Abstract
Extramammary Paget’s disease (EMPD) is a rare cutaneous neoplasm. The aim of this study was to elaborate the clinical and pathological features of Chinese EMPD male patients. The study comprised 246 patients with EMPD at our institute from January 1993 to December 2012. Scrotum was the most common initial site. The average age of onset was 63.9 years but the mean delay in diagnosis was 3.6 years. EPMD spread exclusively to the inguinal lymph nodes and the right inguinal lymph nodes are more likely to suffered Paget cells infiltration. Accompanying malignancies were found in 20 patients. Pathological examination revealed 63 patients defined as invasive EMPD. Immunohistochemical detection showed various expression levels of EMA, CEA, CK7, HER2/neu, Ki67, P53, CK20 and S100 in tumor tissues, but negative expression of VIM, LCA and HMB45. HER2/neu protein exhibited a significant association with invasive EMPD. A novel histological type of EMPD with CK7-/S100+ was identified. Elevated serum PSA level was observed in only 16% patients. Invasive EMPD often had advanced age of onset. Metastatic EMPD showed significantly shorter in the delay in diagnosis and the greater length of skin lesion in contrast to others. This study demonstrates the clinical and pathological features of Chinese male EMPD patients, and may provide implications for the management of Chinese EMPD patients.
Keywords: Extramammary Paget’s disease, pathology, invasion, metastases
Introduction
Extramammary Paget’s disease (EMPD) is a rare cutaneous neoplasm [1]. Due to the rarity of EMPD, our knowledge on the pathogenesis and clinical features of this disease remain limited [2]. The literature reports that EMPD represents just 6.5% of all Paget’s disease [3]. Clinically, EMPD results in skin inflammatory-like manifestations, including pruritus, burning or bleeding, which ultimately mimic treatment-refractory eczema [4]. It predominantly affects skin areas that are full of apocrine glands, such as the genital region, anus or axilla, but the disease can also affect less common areas such as the eyelid and umbilicus [5,6]. EMPD occurs mainly among in Caucasian women and Asian men in their 60 s and 70 s [7].
Since Dr.Croker described the first case of EMPD in 1889, no clear guidelines have been established for the diagnosis, treatment and follow-up of patients with EMPD [8]. Especially, there have been few documented studies of EMPD in China. The purpose of our study was to investigate the clinical and pathological characteristics of Chinese male patients with EMPD. This may improve our knowledge of EMPD and help to establish more effective diagnostic and management guidelines for Chinese EMPD patients.
Materials and methods
This study was based largely on a review of 246 EMPD male patients at Huashan Hospital, Fudan University in Shanghai, China over a 20-year period from January 1993 to December 2012. At diagnosis, all patients received a thorough physical examination and imageological tests including chest X-ray and abdominal and pelvic computed tomography scan to exclude internal malignancies. Clinical data for each EMPD patient were identified from the pathology registry and medical history via the Huashan Hospital archives. The following parameters were reviewed: age of onset and at diagnosis, clinical presentation and symptoms, duration of illness, tumor location and size (Longest diameter of the skin lesion (cm) was used to represent as tumor size.), evaluation of metastases, presence of non-malignant diseases and tumor history, laboratory tests and other information, if applicable. Primary EMPD was defined as patients without tumor history. For those patients with tumor history, according to the guidelines proposed by Lee et al. [9], an internal malignancy was considered to be genuinely associated with EMPD if the two malignancies occurred within the same five-year period; these cases were defined as secondary EMPD. If the interval between diagnosis of EMPD and internal malignancy was greater than five years, the uncertain association was determined between other malignancy and EMPD. The study protocol (2013-021) was approved by the Ethics Committee of Huashan Hospital, and all study participants provided written informed consent prior to study inclusion.
Histological sections were stained with hematoxylin and eosin (HE) and immunohistochemical stains including EMA, CEA, CK7, HER2/neu, Ki67, P53, CK20, S100, VIM, LCA and HMB45 (Antibodies for these eleven proteins were purchased from Dako). All stained histological sections were independently reviewed by the dermatopathologists. Invasiveness was evaluated by the presence and depth of the Paget cells’ dermal invasion.
χ2 and Fisher’s exact tests were used for analysis of categorical variables, and the t-test was used for analysis of continuous variables. Statistical significance was considered as P<0.05.
