Abstract
Background: The chemokine CXCL12 and its receptors CXCR4 and CXCR7 play important roles in cancer invasion and metastasis. This study investigated the mRNA expressions of CXCL12, CXCR4, and CXCR7 to illustrate the role of these biomarkers in breast cancer metastasis and prognosis. Methods: The mRNA expressions of CXCL12, CXCR4, and CXCR7 in 115 primary breast cancer and regional lymph node specimens were detected by quantitative reverse-transcription polymerase chain reaction. Survival time was analyzed by Kaplan-Meier survival curves using log-rank test. Univariable and multivariable Cox regression analyses were performed to assess independent prognostic factors for survival. Results: The expression levels of CXCR4 and CXCR7 in breast cancer tissues were significantly higher than that in adjacent normal tissues (P=0.022 and P<0.001, respectively), while the expression level of CXCL12 in breast cancer tissues did not differ from that in adjacent normal tissues (P=0.156). Furthermore, CXCL12 exhibited significant differences in expression between primary tumor and lymph node metastasis tumor (P=0.039). CXCR4 and CXCR7 expressions in metastasis tumor were also higher, although no significant difference was observed (P=0.067 and P=0.054, respectively). Kaplan-Meier survival analysis revealed that patients exhibiting high CXCR4 and CXCR7 expression experienced a shorter survival period compared with those with low expression. When analyzed with a Cox regression model, the expressions of CXCL12, CXCR4 and CXCR7 were independent prognostic factors for overall survival. Conclusions: The mRNA expressions of CXCL12, CXCR4, and CXCR7 play important roles in the progression and metastasis of breast cancer and may act as predictive factors significantly affecting the prognosis.
Keywords: CXCL12, CXCR4, CXCR7, breast cancer, prognosis
Introduction
Breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death in females worldwide, accounting for 23% (1.38 million) of the total new cancer cases and 14% (458,400) of the total cancer deaths [1]. Despite a major decline in mortality rates over the last two decades, breast cancer remains an important public health burden worldwide, especially in economically developing countries [1]. Metastasis is one characteristic of breast cancer which determines the course of therapy and prognosis. It is a multifactorial, nonrandom, and sequential process with an organ-selective characteristic [2]. Due to the inability to accurately predict the risk of metastasis, more than 80% of patients receive adjuvant chemotherapy when diagnosed with breast cancer, and approximately 40% of these patients still relapse and die of metastatic breast cancer within five years, thus attracting increased attention from researchers [3].
Although several molecules are involved in breast cancer metastasis, precise mechanism of tumor cell migration to specific organs remains to be established [4]. Previously, the “seed and soil” theory was employed to explain directional metastasis, considering that certain metastasis organs possess the congenial environment of the primary organ [5]. More recently, a “chemokine-receptor” model has been proposed to explain the homing of tumor cells to specific organs [6]. Chemokines belongs to a super-family of small, cytokine-like proteins that induce cytoskeletal rearrangement and adhesion to endothelial and directional migration through their interaction with G-protein-coupled receptors (GPCRs) [7]. Among the cytokines/receptor systems studied in the last fifteen years, the CXCL12/CXCR4 axis has been shown to play a relevant role in experimental models of metastases. Müller et al. demonstrated that CXCR4 is consistently expressed in human breast cancer cells, while its ligand CXCL12 is preferentially expressed in the lungs, liver, and lymph nodes [4]. CXCR4 expression was also identified as a predictive factor of worse outcome in some metastatic tumors and in malignant gliomas [8-10] and CXCL12/CXCR4 axis is supposed to be crucial in metastases formation from breast cancer [11]. Recently, another CXCL12 receptor has been identified: the orphan GPCR RDC1, now called CXCR7 [12]. This receptor does not mediate typical GPCR signaling through Gi or Ca2+ mobilization and recent findings in zebrafish primordial germ cells showed a scavenger activity of CXCR7 generating a CXCL12 gradient that would lead to the formation of a guidance cue for CXCR4-positive cells [13]. On the other hand, formation of CXCR4/CXCR7 heterodimers enhancing CXCL12 signaling in embryonic cells was observed, suggesting a potential interaction between the two receptors [14]. In in vivo models of breast cancer, CXCR7 has been shown to promote lung metastasis development and progression [15]. In addition, in vitro and in vivo studies with prostate cancer cell lines suggest a role of CXCR7 in promoting survival, adhesion to endothelial cells, invasion and angiogenesis of tumor cells, emphasizing the potential involvement of this receptor, as well as of CXCR4 in the metastatic process [16].
