Abstract
Background: Hodgkin Reed-Sternberg (HRS) cells may promote differentiation of CD4+ naïve T cells toward both FoxP3+ T regulatory (Treg) cells and TIA-1+ cytotoxic T lymphocytes (CTL). Previous studies suggest that an overabundance of cytotoxic TIA-1+ cells in relation to FoxP3+ T reg cells portends unfavorable outcomes in classical Hodgkin lymphoma (cHL), raising the possibility that its pathogenesis may be related to immune dysregulation. Sirt1 deacetylates FoxP3 and leads to decreased Treg functionality. Our objective was to compare Sirt1 and FoxP3 expressions in Hodgkin lymphoma infiltrating lymphocytes (HLIL) and confirm Sirt1 expression in HRS cells. Design: Immunohistochemical staining of paraffin-embedded tissue with antibodies to Sirt1, FoxP3, TIA-1, and CD8 was performed. Expression of Sirt1was assessed in both the HRS cells and in the HLILs in twenty-four cases. Adequate tissue was available in 13 cHL cases to permit the enumeration of FoxP3, TIA-1 and CD8 by giving their percent staining of HLILs. Results: In HLILs, nuclear expression of Sirt1 was 32-88% (mean 67%); FoxP3 expression was 9-40% (mean 23.9%); TIA-1 expression was 15-87% (mean 32%); and CD8 expression was 10-45% (mean = 31%). Sirt1 to FoxP3 ratio was 0.96-5.5 (mean 3.2). TIA-1 to FoxP3 ratio was 0.6-5.1 (mean 1.6). CD8 to FoxP3 ratio was 0.43-3.7 (mean 1.5). There was a difference of Sirt1 to FoxP3 ratios between remission and recurrence groups, being significantly higher in the recurrence group (P = 0.005). Sirt1 demonstrated high nuclear expression in the HRS cells of 21 out of 24 (88%) cases analyzed. Conclusion: The relative overexpression of Sirt1 to FoxP3 in HLILs may be considered possible targets for immune modulation. Histone deacetylase inhibitors may increase the efficacy of existing treatment regimens by downregulating SIRT1 gene mRNA/Sirt1 protein function and together with rapamycin could expand the T regulatory/FoxP3 population and functionality and improve prognosis for remission in cHL. Targeting Sirt1 in the HRS cells may facilitate their ability to promote naïve T cell differentiation toward Treg cells over CTL.
Keywords: Sirt1, FoxP3, Hodgkin lymphoma, immune dysregulation, morphoproteomics
Introduction
Classical Hodgkin lymphoma (cHL) is composed of various non-neoplastic, reactive cells with only a minority of malignant cells (Reed-Sternberg cells and Hodgkin cells) [1,2]. Existing evidence in most cases supports the notion that these malignant cells in cHL originate from germinal center B cells [1-6]. The etiopathogenesis of cHL is still a subject of considerable investigation and debate. A role for an autoimmune component in the pathogenesis of cHL has been raised and supported by the following associations: 1. a personal or family history of certain autoimmune conditions was strongly associated with the increased risk of Hodgkin lymphoma in a Scandinavian study [7]; 2. The risk of Hodgkin lymphoma developing in systemic lupus erythematosus, an autoimmune disease, is increased [8]; 3. A link was noted between dys-immunity with autoimmune diseases preceding or following the diagnosis of Hodgkin lymphoma in 11 children, in one study [9]; and 4. An associated autoimmune disease was reported in 14 of 121 (11.5%) Hodgkin lymphoma patients in a report of a single center experience [10].