Results
Clinical data
In this study, data from 246 Chinese male EMPD patients were analyzed. The mean age of onset was 63.9 years (ranging from 32 to 91 years), and the distribution of onset age was showed in Figure 1. According to the data, we found that 95% EMPD cases were over 50 years of age, and a peak age could be seen in 60 s. Average age at the time of diagnosis was 67.5 years. The mean delay in diagnosis was 3.6 years, yet there was a case persisting for up to 30 years before diagnosis. The common initial sites of skin lesions included the scrotum (211 cases, 85.8%), penile shaft (15 cases, 6.1%), pubic area (14 cases, 5.7%) and groin (6 cases, 2.4%). Tumor size (longest diameter of the skin lesion) varied from 1.5 to 16 cm (median, 5.5 cm). Pruritus, rash, erythema, erosion, pain and exudation were frequently observed in patients with EMPD. Of these symptoms, pruritus (180 cases, 73%) was the most common initial symptom. Most of the EMPD patients (232 cases, 94.3%) were initially diagnosed with skin inflammation or eczema, but their conditions failed to respond to the standard topical therapies.
Figure 1.
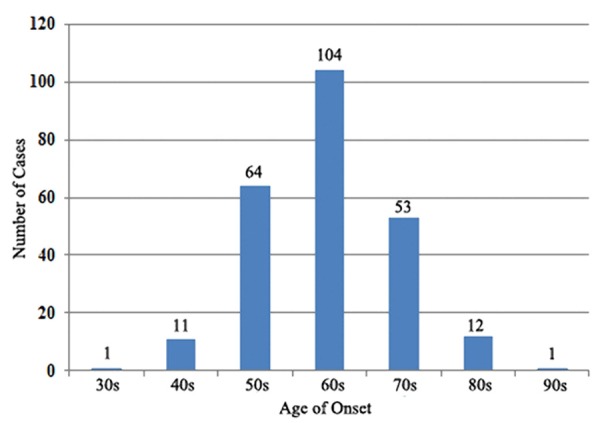
Distribution of age of onset in EMPD patients.
Associated diseases
Some EMPD patients had a basic disease, such as hypertension (31 cases, 12.6%), diabetes mellitus (16 cases, 6.5%), coronary heart disease (8 cases, 3.3%), or benign prostatic hyperplasia (21 cases, 8.5%). Twenty patients (8.1%) possessed a medical history of internal malignancies when diagnosed with EMPD.
The associated internal malignancies included prostate cancer (2 cases), liver cancer (2 cases), colon cancer (2 cases), stomach cancer (2 cases), thyroid cancer (1 case), lung cancer (1 case), basal cell carcinoma (1 case), testicular cancer (1 case), and parotid gland cancer (1 case). These cases were considered as secondary EMPD.
The uncertain associated internal malignancies included liver cancer (2 cases), prostate cancer (1 case), colon cancer (1 case) and thymoma (1 case).
Additionally, with the use of B ultrasonic examination, nearly one-third of the patients were found to have cysts, including simple hepatic cysts (27 cases), simple renal cysts (21 cases), hepatic and renal cysts (16 cases) and testicular and epididymal cysts (9 cases).
Metastases
All patients were screened via imageological examination for metastases evaluation. Lymph node invasion was confirmed in 19 of the 246 patients (7.7%) by lymph node biopsy. A groin lymphadenectomy was performed 2 or 3 weeks following confirmation of invasion. In the population studied, EMPD spread exclusively to the inguinal lymph nodes. Interestingly, there was an imbalance in the metastasis pattern: of these 19 patients, 10 patients suffered from metastases to the right inguinal lymph nodes, 7 patients suffered from metastases to both sides, and only 2 patients suffered from metastases to the left lymph nodes.
Histopathological findings
Paget cells are large, round cells usually characterized by their abundant pale-staining cytoplasm and large nuclei with prominent nucleoli in HE staining. Paget cells were exclusively found in the epidermis in 183 EMPD specimens (74.4%) and in both the epidermis and dermis in 63 cases (25.6%). The ratio of in situ to invasive EMPD was therefore 2.9:1. Additionally, Paget cells could also be observed in the hair follicles (18 cases, 7.3%) and the sweat glands and ducts (19 cases, 7.7%).