The aim of our work was to investigate the role of CXCL12 and its receptors (CXCR4 and CXCR7) in the evaluation of metastasis and the prognosis of breast cancer.
Materials and methods
Patients and tissue specimens
A total of 115 consecutive patients with breast carcinoma who underwent surgical resection at Department of Thyroid and Breast Surgery, the Third Xiangya Hospital of Central South University between January 2008 and December 2012 were retrospectively reviewed. All patients had not been subjected to chemotherapy or radiotherapy prior to surgery but had received chemotherapy following surgical operation. Fresh tissues including breast cancer tissues and adjacent normal tissues were collected and immediately snap-frozen in liquid nitrogen after surgery and were stored at -196°C until used. Patient preoperative demographic and clinical data, including age, details of pathological diagnosis, serum carbohydrate antigen 15-3 (CA 15-3) carbohydrate antigen 19-9CACA levels, follow-up period, and overall survival were collected prospectively. The study has been conducted in accordance with the ethical standards and the principles of the Declaration of Helsinki and has been approved by the Institutional Review Board of the Third Xiangya Hospital of Central South University. All patients gave their informed written consent for their tissues to be retained and analyzed for research purposes.
Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR)
qRT-PCR was utilized to detect CXCL12, CXCR4, and CXCR7 expression in breast cancer tissues. Briefly, total RNA was extracted using TRIzol extraction liquid (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. GAPDH was used as an internal control. The reverse transcriptase (RT) reaction contained 10 ng of total RNA, 50 nmol/l stem-loop RT primer, 1×RT buffer, 0.25 mmol/l each of deoxynucleotide triphosphate (dNTP), 3.33 U/μl MultiScribe reverse transcriptase, and 0.25 U/μl RNase Inhibitor. The 20 μl reaction volumes were incubated at 16°C for 30 min, 40°C for 30 min, and 85°C for 5 min. Real-time PCR was then performed on a StepOnePlus real-time PCR system (Applied Biosystems, Foster City, CA, USA). The sequences of the primers were as follows: human CXCL12 forward 5’-CTCCTGGGGATGTGTAATGG-3’ and reverse 5’-GCCTCCATGGCATACATAGG-3’; human CXCR4 forward 5’-CTCCAAGCTGTCACACTCCA-3’ and reverse 5’-TCGATGCTGATCCCAATGTA-3’; human CXCR7 forward 5’-GCACTACATCCCGTTCACCT-3’ and reverse 5’-AAGGCCTTCATCAGCTCGTA-3’; and human GAPDH 5’-GGGCATCCTGGGCTACACTG-3’ and reverse 5’-GAGGTCCACCACCCTGTTGC-3’. The following PCR parameters were used: 95°C for 2 min, followed by 35 cycles of 95°C for 30 sec and 60°C for 30 sec and a final elongation step of 72°C for 10 min. All reactions were performed in triplicate and the cycle threshold (CT) value in each reaction well was recorded. The relative quantification of HSPA2 mRNA expression was calculated using the 2-∆∆CT method.
Statistical analysis
The mRNA expression levels of CXCL12, CXCR4, and CXCR7 were expressed as mean ± standard deviation (SD); associations between these mRNA expression levels in breast cancer and clinicopathological features were determined using the χ2-test. Survival time was analyzed by Kaplan-Meier survival curves and differences in this parameter between subgroups of patients by log-rank test; this test was used for equality of survival distributions. The variables considered for the univariate analysis consisted of patient-related and tumor-related features, i.e., age, histology of primary tumor, status of metastasis and mRNA expression of CXCL12, CXCR4, and CXCR7. The Cox proportional hazards model for multivariate survival analysis was used to assess predictors related to overall survival. All statistical analyses were performed using SPSS software (SPSS 19.0, Chicago, IL, USA) and P<0.05 was considered statistically significant.
Results
CXCL12, CXCR4, and CXCR7 mRNA expression in breast cancer
Patient and primary tumor characteristics are presented in Table 1. Samples included 115 patients, among which 65 developed lymph node metastasis while 50 did not. The patients were aged from 32 to 77 years with a median of 52 years. The degree of differentiation was well differentiated in 27 cases, moderately differentiated in 34 cases, and poorly differentiated in 53 cases. The TNM stage according to the 7th edition of the AJCC cancer staging manual was 33 cases of stage I, 46 cases of stage II, 32 cases of stage III, and 4 cases of stage IV. CA 15-3 was elevated in 67 of the patients. In addition, the histopathology revealed 42.6% of estrogen receptor (ER), 41.7% of progesterone receptor (PR), and 45.2% of human epidermal growth factor receptor-2 (HER-2) to be positive.
