Abstract
Amount of studies in cells and animal models have proved vitamin D has multifarious antitumor effects. However, epidemiological studies showed inconsistent result on gastric cancer. The antitumor role is mainly mediated by the vitamin D receptor (VDR). Our hypothesis is that VDR may be abnormally (poorly) expressed in gastric cancer tissue. Present study is aimed at discovering and analyzing VDR expression in a series of human gastric tissues, including normal, premalignant, and malignant gastric tissue, and correlated VDR to the clinicopathological parameters of gastric cancer patients. VDR expression was detected by immunohistochemistry. The χ2 test was used to analyze the VDR expression as well as the relationship between VDR and the clinicopathological factors of gastric cancer patients. Compared with normal (82.61%) and premalignant tissues (73.64%), VDR was lower expressed in cancer tissues (57.61%), with a statistically significant difference (P = 0.001). Among cancer tissues, VDR was higher expressed in well and moderate differentiated tissues contrasted with tissues with poor differentiation, and higher expressed in small tumors (< 5 cm) compared with large tumors (≥ 5 cm), with a statistically significant difference respectively (P = 0.016, P = 0.009). A decline linear trend appeared when analyzing the statistical difference of VDR expression among normal, premalignant, and malignant gastric tissues. VDR expression has been on the decline from the premalignant stage, finally low expressed in gastric cancer tissues, especial in poorly differentiated tissues. VDR could be a potential prognostic factor for patients with gastric cancer.
Keywords: Vitamin D, antitumor, gastric cancer, vitamin D receptor, immunohistochemistry
Introduction
Although incidence of gastric cancer is declining in some regions of the world, because of most cases being diagnosed in advanced stages with poor prognosis and limited treatment options, it is still the fourth (after lung, breast and colorectal) most common malignancy and the second (after lung cancer) leading cause of death among all cancers worldwide [1]. Early diagnosis and treatment is absolutely important.
In recent years, with the development of epidemiology and molecular biology, 1,25-dihydroxyvitamin D3, the active metabolite of vitamin D, has been found broadly antitumor effects, including anti-proliferative effects, induction of apoptosis, stimulation of differentiation, inhibition of invasion and metastasis, inhibition of angiogenesis, and also anti-inflammatory effects. Some specific signaling pathways that regulate tumor growth by 1,25-dihydroxyvitamin D3 are particularly showed in breast, colon and prostate cancer cells. Although it still needs some large-scale and long-term human randomized controlled trials to confirm, recent result from amount of preclinical studies in cells and animal models, some observational studies and smaller interventional studies immensely support the anticancer effects of 1,25-dihydroxyvitamin D3. It suggests that avoiding deficiency or adding vitamin D supplements might be an economical and safe way to reduce cancer incidence, or participant into the treatment of tumor as a new chemotherapy drug [2-5].
In gastric cancer, several preclinical studies in cells proved the antitumor effects of 1,25-dihydroxyvitamin D3 [6-11], but epidemiological studies have little support for its protective effects on gastric cancer [12-14]. We have known the biological function of 1,25-dihydroxyvitamin D3, especially anticancer effects, is largely mediated by the vitamin D receptor [2,3,15], which was detected and originally identified as a chromatin-associated protein by Haussler [16] in 1969. VDR is a member of the steroid and thyroid hormone receptor superfamily [17], and also a member of transcription family [18], when free 1,25-dihydroxyvitamin D3 binds to the VDR, causing phosphorylation of the receptor, the ligand-activated VDR interacts with the retinoid X receptor (RXR) to form a heterodimer, and get its translocation to the nucleus, then the 1,25-dihydroxyvitamin D3-VDR-RXR complex binds to the vitamin D response elements (VDREs) in multiple regulatory regions located in the promoters of target genes or at distal sites, and this causes the recruitment of co-activators or co-repressors, which leads to positive or negative transcriptional regulation of gene expression [15,19,20]. Kallay, E. et al. [21] found VDR gene knockout mice show hyperplasia and increased mitotic activity in the descending colon, suggesting a role for 1,25-dihydroxyvitamin D3 mediated signaling way in tumor suppression. Several studies by Zinser GM et al. [22-24] proved the importance of VDR in cancer and that the VDR signalling may be required to suppress tumorigenesis. The anticancer mechanism of 1,25-dihydroxyvitamin D3 mediated by VDR via the gene pathways has been concluded in a recent review that discussing the anticancer role of 1,25-dihydroxyvitamin D3 by Feldman et al. [3].