Through immunohistochemical and PAS staining, EMPD specimens were positive for several stains, including EMA (100%), CEA (98.9%), CK7 (90.3%), PAS (83.3%), HER2/neu (65.1%), Ki67>30% (56.8%), P53 (54.5%), CK20 (29.6%) and S100 (10.5%), while VIM, LCA and HMB45 staining were negative in all these EMPD cases (Figure 2). The expression of HER2/neu, Ki67>30%, P53, CK20 and S100 was analyzed in both invasive EMPD and non-invasive EMPD (Table 1). There is a significant correlation between HER2/neu expression and invasive EMPD.
Figure 2.
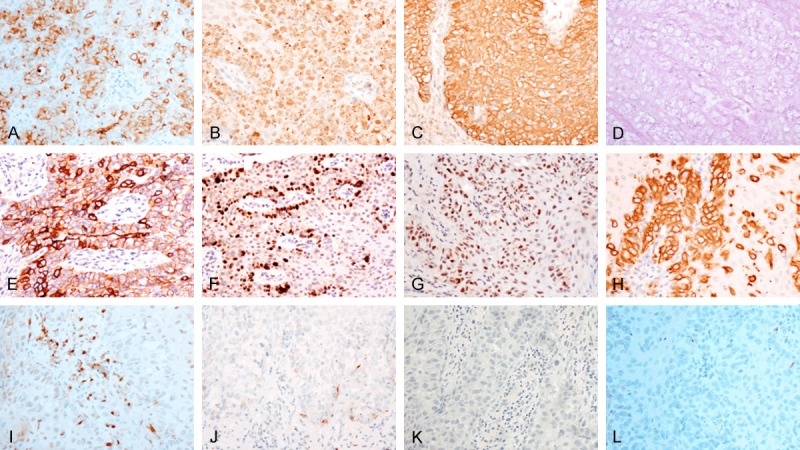
Immunohistochemical staining of proteins expression in EMPD (A. Positive expression of EMA in EMPD section 200×; B. Positive expression of CEA in EMPD section 200×; C. Positive expression of CK7 in EMPD section 200×; D. Positive expression of PAS in EMPD section 200×; E. Positive expression of HER2/neu in EMPD section 200×; F. Positive expression of Ki67 in EMPD section 200×; G. Positive expression of P53 in EMPD section 200×; H. Positive expression of CK20 in EMPD section 200×; I. Partially positive expression of S100 in EMPD section 200×; J. Negative expression of LCA in EMPD section 200×; K. Negative expression of VIM in EMPD section 200×; L. Negative expression of HMB45 in EMPD section 200×).
Table 1.
Expression of HER2/neu, Ki67, P53, CK20 and S100 in EMPD
| Protein Marker | EMPD Invasion | P value | |
|---|---|---|---|
|
| |||
| Invasive | Non-invasive | ||
| HER2/neu | |||
| Expression | 11 | 34 | 0.034 |
| None | 2 | 32 | |
| Ki67>30% | |||
| Expression | 26 | 41 | 0.696 |
| None | 18 | 33 | |
| P53 | |||
| Expression | 25 | 54 | 0.436 |
| None | 17 | 49 | |
| S100 | |||
| Expression | 2 | 9 | 0.195 |
| None | 39 | 55 | |
| CK20 | |||
| Expression | 3 | 13 | 0.338 |
| None | 13 | 25 | |
Laboratory tests
Seventy EMPD patients received laboratory tests to evaluate their serum PSA levels. A total of 11 patients (16%) showed PSA serum levels above 4.0 ng/ml. The average age of onset was 72.5 years, which is significantly higher than others. Among these patients, three had benign prostatic hyperplasia, five had liver cysts, and one had a history of testicular cancer.
Comparison between invasive and non-invasive EMPD
The pivotal pathological characteristic of invasive EMPD lies in that Paget cells break the epidermal basement membrane into dermis. According the histopathological findings, these 246 EMPD cases were divided into 2 groups: 183 patients with non-invasive EMPD and 63 patients with non-invasive EMPD. Comparing the clinical data from two groups indicated that the patients with invasive EMPD were significantly associated advanced age of onset (P=0.043). In contrast, other information such as delay in diagnosis, lesions length, tumor history and lymph node metastasis were not statistically different in these two groups (Table 2).
Table 2.