Table 1.
Association between CXCL12, CXCR4, and CXCR7 mRNA expression and clinicopathological features of breast cancer
| Variables | CXCL12 expression | CXCR4 expression | CXCR7 expression | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Low (n) | High (n) | P value | Low (n) | High (n) | P value | Low (n) | High (n) | P value | |
| Age (years) | 0.545 | 0.386 | 0.457 | ||||||
| <50 | 28 | 26 | 26 | 28 | 26 | 28 | |||
| ≥50 | 32 | 29 | 28 | 33 | 32 | 29 | |||
| Tumor size (cm) | 0.483 | 0.542 | 0.613 | ||||||
| D≤2 | 18 | 22 | 20 | 20 | 19 | 21 | |||
| 2<D≤5 | 34 | 28 | 29 | 33 | 33 | 29 | |||
| D>5 | 8 | 5 | 5 | 8 | 6 | 7 | |||
| Histological grades | 0.216 | 0.437 | 0.189 | ||||||
| Well/Moderate | 35 | 27 | 31 | 31 | 28 | 34 | |||
| Poor | 25 | 28 | 23 | 30 | 30 | 23 | |||
| TNM stagea | 0.392 | 0.184 | 0.064 | ||||||
| I-II | 39 | 40 | 44 | 35 | 36 | 43 | |||
| III-IV | 21 | 15 | 10 | 26 | 22 | 14 | |||
| Lymph node metastasis | 0.001 | 0.022 | 0.006 | ||||||
| Negative | 24 | 26 | 28 | 22 | 31 | 19 | |||
| Positive | 36 | 29 | 26 | 39 | 23 | 42 | |||
| No. of lymph node excised | 0.491 | 0.367 | 0.513 | ||||||
| N≤3 | 13 | 11 | 12 | 12 | 9 | 15 | |||
| 3<N≤9 | 36 | 31 | 32 | 35 | 33 | 34 | |||
| N≥10 | 11 | 13 | 10 | 14 | 12 | 12 | |||
| ER | 0.432 | 0.344 | 0.692 | ||||||
| Negative | 37 | 29 | 33 | 33 | 31 | 35 | |||
| Positive | 23 | 26 | 21 | 28 | 23 | 26 | |||
| PR | 0.281 | 0.081 | 0.096 | ||||||
| Negative | 32 | 35 | 37 | 30 | 29 | 38 | |||
| Positive | 28 | 20 | 17 | 31 | 29 | 19 | |||
| HER-2 | 0.044 | 0.009 | 0.005 | ||||||
| Negative | 36 | 27 | 35 | 28 | 37 | 26 | |||
| Positive | 24 | 28 | 19 | 33 | 21 | 31 | |||
| CA15-3 (U/ml) | 0.096 | 0.335 | 0.218 | ||||||
| <25 | 23 | 25 | 24 | 24 | 22 | 26 | |||
| ≥25 | 37 | 30 | 30 | 37 | 36 | 31 | |||
TNM staging was classified according to the 7th edition of the AJCC cancer staging manual.
n, number of patients; D, diameter, N, number of lymph node excised; ER, estrogen receptor; PR, progesterone receptor; HER-2, human epidermal growth factor receptor-2.
The mRNA expression levels of CXCL12 and its receptors (CXCR4 and CXCR7) were detected in 115 pairs of breast cancer and adjacent normal tissues by qRT-PCR. As shown in Figure 1, the expression levels of CXCR4 and CXCR7 in breast cancer tissues were significantly higher than that in adjacent normal tissues (P=0.022 and P<0.001, respectively), while the expression level of CXCL12 in breast cancer tissues did not differ from that in adjacent normal tissues (P=0.156).
Figure 1.
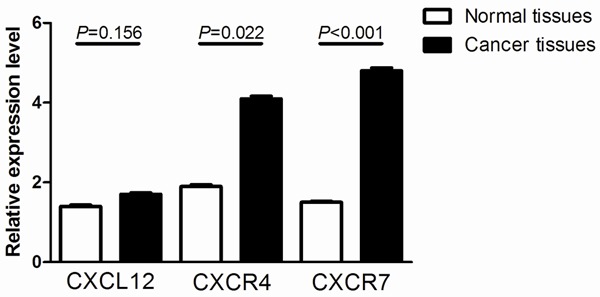
Relative expression of CXCL12, CXCR4, and CXCR7 in 115 pairs of breast cancer tissues (C) and adjacent normal tissues (N).