In support of immune dysregulation and/or autoimmunity in the pathogenesis of Hodgkin lymphoma, it is noteworthy that the presence of cytotoxic TIA-1+ cells was found to be an unfavorable prognostic factor of event-free survival and disease-free survival in Hodgkin lymphoma [11]. Also, activated cytotoxic T cells expressing granzyme B and associated with CD8+ cells had a shortened progression-free survival time in patients with Hodgkin’s lymphoma [12]. Additionally, a ratio of FoxP3 positive T regulatory cells to activated cytotoxic T lymphocytes (identified by positive granzyme B stain) of 1 or less may predict a poor failure-free survival [13]. Alvaro and co-workers reported that a low infiltration of FoxP3+ cells in conjunction with a high infiltration of TIA-1+ cells may represent biological markers predicting an unfavorable outcome [14]. Conversely, the failure-free survival in cHL statistically increased with a higher number of FoxP3+ tumor infiltrating lymphocytes [14] and in another study, an increased number of tumor-infiltrating FoxP3+ cells over the receiver operating characteristic-determined cut-offs positively influenced overall and failure-free survival in cHL [15].
If one accepts, for the sake of argument, this premise of an autoimmune component or immune dysregulation in the pathogenesis of Hodgkin disease, then expanding the T regulatory cells and their functionality would be critical in effecting a positive outcome for patients with this disease. Furthermore, identifying pathways and proteins that reduce the functionality of the T regulatory cells would be important in assessing the resistance signature to conventional therapies, in correlating the clinical response to agents that reduce such inhibitory pathways and in designing future therapies intended to target proliferation while restoring proper immune regulation. Conceivably, an increase in the numbers of functional FoxP3 T regulatory cells could be accomplished by inhibition of Sirt1 (silent mating type information regulation 2 homolog 1), a member of the histone deacetylase Sirtuin family which is known to downregulate FoxP3 [16-20]. To expand on this, FoxP3 expression and stabilization via acetylation is necessary for regulatory T cell differentiation and functionality and Sirt1, as an NAD+ histone deacetylase can lead to destabilization. Inhibition of Sirt1 strengthens the suppressive action of Tregs [16-20]. Panobinostat is a pan-deacetylase inhibitor which was shown by Lemoine et al. to have activity against Hodgkin lymphoma derived cell lines, inducing cell death as well as inhibiting the Sirt1 pathway [21]. Similarly, the combinatorial use of niacinamide and vorinostat, as sirtuin and pan-class I/II deacetylase (DAC) inhibitors, has been shown to result in a response rate of 24% in relapsed or refractory lymphoma patients with an additional 57% reportedly achieving stable disease [22].
Morphoproteomics is a technique which uses bright-field microscopy and immunohistochemistry to study and understand the molecular circuitry of tumors and their microenvironment by observing the expression and when applicable, activation of various protein analytes and their correlative expressions in order to define the biology of pathologic processes [23,24]. Our study was designed to attempt to gain further understanding of the pathologic mechanisms which may be driving classical Hodgkin lymphoma. We sought to confirm that the Hodgkin Reed-Sternberg cells express Sirt1 [25]. In addition, we proposed to analyze the ratios of Sirt1/FoxP3, CD8/FoxP3 and TIA-1/FoxP3 in the Hodgkin lymphoma infiltrating lymphocytes (HLIL) to characterize the T lymphocyte milieu, which may be contributing to tumorigenesis and prognosis. A better understanding of the tumoral microenviroment, particularly immunologically active T lymphocytes may allow favorable interventions via promotion of growth or biological activity of subsets of T cells that leads to immune balance and that may be essential to the successful remission of Hodgkin disease.
Materials and methods
Approval by the Institutional Review Board (IRB) was obtained for this study. Twenty-four cases of cHL were retrospectively examined between 2008 and 2011. All patients were treated with standard frontline chemotherapy as per national guidelines and clinically assessed for response by standard imaging and laboratory evaluations at regular intervals. Clinical follow-up data were available for 10 cases. Seven patients achieved remission and three experienced a recurrence of disease. These patients were divided into remission (n = 7) and recurrence groups (n = 3).
