Abstract
Background: Carcinogenesis is associated with several critical regulatory molecules which are involved in different signaling pathways such as the WNT signaling pathways. Among which the β-catenin dependent pathway has been associated with human endometrial cancer. Genetic and biochemical studies have demonstrated that glypicans can regulate several signaling pathways including those triggered by Wnts. Glypican 3 is one of six mammalian members of the glypican family of proteoglycans. Overexpression of glypican 3 has been reported in some types of cancers but only few data are available about its expression in endometrial carcinoma and its role in endometrial carcinogenesis. The aim of this study was to examine the immunohistochemical expression of glypican 3 in endometrioid endometrial carcinoma (EEC) and serous endometrial carcinoma (SEC), and to correlate its expression with prognostic factors of endometrial carcinoma. Materials and methods: Immunohistochemical expression of glypican 3 was studied in fifty two EEC and nineteen SEC cases. Results: Glypican 3 expression showed a significant difference between EEC and SEC (P = 0.027) and it was significantly correlated with tumor grade, stage and myometrial invasion (P = 0.001). Conclusion: Glypican 3 expression can be used as an adjunct in the differentiation between EEC and SEC. Glypican 3 is associated with poor prognostic parameters in both EEC and SEC, and it can be a promising molecule for targeted immunotherapy in positive cases.
Keywords: Glypican 3, endometrial carcinoma, immunohistochemistry
Introduction
Endometrial adenocacinoma is the most common gynecologic malignancy [1]. The most common form of endometrial carcinoma (EC) is the endometrioid endometrial carcinoma (EEC) or type I which accounts for more than 80% of all EC. Uterine EEC is usually accompanied by endometrial hyperplasia and usually have favorable prognosis [2]. In contrast, type II endometrial cancers are more aggressive, non estrogen related, high grade, and lack association with hyperplasia [3]. The most common histological subtype in the second group is the serous endometrial carcinoma (SEC); it constitutes about 10% of all endometrial cancers and has a higher fatality than the endometrioid counterpart [4]. It usually develops in an atrophic endometrium and do not respond to conventional chemotherapy, radiotherapy and hormone therapy [3]. Factors associated with poor prognosis in endometrial carcinomas are patient’s age, tumor grade, stage, histological subtype and depth of myometrial invasion [5].
Carcinogenesis of EC is associated with several critical regulatory molecules which are involved in different signaling pathways. The WNT signaling pathway is one of the most evolutionary-conserved signal transduction pathways. The WNT signaling pathways include β-catenin dependent WNT signaling pathway (i.e. canonical WNT/β-catenin) and β-catenin independent WNT signaling pathway (i.e. Non-canonical, such as WNT/JNK pathway, WNT/calcium pathway). Among these WNT signaling pathways the β-catenin dependent pathway has been associated with human endometrial cancer [6].
Glypican 3 is a cell surface heparan sulfate proteoglycan that binds to the cell membrane via glycosyl-phosphatidyl-inositol anchors [7]. Glypican 3 was first identified in patients with Simpson-Golabi-Behmel syndrome, a rare x linked disorder characterized by prenatal and postnatal overgrowth caused by mutation in glypican 3 gene [8]. Several studies elucidated the role of Glypican 3 in regulation of cell proliferation and apoptosis during normal development [9]. Overexpression of Glypican 3 has been reported in some types of cancers such as hepatocellular carcinoma, squamous cell carcinoma of the lung and testicular germ cell tumors [10]. In hepatocellular carcinoma several studies aimed to target Glypican 3 by immunotherapeutic approaches and to inhibit its growth by blocking Glypican 3 function [11]. Genetic and biochemical studies have demonstrated that glypicans can regulate several signaling pathways including those triggered by Wnts [12,13], bone morphogenetic proteins (BMPs) [14,15] and fibroblast growth factors [16].
Since the Wnt/β-catenin dependent pathway has been associated with human endometrial cancer [6], and Glypiacn 3 can regulate several signaling pathways including those triggered by Wnts So it is of interest to study the immunohistochemical expression of Glypican 3 in endometrial carcinoma especially that the data available on this subject are very limited. This can help in better understanding of endometrial carcinogenesis and possible therapeutic targeting.