In general, VDR is an important determinant of tumor cells response to 1,25-dihydroxyvitamin D3. Even though there is adequate or higher serum 25-hydroxyvitamin D in cancer patients or high risk individuals, if the expression of VDR is in a lower level, the anticancer effect of 1,25-dihydroxyvitamin D3 could weaken. Based on the inconformity between epidemiological data and preclinical study results of the anticancer effects mediated by 1,25-dihydroxyvitamin D3 on gastric cancer, we hold a bold speculation here, the VDR may be abnormally (poorly) expressed in gastric cancer tissue. We try to illustrate it from the VDR expression status, so that can better understand the prevention and treatment value of 1,25-dihydroxyvitamin D3 in gastric cancer.
Present study is the first detection and analysis of VDR expression in a series of human gastric tissue types. The local VDR expression was examined in normal, premalignant (chronic atrophic gastritis, intestinal metaplasia, atypical hyperplasia) and malignant gastric tissue simultaneously by immunohistochemical technique. In addition, the relationship between VDR and clinicopathological factors of gastric cancer patients was analyzed.
Materials and methods
Tissue samples selection
All the samples were collected from a cohort of patients enrolled in Gansu Province People’s Hospital from September 2013 to February 2015. It contains gastric cancer tissue and matched para-carcinoma tissue from 92 patients who underwent radical surgery for gastric carcinoma at the Surgical Oncology Department, and 148 cases premalignant gastric tissue, including chronic atrophic gastritis (n = 50), intestinal metaplasia (n = 52), and atypical hyperplasia tissues (n = 46), which were obtained from gastroscopic with biopsies at the Endoscopic Center of Gansu Province People’s Hospital. Among of them, the patients who received surgical tumor resection have been recorded clinical and pathological data clearly, without preoperative radiochemotherapy or other therapies. The staging of gastric cancer was according to the American Joint Committee on Cancer (ACJJ, 7th edition) [25]. The matched normal gastric tissues were isolated ≥ 5 cm away from the cancer lesions. This study was approved by the Ethics Committee of Gansu Province People’s Hospital (syll2015009), and all patients gave their informed consent prior to inclusion in the study.
VDR immunohistochemistry
The detection of VDR expression by Immunohistochemistry was carried out with the rabbit anti-human VDR monoclonal antibodies purchased from Sigma-Aldrich, Inc. All the specimens have been formalin-fixed and paraffin-embedded (FFPE) in advance, prepared for future use. Four-micrometer-thick sections were cut and mounted on slides, after placed at room temperature for 60 min, tissue sections were deparaffinized by xylene 10 min per time for twice, followed by 5 times 10 min per time in gradient ethanol (100%, 95%, 85%, 75%, 50%) for rehydration, then rinsed with water and PBS 5 min per time successively, add 0.3% H2O2 into the section followed by incubation in wet box for 10 min at room temperature, then rinsed with PBS 3 times 5 min each, slides were placed into citrate buffer heated to boiling by microwave and stay 8 min for antigen retrieval, when the slides were naturally cooled, washing with PBS 2 times 5 min reduplicative, then sections were blocked with 10% normal mouse serum for 45 min. Rabbit anti-human VDR antibody (1:50 dilution) were added, and slides were incubated overnight at 4°C. On the next day, washing the slides in PBS firstly, then biotinylated goat anti-rabbit secondary antibody was applied, incubate the sections at room temperature for 30 min subsequently, followed by washing with PBS again. Adding DBP into the slides for incubation, observing and grasping the degree of dyeing under a microscope, then sections were washed and counterstained with hematoxylin, using hydrochloric acid alcohol for differentiation. According the reverse order of rehydration above-mentioned for dehydration, then hyalinizing the slides with xylene, finally, add a coverslip to the slide for microscopic examination.