Clinical data between invasive and non-invasive EMPD
| Clinical Data | EMPD invasion | P value | |
|---|---|---|---|
|
| |||
| Invasive | Non-invasive | ||
| Case, n | 63 | 183 | |
| Age of onset, years | |||
| ≥60 | 48 | 121 | 0.043 |
| <60 | 15 | 63 | |
| Delay in diagnosis, years | 3.5 | 3.5 | |
| Longest diameter of the skin lesion, cm | 5.4 | 5.4 | |
| Lesions sites | |||
| Scrotum | 55 | 156 | 0.417 |
| Others | 8 | 27 | |
| Tumor History | 4 | 16 | 0.789 |
| Metastases | 3 | 16 | 0.687 |
Comparison between metastatic and non-metastatic EMPD
In this study, a total of 19 cases with EMPD were identified with the inguinal lymph node metastases via lymph node biopsy. So it can be divided into two groups: 19 cases with lymph node metastatic EMPD and 227 cases with non-metastatic EMPD. Clinical data analysis showed (Table 3) that the lymph node metastatic EMPD had a significantly shorter in the delay in diagnosis (P=0.038), and the length of lesions was also much greater in this group than those with invasive EMPD (P=0.040). There was no statistically significant difference in age of onset, location of skin lesions and tumor history.
Table 3.
Clinical data between metastatic and non-metastatic EMPD
| Clinical Data | EMPD metastasis | P value | |
|---|---|---|---|
|
| |||
| Metastatic | Non-metastatic | ||
| Case, n | 19 | 227 | |
| Age of onset, years | |||
| ≥60 | 15 | 155 | 0.442 |
| <60 | 4 | 72 | |
| Delay in diagnosis (average), years | 1.9 | 3.5 | 0.038 |
| Longest diameter of the skin lesion, cm | 6.9 | 5.2 | 0.042 |
| Lesions sites | |||
| Scrotum | 14 | 197 | 0.116 |
| Others | 5 | 30 | |
| Tumor History | 1 | 19 | 1.000 |
| Invasive | 4 | 59 | 0.788 |
Discussion
EMPD is a relatively rare cutaneous malignancy [10]. Our knowledge of the clinical and histopathological characteristics of EMPD remains very limited. In this study, the medical archives of 246 Chinese male EMPD patients diagnosed over a 20-year period were retrieved for clinical analysis.
In our population, the most frequently initial site of EMPD was the scrotum (85.8% of patients). The mean age at the time of diagnosis was 67.5 years, and the average age of symptom onset was 63.9 years, there is a peak age of onset in the 60s in EMPD patients. A significant delay in diagnosis could be observed for almost all of the EMPD patients in our study, on average, diagnosis occurred 43.2 months after the onset of symptoms. Such a long delay may be attributed to the relatively ordinary initial symptoms of pruritus, rash, and erythema. These nonspecific clinical manifestations can be misdiagnosed as skin eczema or mycosis. Additionally, the physicians often lack of enough awareness of Paget’s disease due to its rarity, and physical examination of the genital areas is frequently not as thorough as other areas. Accordingly, EMPD should always be in the differential diagnostic consideration of patients with genital pruritus and rash, and a biopsy of skin lesions should be performed if the patient’s symptoms cannot be improved.
The inguinal lymph nodes were the most common localization of metastatic EMPD. Patients with metastases showed a relatively dismal outcome compared with non-metastatic EMPD. In the current study, we found 19 patients suffering from metastases, and this rate (7.7%) is lower compared to two other studies (20%, 23%) [11,12]. Although, such a low rate in our population limits this study’s ability to explore the characteristics of metastatic EMPD, our data showed that EMPD patients with metastases often had a larger lesion size than those with non-metastases. It means that metastatic EMPD has greater malignant degree and rate of growth in contrast to other types. In addition, the right inguinal lymph nodes were prone to invasion by Paget cells compared the left inguinal lymph nodes, but further studies with a larger population of metastatic EMPD patients are necessary to confirm the imbalance of EMPD metastases.
Consistent with previous reports [2,13,14], we found that 20 patients with EMPD have personal history of other neoplasms. According to the interval between diagnosis of EMPD and internal malignancy, 13 cases were considered as secondary EMPD, but the rest of 7 cases remained still hard to be determined. Among these 20 cases, 6 cases (including 3 secondary EMPD cases and 3 uncertain cases) were received CK20 immunohistochemical analysis, which is widely considered as a useful biomarker for distinguishing primary and secondary EMPD. CK20 expression could be detected in 2 secondary EMPD cases but none in the uncertain cases. Until now, accumulating evidence has indicated that EMPD often accompanies internal malignancies; however, the true relationship between EMPD and underlying cancers is still difficult to confirm especially in those cases with the relatively long interval between diagnosis of EMPD and internal malignancy. CK20 might be used to assist in distinguishing primary and secondary EMPD.