Immnuohistochemical analysis was performed on paraffin-embedded tissue for all twenty-four cases to detect the expression of Sirt1 within the malignant Hodgkin Reed-Sternberg (HRS) cells. Protein expression was quantified with bright-field microscopy by assessing the percentage of HRS cells with nuclear staining. Percent expression was scored using bright-field microscopy with high expression designated as more than 50% of the cells with positive stain in the appropriate subcellular compartment. Positive and negative controls were run concomitantly and noted to react appropriately. Tissue was available in thirteen cases to allow for staining with antibodies against FoxP3, T-cell intracellular antigen 1 (TIA-1), and CD8. The expression of these antigens within the HLILs was evaluated in a similar manner using bright-field microscopy. The details of the antibodies used are contained in Table 1; and the staining procedure has been previously described [23,24,26]. Positive immunohistochemical staining was determined in multiple high power fields for each antibody and then compared to other cell types to determine the ratios of interest. For each case, the ratios of both CD8:FoxP3 and TIA-1:FoxP3 and of Sirt1:FoxP3 were calculated as a surrogate for the ratio of cytotoxic T cells:T regulatory tumor infiltrating lymphocytes in the case of the former and in the case of Sirt1:FoxP3, the potential for reduction in functionality of T regulatory tumor infiltrating lymphocytes. In addition, the percent of HRS cells expressing Sirt1 was compared to the percent of HLILs expressing Sirt1. The percent of HLILs expressing Sirt1 was estimated by two of the authors (AQ, BA). The values obtained were averaged and compared to those cases of Hodgkin lymphoma which highly expressed Sirt1 (n = 21).
Table 1.
Immunohistochemical protein markers and antibody specifics
| Protein Analyte | Antibody Specifics | Represents |
|---|---|---|
| CD8 | Dako monoclonal mouse IgG1 anti-human CD8 clone C8/144B | Cytotoxic T cells |
| TIA-1 | Abcam monoclonal mouse IgG1 anti-TIA1 | Cytotoxic T cell marker |
| FoxP3 | Abcam monoclonal mouse IgG1 [236A/E7] | T regulatory cells |
| Sirt1 | Abcam Sirt1 monoclonal rabbit antibody [E104] ab32441 | NAD+ histone deacetylase |
Statistical analysis was also performed using the Student’s t-test to compare the percent expression of Sirt1 in HRS versus HLILs. In addition, linear regression analysis of these two groups was conducted to obtain a correlation coefficient. Finally, statistical analysis to compare the mean ratios from the remission and recurrence groups was also accomplished using the Student’s t-test.
Results
Sirt1, TIA-1 and CD8 expressions and correlation with FoxP3
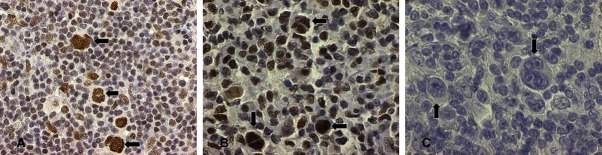
Sirt1 demonstrated high nuclear expression in 21 out of 24 (88%) classical Hodgkin lymphoma cases analyzed (Figure 1). Three cases did not show high expression in the HRS cells. In addition, Sirt1 was also variably positive in the background tumor infiltrating lymphocytes with 16 of the 24 cases (67%) showing nuclear expression of Sirt1 in 50% or more cells (Figure 1).
Figure 1.
Sirt1 highly expressed in the nuclei of Hodgkin Reed-Sternberg cells by immunohistochemistry (A and B, arrows HR-S) and variably in the tumor infiltrating lymphocytes (B) from two cases of cHL in our study, contrast with negative control in (C) (original magnification, ×400 A ×600 B and C).