Evaluation of immunostaining
Under the microscope, slides were examined and evaluated for both staining intensity and percentage of positive cells referring to a scoring method described in a similar study recently published [26], The staining intensity score was classified as 1 (weak), 2 (moderate), and 3 (strong). The percentage of positive cells were scored as 0 (≤ 5%), 1 (6-25%), 2 (26-50%), 3 (51-75%), and 4 (76-100%). The staining intensity and percentage of staining cells were then multiplied to generate the immunoreactivity score for each case, ranging from 0 to 12. Tissue sections with an immunoreactivity score ≥ 4 were considered to be positive (high) expression, while a score < 4 was considered as negative (low) expression. Pictures were taken of representative areas containing the diagnosis of interest for each sample and blindly scored by three observers.
Statistical analysis
Our study was designed to detect and analyze the VDR expression in various human gastric tissues, to test and verify the hypothesis of VDR abnormally (poorly) expressed in gastric cancer tissue. SPSS 16.0 statistical software was used for analysis. The χ2 test were used to analyze the statistical difference among normal, premalignant, and malignant gastric tissues. The relationship between VDR expression and clinicopathological factors of gastric cancer patients was statistically analyzed by χ2 test either. In all analyses, a p value < 0.05 was considered to be statistically significant.
Results
The distribution of VDR
VDR was expressed in the various gastric tissues, at different amounts. And appeared to be localized in the cytoplasm and perinuclear regions, with nuclear staining absent (Figure 1).
Figure 1.
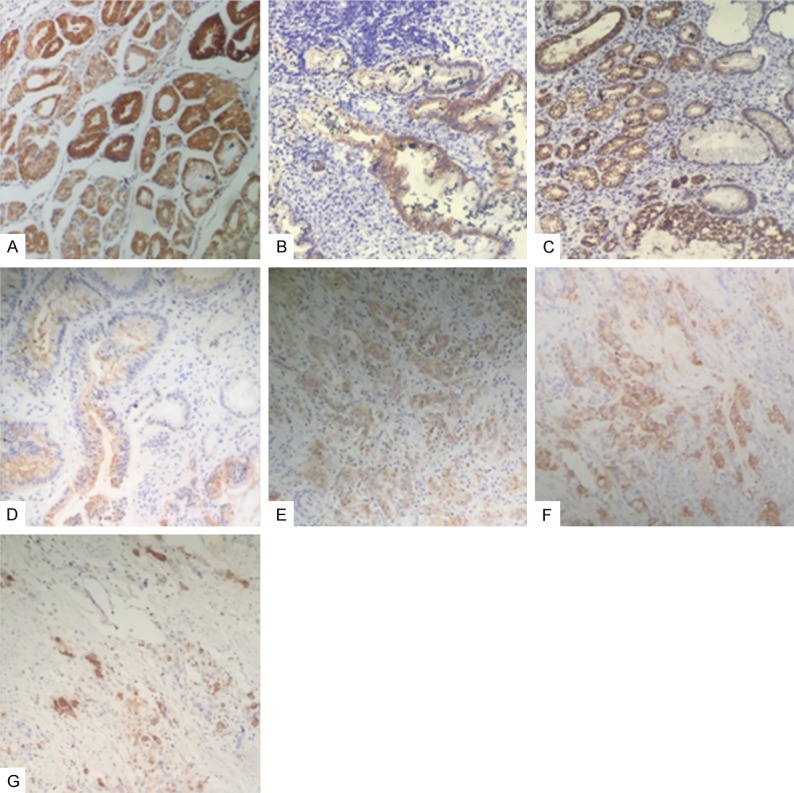
VDR expression across various gastric histologies. (A) Normal mucosa adjacent to gastric carcinoma; (B-D) Premalignant gastric tissue (B. chronic atrophic gastritis, C. intestinal metaplasia, D. atypical hyperplasia); (E-G) Gastric carcinoma (E. well differentiated, F. moderately differentiated, G. poorly differentiated).
Tumor expression of VDR
Compared with normal and premalignant tissues, VDR was lower expressed in gastric cancer tissue. The PR (positive rate) of VDR expression in tumor tissues was 57.61% (53/92), obviously lower than the adjacent normal tissues (82.61%, 76/92) and premalignant tissues (70.41%, 109148), with a significantly statistical difference (P = 0.001) (Table 1).