Interestingly, we observed a high frequency of hepatic, renal, testicular and epididymal cysts in EMPD patients. The overall prevalence of simple hepatic cysts and simple renal cysts were 3.6% and 10.7% [15,16], respectively, in Chinese general population, while the incidence of testicular and epididymal cysts was unclear. Although most of these cysts were asymptomatic and were not harmful to organ function, some pathological changes could also be observed, including abnormal serum creatinine and proteinuria in renal cysts and gastrointestinal discomfort in hepatic cysts [17,18]. However, the pathogenesis of these cysts in EMPD patients is hard to explain.
Histopathological examination is an important tool in the diagnosis of EMPD [19]. In this study, we found that eight immunohistochemical markers and PAS stain showed different expression pattern in EMPD cases. Consistent with other reports, EMA, CEA, CK7 and PAS showed high expression levels in nearly all of the EMPD specimens, so these protein markers can be used to aid in the diagnosis of EMPD. In contrast, LCA, VIM and HMB45 showed negative expression in all EMPD cases. Thus, LCA, VIM and HMB45 might be used in the differential diagnosis of EMPD. HER2/neu, Ki67, P53, CK20 and S100 exhibited various expression levels; analysis of these four markers and EMPD pathological features revealed a significant correlation between HER2/neu expression and invasive EMPD. Combined our and others’ work [20], HER2/neu usually shows expression in a portion of EMPD patients and the expression of this protein is significantly related to invasive EMPD. On the other hand, HER2/neu is also an important biomarker for targeted therapy. This indicates targeted inhibition of HER2/neu may be used for treatment of some types of intractable EMPD with HER2/neu expression.
Surprisingly, we found 5 out of 45 patients, who were received S100 immunohistochemical detection, showed expression of S100 in their Paget cells. Within cells, S100 is involved in aspects of regulation of proliferation, differentiation, apoptosis, Ca2+ homeostasis, energy metabolism, inflammation and migration/invasion through interactions with a variety of target proteins. S100 is present in almost all human benign and malignant melanocytic tumors of the skin and in metastases of human malignant melanomas. In addition, it could also be detected in human tumors of the central and peripheral nervous system or those originating from different salivary glands. A previous study has maintained S100 could aid the separation of pagetoid melanoma from EMPD. In our study, these 5 patients with S100 expression, all of them showed negative HMB45 expression, which could rule out melanoma, but there were 3 of these 5 patients also showing negative CK7 expression. Thus, a novel histological type of EMPD with CK7-/S100+ is identified in our study, which means not only the expression of S100 could not be used to exclude EMPD but also the tumorigenesis of Paget cell is diverse. Several novel histological types might be existence beyond primary and secondary EMPD.
In our study, 70 patients with EMPD received serum PSA tests. A previous study showed elevated serum PSA levels in EMPD patients with associated prostate cancers [21]. As noted, total serum PSA is still the best marker for the detection of prostate cancer. Paget cells from EMPD cases also could be observed PSA expression [22,23]; therefore, we evaluated the potential implication of using serum PSA levels to diagnose EMPD. However, only 16% of EMPD patients presented serum PSA levels exceeding the threshold PSA value (4 ng/ml). Furthermore, there was no significant correlation between elevated serum PSA and invasive EMPD or tumor history, suggesting that the clinical usefulness of serum PSA for EMPD patients may be limited.
Through pathological examination, a total of 63 cases with invasive EMPD were identified. We compared the clinical data from non-invasive and invasive EMPD. Surprisingly, our data showed that invasive EMPD occurred preferentially in those subjects with a higher age of onset. In our previous study, we found a prominent correlation between hypermethylation of DLC1 and the older EMPD cases [24]. Given the known association between aging and EMPD, we speculate that accumulated genetic or epigenetic abnormities might promote the development of this skin cancer. In conclusion, Chinese male EMPD cases in our studied population demonstrate distinct clinical and pathological features, and these findings may have important implications for the management of Chinese EMPD patients.