The ratio between Sirt1 and FoxP3 positive lymphocytes was examined in thirteen cases. The nuclear expression of Sirt1 in HLILs was 32-88% (mean 67%), while FoxP3 expression was 9-40% (mean 23.9%) (Figure 2). The Sirt1 to Foxp3 ratio ranged from 0.96-5.5 (mean = 3.2). TIA-1 expression was 15-87% (mean = 32%). The TIA-1 to FoxP3 ratio ranged from 0.6-5.1 (mean = 1.6). The expression of CD8 was 10-50% (mean = 31%). The CD8 to FoxP3 ratio ranged from 0.43-3.7 (mean = 1.5) (Table 2 and Figure 2).
Figure 2.
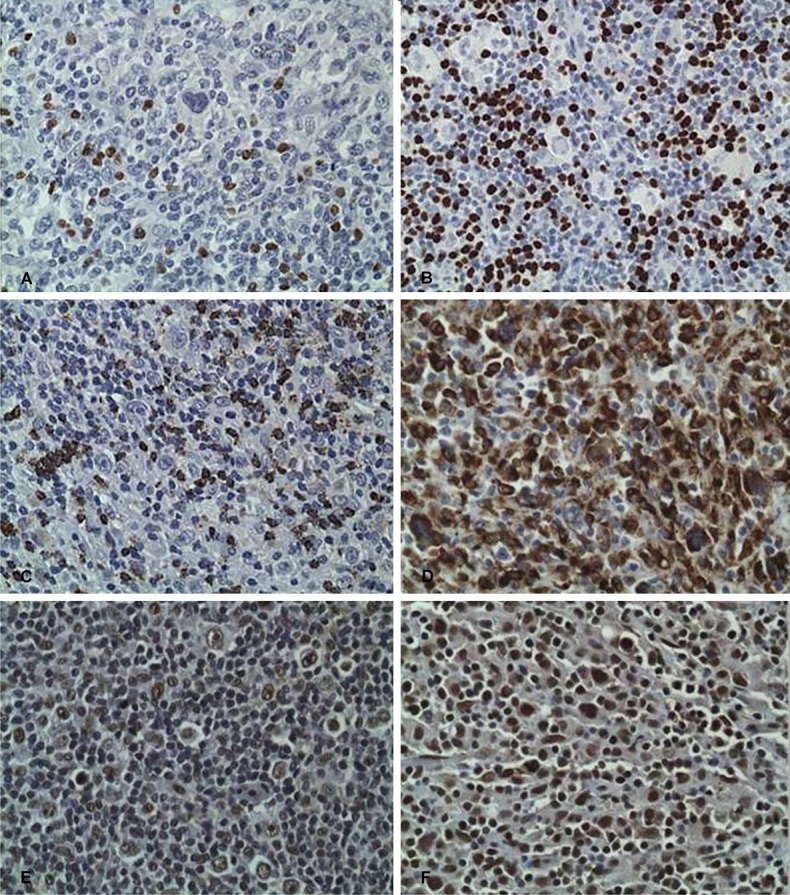
HLIL expression of (A) FoxP3, labeled as 9% staining in case 7, (B) FoxP3, labeled as 40% in case 11, (C) TIA-1, 15% in Case 4, (D) TIA-1, 87% Case 10, (E) Sirt1, 50% in Case 6, (F) Sirt1, 88% Sirt1 in Case 10. Images for CD8 not depicted (original magnification, ×400).
Table 2.