The aim of this study was to examine the immunohistochemical expression of Glypican 3 in endometioid endometrial carcinoma (EEC) and serous endometrial carcinoma (SEC), and to correlate its expression with prognostic factors of endometrial carcinoma.
Materials and methods
Tissue and patient data
The current study was conducted on 52 EEC and 19 SEC. All endometrial carcinoma cases received in the Pathology Lab of Ain Shams Specialized Hospital during the period from January 2008 to June 2015 were retrieved. After exclusion of cases with insufficient information or inadequate material a total number of 52 EEC and 19 SEC were included in our study. All patients underwent total abdominal hysterectomy and bilateral salpinge-oopherectomy. The hematoxylin and eosin slides were reviewed by both authors to confirm the diagnoses and to select the representative sections. The histological classification was done according to the World Health Organization classification [17]. Staging was defined according to the International Federation of Obstetricians and Gynecologists (FIGO) staging system [18].
All patients who participated in this study signed a written informed consent before surgery. The study was approved by the Research Ethical Committee at Faculty of Medicine, Ain Shams University.
Immunohistochemical procedure
Four micrometer sections of formalin-fixed and paraffin-embedded samples from all cases were prepared. They included the tumor and the adjacent normal endometrial tissue in some specimens. Immunohistochemical staining was performed using primary antibody glypican 3 mouse monoclonal antibody (1:200, clone IG 12, Cell Marque, Burlington, VT, USA). Avidin-Biotin immunoperoxidase complex technique was used according to Hsu et al. [19] by applying the super sensitive detection kit (Biogenex, CA, USA). The prepared tissue sections were fixed on poly-L-lysine coated slides overnight at 37°C. They were deparaffinized and rehydrated through graded alcohol series. Then the sections were heated in a microwave oven in 10 mM citrate buffer (pH 6.0) for 20 min. After the blocking of endogenous peroxidase and incubation in Protein Block Serum-Free Solution (Dako Cytomation) for 20 min, the sections were incubated overnight at 4°C with primary antibodies. Biotinylated anti-mouse immunoglobulin and streptavidin conjugated to horseradish peroxidase were then added. Finally, 3, 3-diaminobenzidine as the substrate or chromogen was used to form an insoluble brown product. Finally, the sections were counterstained with hematoxylin and mounted. Sections from hepatocellular carcinoma were used as positive control for Glypican 3. Negative control sections were incubated with normal mouse serum instead of the primary antibody.
Interpretation of immunohistochemical staining
Immunohistochemical analysis of Glypican 3 was performed by both authors separately. Any discrepancies were resolved by consensus using a multihead microscope. Both distribution and intensity of the stain were evaluated. The distribution was defined as the percent of the stained cells and was scored as: positive > 10% positive cells, 1: 10-50% positive cells, 2: > 50% stained cells. The staining intensity was scored as 1: Faint staining (light yellow), 2: Moderate staining (brown), 3: Strong staining (dark brown). Combined scores were obtained by adding the intensity and distribution scores and were considered as 0 in the negative cases.
Statistical analysis
Continuous variables are expressed as mean and SD or as median (interquartile range) in cases of skewed distributions. Categorical variables are expressed as frequencies and percentage. Differences between independent groups were tested using the Student t test for Continuous variables. Categorical variables were compared using the fisher exact test. Spearman’s correlation was used to assess the correlation between variables. A significance level of P < 0.05 was used in all tests. All statistical procedures were carried out using SPSS version 20 for Windows (SPSS Inc, Chicago, IL, USA).