Association between CXCL12, CXCR4, and CXCR7 mRNA expression and clinicopathological parameters of patients with breast cancer
The median mRNA expression level was used as a cut-off point to divide the patients into two groups: the low expression group and the high expression group. The results revealed that low expression of CXCL12 and high expression of CXCR4 and CXCR7 significantly correlated with lymph node metastasis (P=0.001, P=0.022, and P=0.006, respectively). To verify the important effect of CXCR4 and CXCR7 in metastasis, CXCR4, CXCR7, and CXCL12 expression in primary breast cancer were compared with that in lymph node metastasis tumor. It was observed that CXCR4 and CXCR7 expression in metastasis tumor was even higher, although no significant difference was evident (P=0.067 and P=0.054, respectively). More importantly, their ligand, CXCL12 exhibited significant differences in expression between primary tumor and lymph node metastasis tumor (P=0.039, Table 2).
Table 2.
Differences of biomarkers between primary tumor and lymph node metastasis tumor
| CXCL12 | CXCR4 | CXCR7 | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Low (n) | High (n) | P value | Low (n) | High (n) | P value | Low (n) | High (n) | P value | |
| PT | 36 | 29 | 0.039 | 26 | 39 | 0.067 | 23 | 42 | 0.054 |
| MT | 23 | 32 | 24 | 41 | 20 | 45 | |||
PT, primary tumor; MT, lymph node metastasis tumor.
Although neither ER nor PR positivity was associated with degree of the biomarkers, HER-2 over-expression was correlated with high expression of CXCR4 and CXCR7 (P=0.009 and P=0.005, respectively). The expression of HER-2 was 45%, and among these patients, approximately 63% and 59% were associated with high CXCR4 and CXCR7 expression.
Association between CXCL12, CXCR4, and CXCR7 mRNA expression and poor prognosis in patients with breast cancer
Follow-up investigation revealed that the median survival time was 79 months (ranging from 10-89 months), within which 21 patients (18.3%) died because of breast cancer including 14 (21.5%) in the tumor with metastasis group and 7 (14.0%) in the non-metastasis group. Kaplan-Meier analysis revealed that patients suffering from high levels of CXCR4 expression had significantly lower overall survival compared with those with low CXCR4 expression (P=0.034; Figure 2B). Similarly, high levels of CXCR7 expression also revealed poor prognosis (P=0.017; Figure 2C). However, there was no correlation between CXCL12 expression and overall survival (P=0.176; Figure 2A). Univariate analysis demonstrated that histological grades (P=0.037), TNM stage (P=0.008), the status of lymph node metastasis (P=0.003), HER-2 expression (P=0.022), CXCL12 mRNA expression level (P=0.003), CXCR4 mRNA expression level (P=0.001), and CXCR7 mRNA expression level (P<0.001) were significantly associated with overall survival of breast cancer patients (Table 3). Multivariate analysis using the Cox proportional hazards model for all variables that were significant in the univariate analysis showed that TNM stage (P=0.011), the status of lymph node metastasis (P=0.009), CXCL12 mRNA expression level (P=0.019), CXCR4 mRNA expression level (P=0.037), and CXCR7 mRNA expression level (P=0.002) were independent prognostic factors for overall survival in patients with breast cancer (Table 3).
Figure 2.