Thirteen cases of classical Hodgkin lymphoma with the percentage expression of FoxP3, TIA-1, CD8 and Sirt1 in HLILs and the ratios of CD8, TIA-1 and Sirt1 to FoxP3
| Case | FoxP3 | TIA | CD8 | Sirt 1 in HLIL | CD8/FoxP3 | TIA-1/FoxP3 | Sirt1/FoxP3 |
|---|---|---|---|---|---|---|---|
| Case 1 | 30 | 20 | 33 | 57 | 1.1 | 0.66 | 1.9 |
| Case 2 | 23 | 45 | 10 | 82 | 0.43 | 1.95 | 3.56 |
| Case 3 | 33 | 40 | 25 | 32 | 0.75 | 1.21 | 0.96 |
| Case 4 | 25 | 15 | 40 | 72 | 1.6 | 0.6 | 2.88 |
| Case 5 | 27 | 30 | 50 | 70 | 1.85 | 1.11 | 2.59 |
| Case 6 | 30 | 18 | 18 | 50 | 0.6 | 0.6 | 1.66 |
| Case 7 | 9 | 23 | 33 | 50 | 3.66 | 2.55 | 5.55 |
| Case 8 | 15 | 35 | 40 | 65 | 2.66 | 2.33 | 4.33 |
| Case 9 | 18 | 33 | 45 | 65 | 2.5 | 1.83 | 3.61 |
| Case 10 | 17 | 87 | 27 | 88 | 1.58 | 5.11 | 5.17 |
| Case 11 | 40 | 25 | 20 | 82 | 0.5 | 0.62 | 2.05 |
| Case12 | 27 | 33 | 40 | 77 | 1.48 | 1.22 | 2.85 |
| Case 13 | 17 | 15 | 25 | 82 | 1.48 | 0.88 | 4.82 |
| AVERAGE | 23.92 | 32.23 | 31.23 | 67.08 | 1.55 | 1.59 | 3.23 |
Sirt1 expression in Hodgkin Reed-Sternberg cells versus Hodgkin lymphoma infiltrating lymphocytes
There were 21 cases of Hodgkin lymphoma which showed increased expression of Sirt1 within the HRS cells. Statistical analysis showed a significant difference between the mean percent expression within the HRS cells when compared to the mean percent expression within the HLILs (78.3±6.70 versus 59.7±6.52; P = 0.03) (Table 3). The correlation coefficient obtained by linear regression was r = 0.39.
Table 3.
Comparison of the mean and standard error of the mean for percent expression of Sirt1 in Hodgkin Reed-Sternberg (HRS) cells versus Hodgkin lymphoma infiltrating lymphocytes (HLIL)
| Monoclonal Sirt1 in HRS Cells | Monoclonal Sirt1 in HLILs* | |
|---|---|---|
| N | 21 | 21 |
| Mean ± SEM | 78.3±6.70 | 59.7±6.52 |
| p value | 0.03 |
Average of percentage of nuclear expression of Sirt1 in HLIL from two independent observers (AQ and BA).
Sirt1to FoxP3 and cytotoxic T lymphocyte to FoxP3 ratios and clinical outcomes
In the ten cases with follow-up data, seven patients obtained remission whereas three patients had recurrence at the time of the analysis. Statistical analysis revealed a significant difference (P < 0.005) of Sirt1:FoxP3 ratio between the remission and recurrence groups. There was also a trend towards significance in the CD8:FoxP3 ratio (P = 0.052) (Table 4).
Table 4.
Statistical analysis of ratios comparing remission to recurrence groups
| Group | n | CD8/FoxP3 | TIA-1/FoxP3 | Sirt1/FoxP3 |
|---|---|---|---|---|
| Remission | 7 | 1.26±0.31 | 1.14±0.23 | 2.45±0.41 |
| Recurrence | 3 | 2.6±0.77 | 1.92±0.64 | 4.9±0.43 |
| p value | 0.052 | 0.129 | 0.005 |
Discussion
In this study, we documented the expression patterns of Sirt1 in HRS of cHL. We also sought to investigate the tumor milieu in the form of HLILs by determining the ratio of cytotoxic T lymphocytes to T regulatory lymphocytes. Any upregulated proteins in the tumor cells themselves may provide supplemental targets with therapeutic intent, and a better understanding of the environment in which the tumor is growing may provide greater insight into the pathophysiology of the disease. In addition, modifying this environment could suppress tumor growth.