Results
Clinicopathological results
The age of patients with endometrioid endometrial carcinoma ranged from 53 to 57 years (mean = 55.02, SD 1.24), while that of patients with serous endometrial carcinomas ranged from 59 to 68 years (mean = 62.74, SD 3.30). Tumor size in EEC ranged from 4 cm to 7 cm (mean = 5.02, SD 0.82) and in SEC from 6 to 11 cm in longest dimension (mean = 9.13, SD 1.44). The 52 EEC included grade 1 (32 cases), grade 2 (13 cases) and grade 3 (7 cases). The 19 SEC included moderately differentiated tumors i.e. grade 2 (8 cases) and poorly differentiated tumors i.e. grade 3 (11 cases). The moderately differentiated cases showed a well-defined papillary architecture with fibrovascular connective tissue cores with solid areas of tumor cells < 50% of the tumor, while the poorly differentiated carcinomas showed a predominance of solid areas with poorly defined papillae. The nuclei in all SEC were highly atypical with frequent mitotic figures. Myometrial invasion was seen in 5/7 grade 3 EEC, 2/13 grade 2 EEC and in 16/19 SEC (including the 11 poorly differentiated cases and 5/8 moderately differentiated cases). FIGO staging in EEC was stage 1 (47 cases), stage 2 (3 cases), stage 3 (2 cases) while in SEC it was stage 2 (12 cases), stage 3 (6 cases) and stage 4 (1 case).
Immunohistochemical results
Three out of fifty two EEC (5.7%) were positive for Glypican 3. The staining distribution was 2+ in the three cases (i.e. in more than 50% of the tumor cells). The intensity was moderate (2+) in two cases and faint (1+) in one case. The combined scores were 4, 4, and 3. Myometrial invasion was seen in all positive cases. All positive cases were WHO grade 3 (Figure 1). The FIGO staging of the positive cases were (T2a N1 Mx), (T3a N1 Mx), and (T3b N1 Mx).
Figure 1.
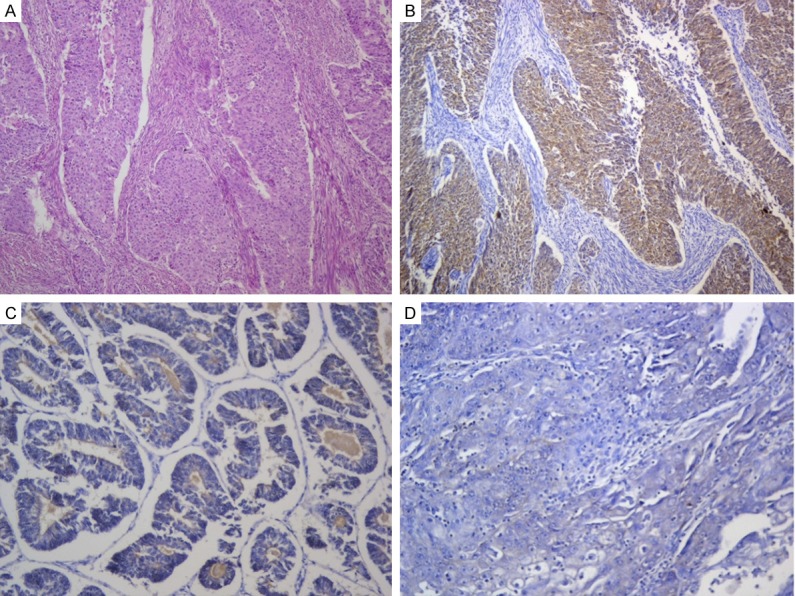
Endometrioid endometrial carcinoma. A. High grade EEC showing deep myometrial invasion (H&E × 100). B. High grade EEC with myometrial invasion positive for Glypican 3 (Glypican 3 × 100). C. Low grade EEC negative for Glypican 3 expression (Glypican 3 × 200). D. Grade 3 EEC negative for Glypican 3 (Glypican 3 × 200).
Five out of the nineteen SEC (26.3%) were positive for Glypican 3. The staining distribution was 2+ in four cases and 1+ in one case. The staining intensity was moderate (2+) in the five cases. The combined scores were 4, 4, 4, 4, and 3. All positive SEC cases showed myometrial invasion and all were WHO grade 3 (Figure 2). The staging in the positive cases were (T3a N1 Mx) in four cases and (T4 N1 Mx) in one case.