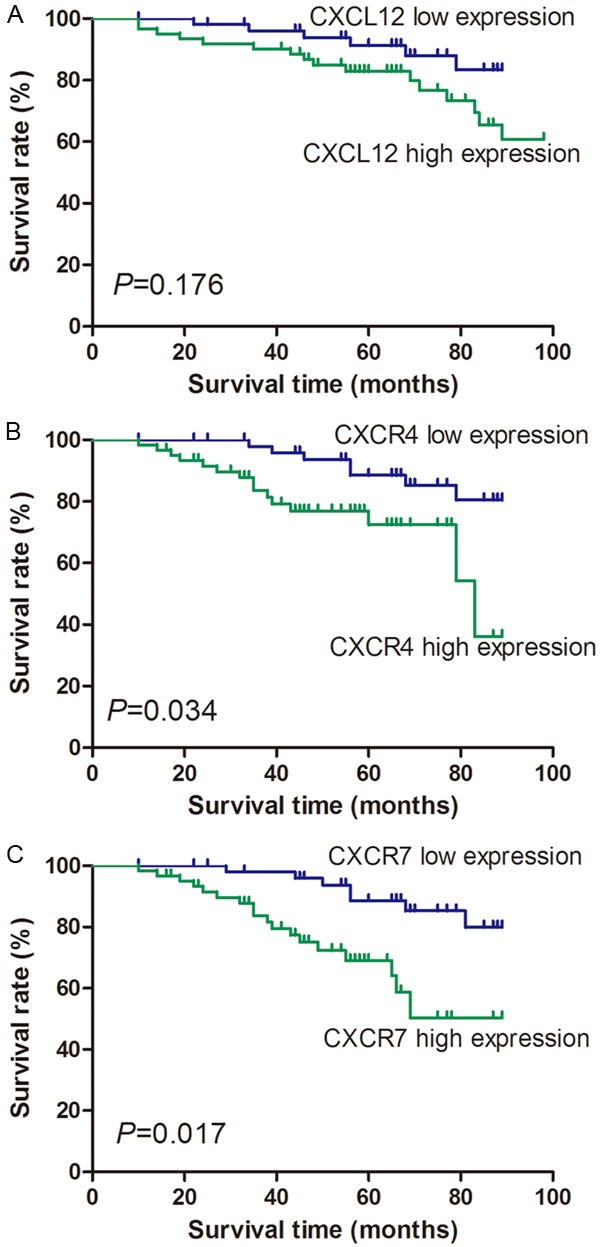
Overall survival analysis for CXCL12, CXCR4, and CXCR7 expression. Kaplan-Meier curves for overall survival in 55 patients with high CXCL12 expression and 60 patients with low CXCL12 expression revealed no significant difference (A). However, Kaplan-Meier curves of CXCR4 expression showed that 61 patients with high CXCR4 expression had lower survival rate than 54 patients with low CXCR4 expression (B). Kaplan-Meier curves for overall survival in 57 patients with high CXCR7 expression and 58 patients with low CXCR7 expression also revealed significant difference (C).
Table 3.
Univariate and multivariate analyses of prognostic factors in patients with breast cancer
| Variables | Univariable P-value (log-rank test) | Multivariable | |
|---|---|---|---|
|
| |||
| HR (95% CI) | P-value | ||
| Age (years) | |||
| <50 vs. ≥50 | 0.472 | ||
| Tumor size (cm) | |||
| D≤5 vs. D>5 | 0.053 | ||
| Histological grades | |||
| Well/Moderate vs. Poor | 0.037 | 1.12 (0.97-1.29) | 0.056 |
| TNM stagea | |||
| I-II vs. III-IV | 0.008 | 1.34 (1.18-1.51) | 0.011 |
| Lymph node metastasis | |||
| Negative vs. Positive | 0.003 | 1.41 (1.20-1.57) | 0.009 |
| No. of lymph node excised | |||
| N≤9 vs. N≥10 | 0.088 | ||
| ER | |||
| Negative vs. Positive | 0.147 | ||
| PR | |||
| Negative vs. Positive | 0.093 | ||
| HER-2 | |||
| Negative vs. Positive | 0.022 | 1.19 (0.96-1.34) | 0.053 |
| CA15-3 (U/ml) | |||
| <25 vs. ≥25 | 0.180 | ||
| CXCL12 mRNA expression | |||
| Low vs. High | 0.003 | 1.29 (1.15-1.44) | 0.019 |
| CXCR4 mRNA expression | |||
| Low vs. High | 0.001 | 1.36 (1.09-1.59) | 0.037 |
| CXCR7 mRNA expression | |||
| Low vs. High | <0.001 | 1.43 (1.27-1.62) | 0.002 |
TNM staging was classified according to the 7th edition of the AJCC cancer staging manual.
n, number of patients; D, diameter; N, number of lymph node excised; ER, estrogen receptor; PR, progesterone receptor; HER-2, human epidermal growth factor receptor-2.