Our finding of high nuclear expression of Sirt1 in HRS cells in 88% of the cases of cHL reaffirms the earlier report of Sirt1 expression in the Hodgkin Reed-Sternberg cells [25]. In this context, the observation that Reed-Sternberg cells, in an in vitro cell line study, have been shown to promote bidirectional differentiation of CD4+ naïve T cells toward both T regulatory and cytotoxic T cells [27] is relevant to the Sirt1 expression in HRS and in the HLIL. We found a statistical difference between the percent expression of Sirt1 in the HRS cells as compared to that in the HLILs. Interestingly, however, there was a mild positive correlation between the two. While the HRS appears to express Sirt1 to a significantly greater extent than the surrounding HLILs, the expression of each seems to increase together.
As previously stated, one of the deacetylation targets of Sirt1 is known to be FoxP3, a transcription factor pivotal in the differentiation and function of T regulatory lymphocytes. Sirt1 deacetylation results in destabilization and enhanced degradation of FoxP3 promoting T regulatory cell decline and/or underactivity and inhibition of Sirt1 increases the suppressive action of Tregulatory cells [16-20]. Thus, Sirt1 inhibition may also allow HRS cells to promote the differentiation of CD4+ naïve T cells toward Tregs.
We suggest the possibility that immune dysregulation may be playing a larger role in the pathogenesis of Hodgkin lymphoma than previously thought. The patients who suffered a recurrence in this study had a statistically significant increase in the ratio of Sirt1 to FoxP3 as compared to the patients who were in remission. The skewed ratio implies a decrease in T-regulatory cells with a resultant imbalance relative to cytotoxic T-cells. In the ten cases with follow-up data, statistical analysis revealed a significant difference (P < 0.005) of Sirt1 to FoxP3 ratio between the remission and recurrence groups. There was also a trend towards significance in the CD8 to FoxP3 ratio (P = 0.052). An analogy drawn from the commonality of germinal center B-cell origins of cHL and germinal center-like diffuse large B-cell and follicular lymphomas is the correlation of high numbers of intratumoral FoxP3+ regulatory T cells and improved survival in both [1-6,15].
The overexpression of Sirt1 in RS and the high mean ratios of Sirt1 and TIA-1 to FoxP3 in the HLILs may be amenable to modulation to influence clinical outcomes. Conceivably, histone deacetylase inhibitors (such as vorinostat [SAHA]) may increase the efficacy of existing treatment regimens by downregulating Sirt1 gene mRNA/Sirt1 protein function and reducing Sirt1 deacetylase activity [28-30]. A decrease in Sirt1-related deacetylation of FoxP3 could reduce the negative modulation of the T regulatory (FoxP3+) cell population. Should immune dysregulation and/or auto-immunity play a contributing role in the pathogenesis of Hodgkin lymphoma, then the inhibition of the Sirt1 pathway could provide additional benefit by upregulating the expression of FoxP3. In turn, this would block the inhibitory modulation of T regulatory cells and potentially restore immune balance in the tumoral microenvironment.
Proofs of concept of the possible role of inhibiting Sirt1 pathway and at the same time expanding the Tregulatory cells to restore immune balance in cHL is contained in the recent report of a case of multiply recurrent Hodgkin lymphoma that experienced complete clinical remission with vorinostat and sirolimus. Morphoproteomics and biomedical analytics, with a focus on the respective roles of vorinostat and sirolimus in targeting Sirt1 and expanding the T regulatory cells and downregulating CD8 cells provided correlates of the response signature in this case [31]. A follow-up study with 28 heavily pretreated refractory cHL patients treated with vorinostat and sirolimus showed a complete response in 32% and a partial response in 25% [32].