Table 1.
VDR expression in normal, premalignant, and malignant gastric tissues
| Histological type | NO. of cases | VDR | p | ||
|---|---|---|---|---|---|
|
|
|||||
| (+) | (-) | PR (%) | |||
| Normal gastric tissue | 92 | 76 | 16 | 82.61 | 0.001* |
| Premalignant gastric tissue | 148 | 109 | 39 | 73.64 | |
| Malignant gastric tissue | 92 | 53 | 39 | 57.61 | |
Indicates statistical significance.
Correlation between VDR and clinicopathological factors
No statistical differences have been found between VDR expression and gender or age of gastric cancer patients, tumor location, histological type, TNM stage, and lymph node metastasis except differentiated degree and tumor size. The PR of VDR expression was 70.00% (28/40) in well differentiated cancer tissues, and 65.00% (13/20) in moderate differentiated cancer tissues, but only 37.50% (12/32) in poor differentiated tissues, with a significantly statistical difference (P = 0.016). The PR of VDR was 66.13% (41/61) in small tumors (< 5 cm), only 38.71% (12/31) in large tumors (≥ 5 cm), with a significantly statistical difference either (P = 0.009) (Table 2).
Table 2.
Correlation of VDR expression with the clinicopathological parameters of gastric cancer patients
| Clinicopathological factors | Discription | NO. of cases | VDR | P | ||
|---|---|---|---|---|---|---|
|
|
||||||
| (+) | (-) | PR (%) | ||||
| Age (years) | 0.684 | |||||
| < 55 | 40 | 24 | 16 | 60.00 | ||
| ≥ 55 | 52 | 29 | 23 | 55.77 | ||
| Gender | 0.557 | |||||
| Male | 63 | 35 | 28 | 55.56 | ||
| Female | 29 | 18 | 11 | 62.07 | ||
| Tumor location | 0.757 | |||||
| Cardia | 13 | 8 | 5 | 61.54 | ||
| Fundic, body, antral | 79 | 45 | 34 | 56.96 | ||
| Tumor size (cm) | 0.009* | |||||
| < 5 | 61 | 41 | 20 | 67.21 | ||
| ≥ 5 | 31 | 12 | 19 | 38.71 | ||
| Differentiation degree | 0.016* | |||||
| High | 40 | 28 | 12 | 70.00 | ||
| Middle | 20 | 13 | 7 | 65.00 | ||
| Low | 32 | 12 | 20 | 37.50 | ||
| Pathological type | 0.606 | |||||
| Pathological type | 37 | 23 | 14 | 62.16 | ||
| Polypoid adenocarcinoma | 23 | 14 | 9 | 60.87 | ||
| Mucinous adenocarcinoma | 24 | 13 | 11 | 54.17 | ||
| Signet-ring cell carcinoma | 8 | 3 | 5 | 37.50 | ||
| Lymphnode metastasis | 0.136 | |||||
| Yes | 58 | 30 | 28 | 51.72 | ||
| No | 34 | 23 | 11 | 67.65 | ||
| TNM stage | 0.635 | |||||
| I+II | 38 | 23 | 15 | 60.53 | ||
| III+IV | 54 | 30 | 24 | 55.56 | ||
Indicates statistical significance.
VDR expression across all samples
Along with the pathological changes of tissues in the light of the gastric cancer progression pattern, which was put forward by Corea et al. [27] and widely accepted by most scholars, the expression of VDR showed a decline linear trend (Figure 2). The PR of VDR expression in normal tissues was 82.61% (76/92), then 73.64% (109/148) and 57.61% (53/92) in premalignant and gastric cancer tissues respectively, with a statistically significant difference (P = 0.001) (Table 1). In the premalignant tissues, no statistically difference (P = 0.888) was found among chronic atrophic gastritis (76.00%, 38/50), intestinal metaplasia (73.07%, 38/52) and atypical hyperplasia tissues (71.73%, 33/46) (Table 3).
Figure 2.