Figure 2.
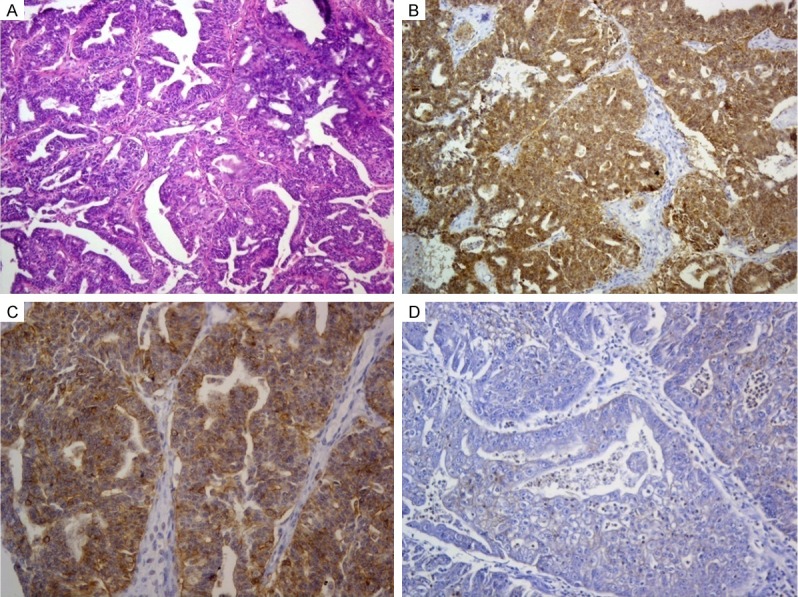
Serous endometrial carcinoma. A. Complex papillary architecture in SEC (H&E × 100). B. Moderately differentiated SEC positive for Glypican 3 expression (Glypican 3 × 100). C. Glypican 3 positive SEC showing foci of cell membrane staining in addition to predominant cytoplasmic staining (Glypican 3 × 200). D. SEC negative for Glypican 3 expression (Glypican 3 × 200).
The stain was localized in the cytoplasm with cell membrane staining in some tumor areas (Figure 2C). Normal tissue adjacent to tumors was present in 13 cases and was totally negative for Glypican 3.
Table 1 summarizes the WHO grading and the FIGO staging for the examined cases.
Table 1.
WHO grading and the FIGO staging for the examined cases
| WHO grading | FIGO staging | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Grade 1 | Grade 2 | Grade 3 | Stage 1 | Stage 2 | Stage 3 | Stage 4 | |
| EEC (n = 52) | 32 | 13 | 7 | 47 | 3 | 2 | - |
| SEC (n = 19) | - | 8 | 11 | - | 12 | 6 | 1 |
Table 2 summarizes the clinicopathological and immunohistochemical results for the positive Glypivan 3 cases.
Table 2.
Clinicopathological and immunohistochemical results for the positive Glypican cases
| Positive cases | Age | Tumor size* | Grade | Myometrial invasion | Stage (TNM) | Glypican 3 distribution | Glypican 3 intensity | Glypican 3 score | |
|---|---|---|---|---|---|---|---|---|---|
| EEC 3/52 (5.7%) | 1 | 54 | 4 | 3 | + | T2a N1 Mx | 2+ | 1+ | 3 |
| 2 | 53 | 5.5 | 3 | + | T3a N1 MX | 2+ | 2+ | 4 | |
| 3 | 53 | 6.5 | 3 | + | T3b N1 Mx | 2+ | 2+ | 4 | |
| SEC 5/19 (26.3%) | 1 | 62 | 7.5 | 3 | + | T3a N1 Mx | 2+ | 2+ | 4 |
| 2 | 65 | 11 | 3 | + | T3a N1 Mx | 1+ | 2+ | 3 | |
| 3 | 59 | 8 | 3 | + | T3a N1 Mx | 2+ | 2+ | 4 | |
| 4 | 67 | 10.5 | 3 | + | T4 N1 Mx | 2+ | 2+ | 4 | |
| 5 | 60 | 10 | 3 | + | T3a N1 Mx | 2+ | 2+ | 4 | |
Tumor longest dimension.