This study has some notable limitations. Even with this large series, there was insufficient information on familial history for most of EMPD patients and the retrospective data from a single center are short of adequate material of female patients with EMPD. In addition, for the lack of follow up data of our studied population, we were unable to estimate the prognosis after their diagnosis of EMPD.
In conclusion, Chinese male EMPD cases in our studied population demonstrate distinct clinical and pathological features, and these findings may have important implications for the management of Chinese EMPD patient.
Acknowledgements
This study was supported by foundation from Shanghai Municipal Health Bureau hundred talents program (XBR2011044), Fudan University (MDJH2012017), and Scientific Research project supported by Huashan Hospital, Fudan University (2014QD02) and the National Science Foundation of China (NSFC no. 81472616). We thank Nature Publishing Group Language Editing for assisting in the preparation of this manuscript.
Disclosure of conflict of interest
None.
References
- 1.Kyriazanos ID, Stamos NP, Miliadis L, Noussis G, Stoidis CN. Extra-mammary Paget’s disease of the perianal region: a review of the literature emphasizing the operative management technique. Surg Oncol. 2011;20:e61–e71. doi: 10.1016/j.suronc.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 2.Funaro D, Krasny M, Lam C, Desy D, Sauthier P, Bouffard D. Extramammary Paget disease: epidemiology and association to cancer in a Quebec-based population. J Low Genit Tract Dis. 2013;17:167–174. doi: 10.1097/LGT.0b013e31825f4b4f. [DOI] [PubMed] [Google Scholar]
- 3.Alo PL, Galati GM, Sebastiani V, Ricci F, Visca P, Mariani L, Romagnoli F, Lombardi G, Tondo UD. Fatty acid synthase expression in Paget’s disease of the vulva. Int J Gynecol Pathol. 2005;24:404–408. doi: 10.1097/01.pgp.0000170065.53813.81. [DOI] [PubMed] [Google Scholar]
- 4.Shaco-Levy R, Bean SM, Vollmer RT, Jewell E, Jones EL, Valdes CL, Bentley RC, Selim MA, Robboy SJ. Paget disease of the vulva: a study of 56 cases. Eur J Obstet Gynecol Reprod Biol. 2010;149:86–91. doi: 10.1016/j.ejogrb.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Sawada Y, Bito T, Kabashima R, Yoshiki R, Hino R, Nakamura M, Shiraishi M, Tokura Y. Ectopic extramammary Paget’s disease: case report and literature review. Acta Derm Venereol. 2010;90:502–505. doi: 10.2340/00015555-0892. [DOI] [PubMed] [Google Scholar]
- 6.Shepherd V, Davidson EJ, Davies-Humphreys J. Extramammary Paget’s disease. BJOG. 2005;112:273–279. doi: 10.1111/j.1471-0528.2004.00438.x. [DOI] [PubMed] [Google Scholar]
- 7.Wagner G, Sachse MM. Extramammary Paget disease - clinical appearance, pathogenesis, management. J Dtsch Dermatol Ges. 2011;9:448–454. doi: 10.1111/j.1610-0387.2010.07581.x. [DOI] [PubMed] [Google Scholar]
- 8.Zhang N, Gong K, Zhang X, Yang Y, Na Y. Extramammary Paget’s disease of scrotum--report of 25 cases and literature review. Urol Oncol. 2010;28:28–33. doi: 10.1016/j.urolonc.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Lee SJ, Choe YS, Jung HD, Ahn SK, Cha YC, Cho KH, Choi HY, Chung KY, Huh CH, Kim IH, Kim KH, Kim MB, Kim MH, Kim YC, Lee JB, Lee MW, Lee MG, Lee WJ, Shin DH, Shin JH, Suh KS, Won YH. A multicenter study on extramammary Paget’s disease in Korea. Int J Dermatol. 2011;50:508–515. doi: 10.1111/j.1365-4632.2010.04661.x. [DOI] [PubMed] [Google Scholar]