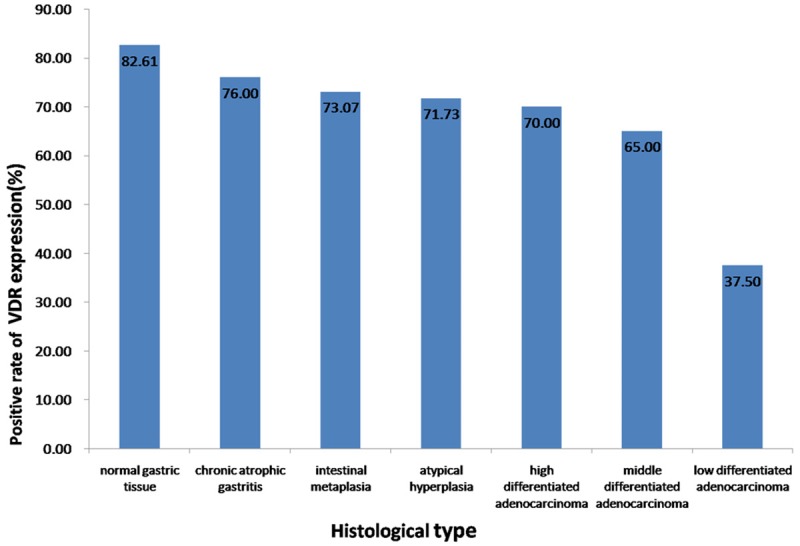
VDR was expressed in the various gastric tissues at different amounts, which was showed a declined linear trend with the pathological change from normal to premaligant (chronic atrophic gastritis, intestinal metaplasia, atypical hyperplasia), then malignant as well as the differentiation degree of gastric carcinoma (well differentiated, moderately differentiated, poorly differentiated).
Table 3.
VDR expression in the various types of premalignant gastric tissues
| Histological type | NO. of cases | VDR | p | ||
|---|---|---|---|---|---|
|
|
|||||
| (+) | (-) | PR (%) | |||
| Chronic atrophic gastritis | 50 | 38 | 12 | 76.00 | 0.888 |
| Intestinal metaplasia | 52 | 38 | 14 | 73.07 | |
| Atypical hyperplasia | 46 | 33 | 13 | 71.74 | |
Discussion
Since Colston et al. [28] found 1,25-dihydroxyvitamin D3 can inhibit the proliferation of human melanoma cells in 1981, and Abe et al.[29] reported mouse myeloid leukemia cells can be induced to differentiate into macrophages in vitro by 1,25-dihydroxyvitamin D3 in the same year, the anticancer effects of 1,25-dihydroxyvitamin D3 have been shown in vitro and in vivo among various malignancies, recently reviewed by Feldman et al. [3]. In general, accumulating results from preclinical and some clinical studies have strongly suggested the anticancer action of 1,25-dihydroxyvitamin D3.
Specific to gastric cancer, several studies in vitro proved 1,25-dihydroxyvitamin D3 have the anticarcinoma effects of anti-proliferation, promoting apoptosis [7,8,11], regulating cell viability [10], inhibiting gastric cancer cell growth and inducing cell cycle arrest [7,9]. However, epidemiological studies have little support for its protective effects on gastric cancer [13,14]. Chen, W. et al. [12] found no relationship between serum vitamin D status and the risk of gastric cancer in a cohort of Chinese patients. Genomic and proteomic screening approaches have identified the antitumor effects of 1,25-dihydroxyvitamin D3 is particularly mediated by VDR [3,11]. In other words, VDR is an important determinant of tumor cell response to 1,25-dihydroxyvitamin D3. Therefore, we hold a bold hypothesis that VDR may be abnormally (poorly) expressed in gastric cancer tissue here, trying to explain the inconsistent conclusion between epidemiological data and preclinical study results of the anticancer effects of 1,25-dihydroxyvitamin D3 on gastric cancer from the VDR expression status.