Statistical results
A statistically significant correlation was found between EEC and SEC regarding age, tumor size, stage, grade and myometrial invasion being higher in SEC than EEC (P = 0.001 for each parameter) (Table 3). A statistically significant difference was found between Glypican 3 expression in EEC and SEC (P = 0.027) (Table 4). There was a high statistically significant correlation between tumor grade, stage, myometrial invasion and Glypican 3 positivity (P < 0.001 in each parameter), all positive cases in EEC and SEC were of high grade, stage and all of which showed myometrial invasion (Table 5).
Table 3.
Comparison between EEC and SEC as regard clinicopathological data
| Group | P | Sig | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| EEC (N = 52) | SEC (N = 19) | ||||||
|
| |||||||
| Mean | ± SD | Mean | ± SD | ||||
| Age | 55.02 | 1.24 | 62.74 | 3.30 | 0.001* | HS | |
| Size (cm) | 5.02 | .82 | 9.13 | 1.44 | 0.001* | HS | |
| Stage | 1 (n %) | 46 | 88.5% | 0 | 0.0% | 0.001** | HS |
| 2 (n %) | 3 | 5.8% | 12 | 63.2% | |||
| 3 (n %) | 3 | 5.8% | 6 | 31.6% | |||
| 4 (n %) | 0 | 0.0% | 1 | 5.3% | |||
| Grade | 1 (n %) | 32 | 61.5% | 0 | 0.0% | 0.001** | HS |
| 2 (n %) | 13 | 25.0% | 8 | 42.1% | |||
| 3 (n %) | 7 | 13.5% | 11 | 57.9% | |||
| Myometrial invasion | Negative (n %) | 45 | 86.5% | 3 | 15.8% | 0.001** | HS |
| Positive (n %) | 7 | 13.5% | 16 | 84.2% | |||
Student t test.
N = number of cases.
Fisher exact test.
Table 4.
Comparison between EEC and SEC as regard Glypican 3 expresssion
| Group | P | Sig | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| EEC | SEC | ||||||
|
| |||||||
| N | % | N | % | ||||
| Expression of gp3 | Negative | 49 | 94.2% | 14 | 73.7% | 0.027* | S |
| Positive | 3 | 5.7% | 5 | 26.3% | |||
Fisher exact test.
S = significant.
Table 5.
Relation between myometrial invasion, tumor stage, and grade with Glypican 3 expression in all examined endometrial carcinoma cases (EEC and SEC)
| Glypican 3 expression | P | Sig | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Positive | Negative | ||||||
|
| |||||||
| N | % | N | % | ||||
| Myometrial invasion | Positive | 8 | 34.8% | 15 | 65.2% | .001* | HS |
| Negative | 0 | 0.0% | 48 | 100.0% | |||
| Stage | 1 | 0 | 0.0% | 46 | 100.0% | .001* | HS |
| 2 | 1 | 6.7% | 14 | 93.3% | |||
| 3 | 6 | 66.7% | 3 | 33.3% | |||
| 4 | 1 | 100.0% | 0 | 0.0% | |||
| Grade | 1 | 0 | 0.0% | 32 | 100.0% | .001* | HS |
| 2 | 0 | 0.0% | 21 | 100.0% | |||
| 3 | 8 | 44.4% | 10 | 55.6% | |||
Fisher exact test.
Discussion
Carcinogenesis is associated with several critical regulatory molecules that are involved in different signaling pathways [20]. Glypican 3 is one of six mammalian members of the Glypican family of proteoglycans [21]. It regulates cell growth and apoptosis by interacting with various morphogenic or growth factors such as Wnt, fibroblast growth factor 2, and bone morphogenic protein [22]. It is normally expressed in trophoblastic and fetal tissues but has a limited expression in adult tissue [23]. Over-expression of Glypican 3 has been reported in several malignant tumors such as hepatocellular carcinoma, malignant melanoma, squamous cell carcinoma of the lung and testicular germ cell tumors [10]. However very few data are available about the expression of Glypican 3 in endometrial carcinoma.