Discussion
Recently, reports have demonstrated that chemokines and their receptors play critical roles in the development of cancer, including tumor cell growth, migration, and angiogenesis. Further, they influence the infiltration of immune cells in a tumor [17]. The mechanism of chemokines in malignant tumor metastasis may be reflected by the production of chemokine receptors by tumor cells, which respond to their homologous ligands (produced by certain organs) and migrate along the chemokine gradients to trigger specific organ metastasis [18]. Out of all the known chemokine receptors, breast cancer cells specifically express active CXCR4 and CXCR7, the ligand of which is CXCL12 [4]. Several studies have observed an association between CXCR4 expression and distant metastasis in primary breast cancer patients [19-21]. Furthermore, Hassan et al. identified circulating levels of CXCL12 as a prognostic blood marker in a series of patients with primary breast cancers [22]. However, the contribution of CXCL12 via its receptor CXCR7 is less understood. CXCR7 has been implicated in enhancing cancer cell adhesion to fibronectin and endothelial cells [16], increasing cell survival by decreasing apoptosis [23], and promoting primary tumor growth of lymphoma, lung, prostate and hepatocellular cancer cells [16,23,24]. It has been reported to contribute to tumor angiogenesis through the secretion of angiogenic factors such as vascular endothelial growth factor (VEGF) [16,23], as well as to promote experimental metastasis formation of breast cancer cells [15]. A recent study [25] showed that CXCR7 overexpression alone did not result in CXCL12-induced chemotaxis or invasion in vitro; however, in the context of high CXCR4 expression it further increased the in vitro chemotactic response of MTLn3 CXCR4 cells to CXCL12, while reducing invasion and matrix degradation. In vivo, CXCR7 increased primary tumor growth while it impaired invasion to CXCL12, intravasation and spontaneous metastasis formation. CXCR7 over-expression downregulated the effects of CXCR4 in motility within the primary tumor, intravasation, and spontaneous metastasis formation. The authors concluded that CXCR4 and CXCR7 play different roles in metastasis, with CXCR4 mediating breast cancer invasion and CXCR7 impairing invasion but enhancing primary tumor growth through angiogenesis.
In this study, we investigated a series of breast cancer to demonstrate whether the expression of the CXCR4 and CXCR7 chemokine receptors, along with expression of CXCL12, predicts the risk of metastasis and mortality. Present data showed that the expression of CXCR4, CXCR7, and CXCL12 was correlated with the status of lymph node metastasis and CXCL12 exhibited significant differences in expression between primary tumor and lymph node metastasis tumor. Besides, Survival analysis revealed patients with highly expressed CXCR4 and CXCR7 are subject to a more undesirable prognosis compared with those who expressed low CXCR4 and CXCR7. Furthermore, when analyzed with a multivariate Cox regression model, the expression levels of CXCR4, CXCR7, and CXCL12 were independent prognostic factors for overall survival. Findings of this study coincide with those of other studies [26]. In view of all the evidences, there is reason to believe that the CXCL12-CXCR4-CXCR7 axis is a crucial factor in tumor lymph node metastasis.
Finally, several therapeutic agents have been designed to target the CXCL12-CXCR4 axis, one of which is presently being tested in a clinical trial [27]. In preclinical models, such treatments have been shown to be effective not only in decreasing metastasis from breast cancer, but also in inhibiting primary tumor growth in breast cancer [28,29]. As most breast cancers express at least moderate to high levels of CXCR4, there is a risk that these therapeutic agents may not be adequately targeted, perhaps impeding their clinical development. Since we found that HER-2 over-expression was correlated with high expression of CXCR7 and CXCR7 was also an independent prognostic factor for overall survival, patients with double positive of HER-2 and CXCR7 who do not benefit from anti-HER-2 therapy may potentially benefit from anti-CXCR7 therapy.
In summary, by examining the expression of CXCL12 and its receptors (CXCR4 and CXCR7) in both primary tumors and corresponding lymph node metastasis tumors, data indicate that CXCL12, CXCR4, and CXCR7 are differentially expressed in the primary and metastatic sites of breast cancer. Results revealed the significant association of CXCL12-CXCR4-CXCR7 axis with metastasis and poor prognosis. The correlation between CXCL12-CXCR4-CXCR7 axis and prognosis may provide a new method of understanding breast cancer metastasis and therapy, which are worthy of further study.
Acknowledgements
This work was supported by a grant from Hunan Provincial Department of Science and Technology (No. 2013SK5074).