To verify our conjecture, we retrieved literatures on VDR distribution in human tissues in advance, different types of tissue or cell have diverse VDR expression [30]. The VDR is overexpressed or repressed in several histological types of cancer, demonstrating tissue-type variations in 1,25-dihydroxyvitamin D3 signaling [2]. F.C. et al. reviewed the Yin and Yang of VDR signaling in neoplastic progression [31]. Some studies have described the VDR was expressed in human normal gastric tissue [32,33]. Here we detected the expression of VDR in a series of human gastric tissues, including normal, premalignant, and malignant gastric tissues. This study first illustrated that VDR expression differs in various types of gastric tissue by immunohistochemical detection. We found VDR expression does declined in tumor cells and revealed a decline trend along with the advance of gastric cancer. Furthermore, VDR was highly expressed in more tumors with high or middle differentiation degree, but poorly expressed in low differentiated cancer tissues, and higher expressed in small tumors contrasted with large tumors. It suggests VDR could be a potential prognostic factor for patients with gastric cancer. On account of the samples were collected from the patients who accepted radical surgery for gastric carcinoma in our department in recent one and a half years, this cohort of patients are still in a timely follow-up status, the relationship between VDR expression and the five-year survival rate of the patients will be statistically analyzed in the near future, which may further declare its role as a potential prognostic factor.
Even though there is no study covering the dose-response relationship between the VDR and 1,25-dihydroxyvitamin D3 so far. On the basis of the VDR pathway inducing the anticancer action of 1,25-dihydroxyvitamin D3, and the result from our study that VDR abnormally expressed in gastric cancer tissue, we suggest 1,25-dihydroxyvitamin D3 may better provide a therapeutic choice on the patients with well or moderate differentiated degree, which have a higher VDR level, but not poorly differentiated degree group, which has a lower VDR level, supposedly not enough to induce 1,25-dihydroxyvitamin D3 play its anticancer role well. In the case of how much 1,25-dihydroxyvitamin D3 or its analogue should be used for tumor treatment, it is a huge research project, which needs some large-scale and long-term human randomized controlled trials to complete.
As to the cancer prevention value of 1,25-dihydroxyvitamin D3, similarly, no exactly conclusion has been published and no right dose have been recommended, especially the inconsistent results reported by IOM and Endocrine Society [34,35]. We just suggest the population in the regions with high incidence of gastric cancer, especially those who cannot receive adequate sunlight exposure to natural sunlight appropriately as a way for reducing the incidence of gastric cancer at present. It is worth stressing that our study shows VDR has been declined in precancerous conditions of gastric cancer, we particularly suggest 1,25-dihydroxyvitamin D3, as a new anticancer agent, if possible, should better be used in the patients who stay in the early stage of gastric cancer progression, such as intestinal metaplasia and atypical hyperplasia.
Clearly, our study is limited to detecting the VDR expression by immunohistochemistry, and theoretically assess the anticancer effects of 1,25-dihydroxyvitamin D3 only from the VDR expression status. The activity of CYP24A1, which catabolizes 1,25-dihydroxyvitamin D3, also influences the anticancer effect of 1,25-dihydroxyvitamin D3 [3,36], has not been involved in present study. Further studies of VDR expression and the anticancer effects of 1,25-dihydroxyvitamin D3 on gastric cancer still be needed.
In summary, this is the first study to detect and analyze the expression of the VDR in a series of human gastric tissues. We found VDR was expressed in the various types of gastric tissues, but in different quantity. VDR expression has been declined from the premalignant stage, finally low expressed in gastric cancer tissues, especial in poorly differentiated tissues. That suggests 1,25-dihydroxyvitamin D3, as a new anticancer agent, may better be selectively used in the gastric cancer patients with well or moderate differentiated degree for treatment; or be added to the patients who stay in a premalignant status for gastric cancer prevention, which have a higher VDR level, to induce 1,25-dihydroxyvitamin D3 to play its anticancer role well. In addition, except histology differentiated degree, we correlated the expression of VDR to the tumor size. VDR could be a potential prognostic factor for patients with gastric cancer.
Disclosure of conflict of interest
None.