The Wnt signaling pathways represent a group of pathways that comprise of proteins involved in the transduction of signals via cell surface receptors. First identified in 1982 by Nusse in mouse models of mammary cancer, they can be divided into two major groups: canonical and non-canonical pathways, with the differentiating factor being the involvement of β-catenin in the former [24]. Both are activated by the binding of a Wnt-protein ligand to a Frizzled family receptor (Fz), which in turn transfers the signal to the intracellular protein, Dishevelled (Dsh) [25]. Cannonical Wnt activity has been shown to play a role in cancer progression in many tumor types including hepatocellular carcinoma [26]. It has also been associated with human endometrial carcinoma [20].
Because Glypican 3 interacts with Wnts it has been proposed that it stimulates signaling by increasing the amount of Wnt at the cell membrane and thus facilitating the interaction between this growth factor and its signaling receptor Frizzled [27]. Therapies targeting the Wnt pathway may play an essential role in the future of anticancer therapeutics either alone or in conjunction with traditional therapies [28].
Since only few data are available about Glypican 3 in endometrial carcinoma, so in our study we examined the immunohistochemical expression of Glypican 3 in EEC and SEC, we compared its expression in both types and we correlated it with prognostic parameters of endometrial carcinoma. Our results revealed Glypican 3 expression in 5.7% of EEC and in 26.3% of SEC. This was close to the findings of Baumhoer, et al. in EEC (6%) but higher in SEC cases (13% in contrast to 26.3% in our study) [29]. To the best of our knowledge our study is the first one to investigate the correlation between Glypican 3 expression and prognostic factors in endometrial carcinoma. We found Glypican 3 to be significantly associated with poor prognostic parameters as high grade, myometrial invasion and advanced stage (P = 0.001). In the study of Bing et al. [30] Glypican 3 reactivity in malignant mixed mullerian tumors was detected in both sarcomatous and poorly differentiated epithelial component, and in accordance to our study and the study of Baumhoer, et al. [29] the normal tissue adjacent to tumors was totally negative for Glypican 3 [30].
Despite the few number of positive cases in EEC and SEC there was a statistically significant difference between Glypican 3 expression in both types being more expressed in SEC so it can be used as an adjunct with other markers in their differentiation in problematic cases. The small percent of positive Glypican 3 in EEC suggests that Glypican 3-Wnt interaction has a limited role in its carcinogenesis. The larger percent of Glypican 3 positivity in SEC (in which Wnt pathway is not a major player in its carcinogenesis [31]) suggests involvement of Glypican 3 in serous endometrial carcinogenesis through a different regulatory pathway other than Wnt. Further molecular studies are needed to clarify the role of Glypican 3 in endometrial carcinogenesis.
We concluded that Glypican 3 expression is significantly higher in SEC than EEC and it can be used as an adjunct in their differentiation. Glypican 3 is associated with poor prognostic parameters in both EEC and SEC, and it can be a promising molecule for targeted immunotherapy in positive cases. Further studies are needed to clarify the possible Glypican 3 role in endometrial carcinogenesis.
Acknowledgements
The authors acknowledge Mrs. Manal Hossam El Din for her technical support in immunostaining procedure.
Disclosure of conflict of interest
None.