Disclosure of conflict of interest
None.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y, Ji R, Li J, Gu Q, Zhao X, Sun T, Wang J, Li J, Du Q, Sun B. Correlation effect of EGFR and CXCR4 and CCR7 chemokine receptors in predicting breast cancer metastasis and prognosis. J Exp Clin Cancer Res. 2010;29:16. doi: 10.1186/1756-9966-29-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weigelt B, Peterse JL, van’t Veer LJ. Breast cancer metastasis: markers and models. Nat Rev Cancer. 2005;5:591–602. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 4.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verástegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 5.Paget S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 6.Hassan S, Ferrario C, Saragovi U, Quenneville L, Gaboury L, Baccarelli A, Salvucci O, Basik M. The influence of tumor-host interactions in the stromal cell-derived factor-1/CXCR4 ligand/receptor axis in determining metastatic risk in breast cancer. Am J Pathol. 2009;175:66–73. doi: 10.2353/ajpath.2009.080948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabioglu N, Gong Y, Islam R, Broglio KR, Sneige N, Sahin A, Gonzalez-Angulo AM, Morandi P, Bucana C, Hortobagyi GN, Cristofanilli M. Expression of growth factor and chemokine receptors: new insights in the biology of inflammatory breast cancer. Ann Oncol. 2007;18:1021–1029. doi: 10.1093/annonc/mdm060. [DOI] [PubMed] [Google Scholar]
- 8.Scala S, Ottaiano A, Ascierto PA, Cavalli M, Simeone E, Giuliano P, Napolitano M, Franco R, Botti G, Castello G. Expression of CXCR4 predicts poor prognosis in patients with malignant melanoma. Clin Cancer Res. 2005;11:1835–1841. doi: 10.1158/1078-0432.CCR-04-1887. [DOI] [PubMed] [Google Scholar]
- 9.Ottaiano A, Franco R, Aiello Talamanca A, Liguori G, Tatangelo F, Delrio P, Nasti G, Barletta E, Facchini G, Daniele B, Di Blasi A, Napolitano M, Lerano C, Calemma R, Leonardi E, Albino V, De Angelis V, Falanga M, Boccia V, Capuozzo M, Parisi V, Botti G, Castello G, Vincenzo Iaffaioli R, Scala S. Overexpression of both CXC chemokine receptor 4 and vascular endothelial growth factor proteins predicts early distant relapse in stage II-III colorectal cancer patients. Clin Cancer Res. 2006;12:2795–2803. doi: 10.1158/1078-0432.CCR-05-2142. [DOI] [PubMed] [Google Scholar]
- 10.Bian XW, Yang SX, Chen JH, Ping YF, Zhou XD, Wang QL, Jiang XF, Gong W, Xiao HL, Du LL, Chen ZQ, Zhao W, Shi JQ, Wang JM. Preferential expression of chemokine receptor CXCR4 by highly malignant human gliomas and its association with poor patient survival. Neurosurgery. 2007;61:570–578. doi: 10.1227/01.NEU.0000290905.53685.A2. [DOI] [PubMed] [Google Scholar]
- 11.Hinton CV, Avraham S, Avraham HK. Role of the CXCR4/CXCL12 signaling axis in breast cancer metastasis to the brain. Clin Exp Metastasis. 2010;27:97–105. doi: 10.1007/s10585-008-9210-2. [DOI] [PubMed] [Google Scholar]
- 12.Balabanian K, Lagane B, Infantino S, Chow KY, Harriague J, Moepps B, Arenzana-Seisdedos F, Thelen M, Bachelerie F. The chemokine SDF-1/CXCL12 binds to and signals through the orphan receptor RDC1 in T lymphocytes. J Biol Chem. 2005;280:35760–35766. doi: 10.1074/jbc.M508234200. [DOI] [PubMed] [Google Scholar]
- 13.Luker KE, Lewin SA, Mihalko LA, Schmidt BT, Winkler JS, Coqqins NL, Thomas DG, Luker GD. Scavenging of CXCL12 by CXCR7 promotes tumor growth and metastasis of CXCR4-positive breast cancer cells. Oncogene. 2012;31:4750–4758. doi: 10.1038/onc.2011.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boudot A, Kerdivel G, Habauzit D, Eeckhoute J, Le Dily F, Flouriot G, Samson M, Pakdel F. Differential estrogen-regulation of CXCL12 chemokine receptors, CXCR4 and CXCR7, contributes to the growth effect of estrogens in breast cancer cells. PLoS One. 2011;6:e20898. doi: 10.1371/journal.pone.0020898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miao Z, Luker KE, Summers BC, Berahovich R, Bhojani MS, Rehemtulla A, Kleer CG, Essner JJ, Nasevicius A, Luker GD, Howard MC, Schall TJ. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proc Natl Acad Sci U S A. 2007;104:15735–15740. doi: 10.1073/pnas.0610444104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Shiozawa Y, Wang J, Wang Y, Jung Y, Pienta KJ, Mehra R, Loberg R, Taichman RS. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J Biol Chem. 2008;283:4283–4294. doi: 10.1074/jbc.M707465200. [DOI] [PubMed] [Google Scholar]
