrointestinal stromal tumors in adults: distinctive histology predicts genotype and clinical behavior. Am J Surg Pathol. 2011;35:495–504. doi: 10.1097/PAS.0b013e31820e5f7d. [DOI] [PubMed] [Google Scholar]
- 29.Changchien YC, Bocskai P, Kovacs I, Hargitai Z, Kollar S, Torok M. Pleomorphic hyalinizing angiectatic tumor of soft parts: case report with unusual ganglion-like cells and review of the literature. Pathol Res Pract. 2014;210:1146–1151. doi: 10.1016/j.prp.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 30.Kovarik CL, Barrett T, Auerbach A, Cassarino DS. Acral myxoinflammatory fibroblastic sarcoma: case series and immunohistochemical analysis. J Cutan Pathol. 2008;35:192–196. doi: 10.1111/j.1600-0560.2007.00791.x. [DOI] [PubMed] [Google Scholar]