References
- 1.Piazuelo MB, Correa P. Gastric cancer: Overview. Colomb Med (Cali) 2013;44:192–201. [PMC free article] [PubMed] [Google Scholar]
- 2.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 3.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 4.Woloszynska-Read A, Johnson CS, Trump DL. Vitamin D and cancer: clinical aspects. Best Pract Res Clin Endocrinol Metab. 2011;25:605–615. doi: 10.1016/j.beem.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leyssens C, Verlinden L, Verstuyf A. Antineoplastic effects of 1,25(OH)2D3 and its analogs in breast, prostate and colorectal cancer. Endocr Relat Cancer. 2013;20:R31–47. doi: 10.1530/ERC-12-0381. [DOI] [PubMed] [Google Scholar]
- 6.Chang S, Gao L, Yang Y, Tong D, Guo B, Liu L, Li Z, Song T, Huang C. miR-145 mediates the antiproliferative and gene regulatory effects of vitamin D3 by directly targeting E2F3 in gastric cancer cells. Oncotarget. 2015;6:7675–85. doi: 10.18632/oncotarget.3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bao A, Li Y, Tong Y, Zheng H, Wu W, Wei C. 1,25-Dihydroxyvitamin D3 and cisplatin synergistically induce apoptosis and cell cycle arrest in gastric cancer cells. Int J Mol Med. 2014;33:1177–1184. doi: 10.3892/ijmm.2014.1664. [DOI] [PubMed] [Google Scholar]
- 8.Wang W, Zhao CH, Zhang N, Wang J. Vitamin D analog EB1089 induces apoptosis in a subpopulation of SGC-7901 gastric cancer cells through a mitochondrial-dependent apoptotic pathway. Nutr Cancer. 2013;65:1067–1075. doi: 10.1080/01635581.2013.811273. [DOI] [PubMed] [Google Scholar]
- 9.Park MR, Lee JH, Park MS, Hwang JE, Shim HJ, Cho SH, Chung IJ, Bae WK. Suppressive effect of 19-nor-1alpha-25-dihydroxyvitamin D2 on gastric cancer cells and peritoneal metastasis model. J Korean Med Sci. 2012;27:1037–1043. doi: 10.3346/jkms.2012.27.9.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baek S, Lee YS, Shim HE, Yoon S, Baek SY, Kim BS, Oh SO. Vitamin D3 regulates cell viability in gastric cancer and cholangiocarcinoma. Anat Cell Biol. 2011;44:204–209. doi: 10.5115/acb.2011.44.3.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pan L, Matloob AF, Du J, Pan H, Dong Z, Zhao J, Feng Y, Zhong Y, Huang B, Lu J. Vitamin D stimulates apoptosis in gastric cancer cells in synergy with trichostatin A/sodium butyrate-induced and 5-aza-2’-deoxycytidine-induced PTEN upregulation. FEBS J. 2010;277:989–999. doi: 10.1111/j.1742-4658.2009.07542.x. [DOI] [PubMed] [Google Scholar]
- 12.Chen W, Dawsey SM, Qiao YL, Mark SD, Dong ZW, Taylor PR, Zhao P, Abnet CC. Prospective study of serum 25(OH)-vitamin D concentration and risk of oesophageal and gastric cancers. Br J Cancer. 2007;97:123–128. doi: 10.1038/sj.bjc.6603834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Helzlsouer KJ, Gallicchio L. Shedding light on serum vitamin D concentrations and the risk of rarer cancers. Anticancer Agents Med Chem. 2013;13:65–69. [PubMed] [Google Scholar]
- 14.Trowbridge R, Mittal SK, Agrawal DK. Vitamin D and the epidemiology of upper gastrointestinal cancers: a critical analysis of the current evidence. Cancer Epidemiol Biomarkers Prev. 2013;22:1007–1014. doi: 10.1158/1055-9965.EPI-13-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haussler MR, Jurutka PW, Mizwicki M, Norman AW. Vitamin D receptor (VDR)-mediated actions of 1alpha,25(OH)2vitamin D3: genomic and non-genomic mechanisms. Best Pract Res Clin Endocrinol Metab. 2011;25:543–559. doi: 10.1016/j.beem.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Haussler MR, Norman AW. Chromosomal receptor for a vitamin D metabolite. Proc Natl Acad Sci U S A. 1969;62:155–162. doi: 10.1073/pnas.62.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlberg C, Campbell MJ. Vitamin D receptor signaling mechanisms: integrated actions of a well-defined transcription factor. Steroids. 2013;78:127–136. doi: 10.1016/j.steroids.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, Hsieh JC, Jurutka PW. Molecular mechanisms of vitamin D action. Calcif Tissue Int. 2013;92:77–98. doi: 10.1007/s00223-012-9619-0. [DOI] [PubMed] [Google Scholar]