- 17.Feng LY, Ou ZL, Wu FY, Shen ZZ, Shao ZM. Involvement of a novel chemokine decoy receptor CCX-CKR in breast cancer growth, metastasis and patient survival. Clin Cancer Res. 2009;15:2962–2970. doi: 10.1158/1078-0432.CCR-08-2495. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Seethala RR, Zhang Q, Gooding W, van Waes C, Hasegawa H, Ferris RL. Autocrine and paracrine chemokine receptor 7 activation in head and neck cancer: implications for therapy. J Natl Cancer Inst. 2008;100:502–512. doi: 10.1093/jnci/djn059. [DOI] [PubMed] [Google Scholar]
- 19.Salvucci O, Bouchard A, Baccarelli A, Deschenes J, Sauter G, Simon R, Bianchi R, Basik M. The role of CXCR4 receptor expression in breast cancer: a large tissue microarray study. Breast Cancer Res Treat. 2006;97:275–283. doi: 10.1007/s10549-005-9121-8. [DOI] [PubMed] [Google Scholar]
- 20.Blot E, Laberge-Le Couteulx S, Jamali H, Cornic M, Guillemet C, Duval C, Hellot MF, Pille JY, Picquenot JM, Veyret C. CXCR4 membrane expression in node-negative breast cancer. Breast J. 2008;14:268–274. doi: 10.1111/j.1524-4741.2008.00573.x. [DOI] [PubMed] [Google Scholar]
- 21.Holm NT, Byrnes K, Li BD, Turnage RH, Abreo F, Mathis JM, Chu QD. Elevated levels of chemokine receptor CXCR4 in HER-2 negative breast cancer specimens predict recurrence. J Surg Res. 2007;141:53–59. doi: 10.1016/j.jss.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 22.Hassan S, Baccarelli A, Salvucci O, Basik M. Plasma stromal cell-derived factor-1: host derived marker predictive of distant metastasis in breast cancer. Clin Cancer Res. 2008;14:446–454. doi: 10.1158/1078-0432.CCR-07-1189. [DOI] [PubMed] [Google Scholar]
- 23.Burns JM, Summers BC, Wang Y, Melikian A, Berahovich R, Miao Z, Penfold ME, Sunshine MJ, Littman DR, Kuo CJ, Wei K, McMaster BE, Wright K, Howard MC, Schall TJ. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng K, Li HY, Su XL, Wang XY, Tian T, Li F, Ren GS. Chemokine receptor CXCR7 regulates the invasion, angiogenesis and tumor growth of human hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2010;29:31. doi: 10.1186/1756-9966-29-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernandez L, Magalhaes MA, Coniglio SJ, Condeelis JS, Seqall JE. Opposing roles of CXCR4 and CXCR7 in breast cancer metastasis. Breast Cancer Res. 2011;13:R128. doi: 10.1186/bcr3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattern J, Koanagi R, Volm K. Association of vascular endothelium growth factor expression with intratumoral microvessel density and tumor cell proliferation in human epidermoid lung cancer. Br J Cancer. 1996;73:931–934. doi: 10.1038/bjc.1996.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong D, Korz W. Translating an Antagonist of Chemokine Receptor CXCR4: from bench to bedside. Clin Cancer Res. 2008;14:7975–7980. doi: 10.1158/1078-0432.CCR-07-4846. [DOI] [PubMed] [Google Scholar]
- 28.Smith MC, Luker KE, Garbow JR, Prior JL, Jackson E, Piwnica-Worms D, Luker GD. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004;64:8604–8612. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- 29.Liang Z, Wu T, Lou H, Yu X, Taichman RS, Lau SK, Nie S, Umbreit J, Shim H. Inhibition of breast cancer metastasis by selective synthetic polypeptide against CXCR4. Cancer Res. 2004;64:4302–4308. doi: 10.1158/0008-5472.CAN-03-3958. [DOI] [PubMed] [Google Scholar]