References
- 1.Macwhinnie N, Monaghan H. The use of P53, PTEN, and CerbB-2 to differentiate uterine serous papillary carcinoma from endometrioid endometrial carcinoma. Int J Gynecol Cancer. 2004;14:938–46. doi: 10.1111/j.1048-891X.2004.14533.x. [DOI] [PubMed] [Google Scholar]
- 2.Silverberg SG, Kurman RJ, Nogales F, Mutter GL, Kubik-Huch RA, Tavassoli FA. Epithelial tumours and related lesions. In: Tavassoli FA, Devilee P, editors. Tumours of the Breast and Femal Genital organs. World Health Organization Classification of Tumours. Lyon: IARC Press; 2003. pp. 221–32. [Google Scholar]
- 3.Goff BA. Uterine papillary serous carcinoma: what have we learned over the past quarter century? Gynecol Oncol. 2005;98:341–3. doi: 10.1016/j.ygyno.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Lampe B, Kurzl R, Kindermann G. Serous papillary adenocarcinoma of the endometrium. Geburtshilfe Frauenheilkd. 1991;51:45–50. doi: 10.1055/s-2008-1026331. [DOI] [PubMed] [Google Scholar]
- 5.Deligdisch L, Holinka CF. Endometrial carcinoma: two diseases? Cancer Detect Prev. 1987;10:237–46. [PubMed] [Google Scholar]
- 6.Kestler HA, Kuhl M. From Individual Wnt Pathways towards a Wnt Signalling Network. Philos Trans R Soc Lond B Biol Sci. 2008;363:1333–1347. doi: 10.1098/rstb.2007.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filmus J, Selleck SB. Glypicans: proteoglycans with a surprise. J Clin Invest. 2001;108:497–501. doi: 10.1172/JCI13712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pilia G, Hughes-Benzie RM, MacKenzie A, Baybayan P, Chen EY, Huber R, Neri G, Cao A, Farabosco A, Schlessinger D. Mutations in GPC3, a glypican gene, cause the Simpson-Golabi-Behmel overgrowth syndrome. Nat Genet. 1996;12:241–247. doi: 10.1038/ng0396-241. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez AD, Kaya M, Shi W, Song H, Testa JR, Penn LZ, Filmus J. OCI-5/GPC3, a glypican encoded by a gene that is mutated in the Simpson-Golabi-Behmel overgrowth syndrome, induces apoptosis in a cell line-specific manner. J Cell Biol. 1998;141:1407–1414. doi: 10.1083/jcb.141.6.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ota S, Hishinuma M, Yamauchi N, Goto A, Morikawa T, Fujimura T, Kitamura T, Kodama T, Aburatani H, Fukayama M. Oncofetal protein glypican-3 in testicular germ-cell tumor. Virchows Arch. 2006;449:308–14. doi: 10.1007/s00428-006-0238-x. [DOI] [PubMed] [Google Scholar]
- 11.Zittermann SI, Capurro MI, Filmus J. Soluble glypican 3 inhibits the growth of hepatocellular carcinoma in vitro and in vivo. Int J Cancer. 2010;126:1291–1301. doi: 10.1002/ijc.24941. [DOI] [PubMed] [Google Scholar]
- 12.Topczewski J, Sepich DS, Myers DC, Walker C, Amores A, Lele Z, Hammerschmidt M, Postlethwait J, Solnica-Krezel L. The zebrafish glypican knypek controls cell polarity during gastrulation movements of convergent extension. Dev Cell. 2001;1:251–264. doi: 10.1016/s1534-5807(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 13.Song HH, Shi W, Xiang YY, Filmus J. The loss of glypican-3 induces alterations in Wnt signaling. J Biol Chem. 2005;280:2116–2125. doi: 10.1074/jbc.M410090200. [DOI] [PubMed] [Google Scholar]