- 20.Pike JW, Meyer MB. Fundamentals of vitamin D hormone-regulated gene expression. J Steroid Biochem Molecul Biol. 2014;144 Pt A:5–11. doi: 10.1016/j.jsbmb.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kallay E, Pietschmann P, Toyokuni S, Bajna E, Hahn P, Mazzucco K, Bieglmayer C, Kato S, Cross HS. Characterization of a vitamin D receptor knockout mouse as a model of colorectal hyperproliferation and DNA damage. Carcinogenesis. 2001;22:1429–1435. doi: 10.1093/carcin/22.9.1429. [DOI] [PubMed] [Google Scholar]
- 22.Zinser GM, Sundberg JP, Welsh J. Vitamin D(3) receptor ablation sensitizes skin to chemically induced tumorigenesis. Carcinogenesis. 2002;23:2103–2109. doi: 10.1093/carcin/23.12.2103. [DOI] [PubMed] [Google Scholar]
- 23.Zinser GM, Welsh J. Vitamin D receptor status alters mammary gland morphology and tumorigenesis in MMTV-neu mice. Carcinogenesis. 2004;25:2361–2372. doi: 10.1093/carcin/bgh271. [DOI] [PubMed] [Google Scholar]
- 24.Zinser GM, Suckow M, Welsh J. Vitamin D receptor (VDR) ablation alters carcinogen-induced tumorigenesis in mammary gland, epidermis and lymphoid tissues. J Steroid Biochem Mol Biol. 2005;97:153–164. doi: 10.1016/j.jsbmb.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 25.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 26.Wang K, Dong M, Sheng W, Liu Q, Yu D, Dong Q, Li Q, Wang J. Expression of Vitamin D Receptor as a Potential Prognostic Factor and Therapeutic Target in Pancreatic Cancer. Histopathology. 2015;67:386–97. doi: 10.1111/his.12663. [DOI] [PubMed] [Google Scholar]
- 27.Correa P. A human model of gastric carcinogenesis. Cancer Res. 1988;48:3554–3560. [PubMed] [Google Scholar]
- 28.Colston K, Colston MJ, Feldman D. 1,25-dihydroxyvitamin D3 and malignant melanoma: the presence of receptors and inhibition of cell growth in culture. Endocrinology. 1981;108:1083–1086. doi: 10.1210/endo-108-3-1083. [DOI] [PubMed] [Google Scholar]
- 29.Abe E, Miyaura C, Sakagami H, Takeda M, Konno K, Yamazaki T, Yoshiki S, Suda T. Differentiation of mouse myeloid leukemia cells induced by 1 alpha,25-dihydroxyvitamin D3. Proc Natl Acad Sci U S A. 1981;78:4990–4994. doi: 10.1073/pnas.78.8.4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Zhu J, DeLuca HF. Where is the vitamin D receptor? Arch Biochem Biophys. 2012;523:123–133. doi: 10.1016/j.abb.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Campbell FC, Xu H, El-Tanani M, Crowe P, Bingham V. The yin and yang of vitamin D receptor (VDR) signaling in neoplastic progression: operational networks and tissue-specific growth control. Biochem Pharmacol. 2010;79:1–9. doi: 10.1016/j.bcp.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trowbridge R, Mittal SK, Sharma P, Hunter WJ, Agrawal DK. Vitamin D receptor expression in the mucosal tissue at the gastroesoph-ageal junction. Exp Mol Pathol. 2012;93:246–249. doi: 10.1016/j.yexmp.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berger U, Wilson P, McClelland RA, Colston K, Haussler MR, Pike JW, Coombes RC. Immunocytochemical detection of 1,25-dihydroxyvitamin D receptors in normal human tissues. J Clin Endocrinol Metab. 1988;67:607–613. doi: 10.1210/jcem-67-3-607. [DOI] [PubMed] [Google Scholar]
- 34.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 35.Rosen CJ, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Manson JE, Mayne ST, Ross AC, Shapses SA, Taylor CL. IOM committee members respond to Endocrine Society vitamin D guideline. J Clin Endocrinol Metab. 2012;97:1146–1152. doi: 10.1210/jc.2011-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21:319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