- 10.Mengjun B, Zheng-Qiang W, Tasleem MM. Extramammary Paget’s disease of the perianal region: a review of the literature emphasizing management. Dermatol Surg. 2013;39:69–75. doi: 10.1111/dsu.12019. [DOI] [PubMed] [Google Scholar]
- 11.Hatta N, Yamada M, Hirano T, Fujimoto A, Morita R. Extramammary Paget’s disease: treatment, prognostic factors and outcome in 76 patients. Br J Dermatol. 2008;158:313–318. doi: 10.1111/j.1365-2133.2007.08314.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Y, Ye DW, Yao XD, Zhang SL, Dai B, Zhang HL, Shen YJ, Mao HR. Clinicopathological characteristics, management and outcome of metastatic penoscrotal extramammary Paget’s disease. Br J Dermatol. 2009;161:577–582. doi: 10.1111/j.1365-2133.2009.09203.x. [DOI] [PubMed] [Google Scholar]
- 13.Zollo JD, Zeitouni NC. The Roswell Park Cancer Institute experience with extramammary Paget’s disease. Br J Dermatol. 2000;142:59–65. doi: 10.1046/j.1365-2133.2000.03242.x. [DOI] [PubMed] [Google Scholar]
- 14.Karam A, Dorigo O. Increased risk and pattern of secondary malignancies in patients with invasive extramammary Paget disease. Br J Dermatol. 2014;170:661–671. doi: 10.1111/bjd.12635. [DOI] [PubMed] [Google Scholar]
- 15.Chang CC, Kuo JY, Chan WL, Chen KK, Chang LS. Prevalence and clinical characteristics of simple renal cyst. J Chin Med Assoc. 2007;70:486–491. doi: 10.1016/S1726-4901(08)70046-7. [DOI] [PubMed] [Google Scholar]
- 16.Huang JF, Chen SC, Lu SN, Lin ZY, Chuang WL, Hsieh MY, Wang LY, Tasi JF, Chang WY, Chen CJ. Prevalence and size of simple hepatic cysts in Taiwan: community- and hospital-based sonographic surveys. Gaoxiong Yi Xue Ke Xue Za Zhi. 1995;11:564–567. [PubMed] [Google Scholar]
- 17.Macedo FI. Current management of noninfectious hepatic cystic lesions: A review of the literature. World J Hepatol. 2013;5:462–469. doi: 10.4254/wjh.v5.i9.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terada N, Arai Y, Kinukawa N, Terai A. The 10-year natural history of simple renal cysts. Urology. 2008;71:7–11. doi: 10.1016/j.urology.2007.07.075. discussion 11-2. [DOI] [PubMed] [Google Scholar]
- 19.Lam C, Funaro D. Extramammary Paget’s disease: Summary of current knowledge. Dermatol Clin. 2010;28:807–826. doi: 10.1016/j.det.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 20.Richter CE, Hui P, Buza N, Silasi DA, Azodi M, Santin AD, Schwartz PE, Rutherford TJ. HER-2/NEU overexpression in vulvar Paget disease: the Yale experience. J Clin Pathol. 2010;63:544–547. doi: 10.1136/jcp.2010.077446. [DOI] [PubMed] [Google Scholar]
- 21.Shi G, Ye DW, Yao X, Zhang S, Dai B, Zhang H, Shen Y, Zhu Y, Zhu Y, Xiao W, Ma C. Extramammary Paget’s diseases in men from the Shanghai area: its association with PSA level increase. APMIS. 2010;118:777–781. doi: 10.1111/j.1600-0463.2010.02657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammer A, Hager Hand Steiniche T. Prostate-specific antigen-positive extramammary Paget’s disease--association with prostate cancer. APMIS. 2008;116:81–88. doi: 10.1111/j.1600-0463.2008.00816.x. [DOI] [PubMed] [Google Scholar]
- 23.Inoguchi N, Matsumura Y, Kanazawa N, Morita K, Tachibana T, Sakurai T, Utani A, Miyachi Y. Expression of prostate-specific antigen and androgen receptor in extramammary Paget’s disease and carcinoma. Clin Exp Dermatol. 2007;32:91–94. doi: 10.1111/j.1365-2230.2006.02304.x. [DOI] [PubMed] [Google Scholar]
- 24.Kang Z, Xu F, Zhang QA, Lin J, Wu Z, Zhang X, Luo Y, Xu J, Guan M. Correlation of DLC1 gene methylation with oncogenic PIK3CA mutations in extramammary Paget’s disease. Mod Pathol. 2012;25:1160–1168. doi: 10.1038/modpathol.2012.65. [DOI] [PubMed] [Google Scholar]