- 14.Belenkaya TY, Han C, Yan D, Opoka RJ, Khodoun M, Liu H, Lin X. Drosophila Dpp morphogen movement is independent of dynamin mediated endocytosis but regulated by the glypican members of heparan sulfate proteoglycans. Cell. 2004;119:231–244. doi: 10.1016/j.cell.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 15.Dwivedi PP, Grose RH, Filmus J, Hii CS, Xian CJ, Anderson PJ, Powell BC. Regulation of bone morphogenetic protein signaling and cranial osteogenesis by Gpc1 and Gpc3. Bone. 2013;55:367–376. doi: 10.1016/j.bone.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 16.Gutiérrez J, Brandan E. A novel mechanism of sequestering FGF-2 by glypican in lipid rafts, allowing skeletal muscle differentiation. Mol Cell Biol. 2010;30:1634–1649. doi: 10.1128/MCB.01164-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tavassoli FA, Devilee P, editors. Pathology and genetics of tumors of the breast and female genital organs. Lyon: IARC press; 2003. World Health Organization classification of tumors. [Google Scholar]
- 18.Mutch DG. The New FIGO staging system for cancers of the vulva, cervix, endometrium, and sarcomas. Gynecol Oncol. 2009;115:325–328. [Google Scholar]
- 19.Hsu SM, Raine L, Fanger H. Use of avidin-biotin- peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabelled antibody (PAP) procedures. J Histochem Cytochem. 1981;29:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- 20.Ma XY, Ma CX, Wang JH. Endometrial Carcinogenesis and Molecular Signaling Pathways. American Journal of Molecular Biology. 2014;4:134–149. [Google Scholar]
- 21.Filmus J, Capurro M. The glypican family. In: Karamanos NK, editor. Extracellular Matrix: Pathobiology and Signaling. Berlin/Boston: De Gruyter; 2012. pp. 209–220. [Google Scholar]
- 22.De Cat B, Muyldermans SY, Coomans C, Degeest G, Vanderschueren B, Creemers J, Biemar F, Peers B, David G. Processing by proprotein convertases is required for glypican-3 modulation of cell survival, Wnt signaling, and gastrulation movements. J Cell Biol. 2003;163:625–35. doi: 10.1083/jcb.200302152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiang YY, Ladeda V, Filmus J. Glypican-3 expression is silenced in human breast cancer. Oncogene. 2001;20:7408–7412. doi: 10.1038/sj.onc.1204925. [DOI] [PubMed] [Google Scholar]
- 24.Rao TP, Kühl M. An updated overview on Wnt signaling pathways: a prelude for more. Circ Res. 2010;106:1798–1806. doi: 10.1161/CIRCRESAHA.110.219840. [DOI] [PubMed] [Google Scholar]
- 25.Perrimon N, Mahowald AP. Multiple functions of segment polarity genes in Drosophila. Dev Biol. 1987;119:587–600. doi: 10.1016/0012-1606(87)90061-3. [DOI] [PubMed] [Google Scholar]
- 26.White BD, Chien AJ, Dawson DW. Dysregulation of Wnt/β-catenin signaling in gastrointestinal cancers. Gastroenterology. 2012;142:219–32. doi: 10.1053/j.gastro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Capurro M, Martin T, Shi W, Filmus J. Glypican-3 binds to Frizzled and plays a direct role in the stimulation of canonical Wnt signaling. J Cell Sci. 2014;127:1565–1575. doi: 10.1242/jcs.140871. [DOI] [PubMed] [Google Scholar]
- 28.Sheikh A, Niazi AK, Ahmed MZ, Iqbal B, Muhammad S, Anwer S, Khan HH. The role of Wnt signaling pathway in carcinogenesis and implications for anticancer therapeutics. Hered Cancer Clin Pract. 2014;12:13. doi: 10.1186/1897-4287-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baumhoer D, Tornillo L, Stadlmann S, Roncalli M, Diamantis EK, Terracciano LM. Glypican 3 Expression in Human Nonneoplastic, Preneoplastic, and Neoplastic Tissues, A Tissue Microarray Analysis of 4,387 Tissue Samples. Am J Clin Pathol. 2008;129:899–906. doi: 10.1309/HCQWPWD50XHD2DW6. [DOI] [PubMed] [Google Scholar]
- 30.Bing Z, Pasha T, Wang LP, Zhang PJ. Malignant Mixed Mullerian Tumor: An Immunohistochemical Study. Patholog Res Int. 2012;2012:569609. doi: 10.1155/2012/569609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Markowska A, Pawalowska M, Lubin J, Markowska J. Signaling pathways in endometrial cancer. Contemp Oncol (pozn) 2014;18:143–148. doi: 10.5114/wo.2014.43154. [DOI] [PMC free article] [PubMed] [Google Scholar]