In summary, this study demonstrates that, in a limited number of patients with cHL, the remission and relapse status can be correlated with the ratio of Sirtl and FoxP3 expression and therefore, carries prognostic implications. Our findings also provide a basis to relate the apparent clinical significance of the relative overexpression of Sirtl vis-à-vis FoxP3 cells in the HLIL with dysregulation of the T cell ratios in the tumoral microenviroment and dysimmunity in cHL. Histone deacetylase inhibitors may increase the efficacy of existing treatment regimens by downregulating SIRT1 gene mRNA/ Sirt1 protein function and together with rapamycin could expand the T regulatory/FoxP3 population and functionality and improve prognosis for remission in cHL [16-20,28-31]. Targeting Sirt1 in the HRS cells also may facilitate their ability to promote naïve T cell differentiation toward Treg cells over CTL [27]. Further examination of this pathway and its relationship to T cell microenvironment and immune dysregulation in cHL is warranted, as it carries therapeutic implications for improved patient outcomes.
Acknowledgements
The authors thank Pamela K. Johnston, HT (ASCP) for technical assistance and Ms. Bheravi Patel for secretarial and graphic support.
Disclosure of conflict of interest
None.
References
- 1.Kuppers R. Molecular biology of Hodgkin’s lymphoma. Adv Cancer Res. 2002;84:277–312. doi: 10.1016/s0065-230x(02)84009-x. [DOI] [PubMed] [Google Scholar]
- 2.Kuppers R, Hansmann ML. The Hodgkin and Reed/Sternberg cell. Int J Biochem Cell Biol. 2005;37:511–517. doi: 10.1016/j.biocel.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 3.Hummel M, Ziemann K, Lammert H, Pileri S, Sabattini E, Stein H. Hodgkin’s disease with monoclonal and polyclonal populations of Reed-Sternberg cells. N Engl J Med. 1995;333:901–906. doi: 10.1056/NEJM199510053331403. [DOI] [PubMed] [Google Scholar]
- 4.Stein H, Hummel M. Cellular origin and clonality of classic Hodgkin’s lymphoma: immunophenotypic and molecular studies. Semin Hematol. 1999;36:233–241. [PubMed] [Google Scholar]
- 5.Kuppers R. Identifying the precursors of Hodgkin and Reed-Sternberg cells in Hodgkin’s disease: role of the germinal center in B-cell lymphomagenesis. J Acquir Immune Defic Syndr. 1999;21(Suppl 1):S74–79. [PubMed] [Google Scholar]
- 6.Marafioti T, Hummel M, Foss HD, Laumen H, Korbjuhn P, Anagnostopoulos I, Lammert H, Demel G, Theil J, Wirth T, Stein H. Hodgkin and reed-sternberg cells represent an expansion of a single clone originating from a germinal center B-cell with functional immunoglobulin gene rearrangements but defective immunoglobulin transcription. Blood. 2000;95:1443–1450. [PubMed] [Google Scholar]
- 7.Landgren O, Engels EA, Pfeiffer RM, Gridley G, Mellemkjaer L, Olsen JH, Kerstann KF, Wheeler W, Hemminki K, Linet MS, Goldin LR. Autoimmunity and susceptibility to Hodgkin lymphoma: a population-based case-control study in Scandinavia. J Natl Cancer Inst. 2006;98:1321–1330. doi: 10.1093/jnci/djj361. [DOI] [PubMed] [Google Scholar]
- 8.Bernatsky S, Ramsey-Goldman R, Isenberg D, Rahman A, Dooley MA, Sibley J, Boivin JF, Joseph L, Armitage J, Zoma A, Clarke A. Hodgkin’s lymphoma in systemic lupus erythematosus. Rheumatology (Oxford) 2007;46:830–832. doi: 10.1093/rheumatology/kel444. [DOI] [PubMed] [Google Scholar]
- 9.Jarrasse C, Pagnier A, Edan C, Landman-Parker J, Mazingue F, Mansuy L, Bertrand Y, Paillard C, Pellier I, Margueritte G, Plantaz D. [Hodgkin disease and autoimmunity in children: 11 case reports] . Arch Pediatr. 2011;18:376–382. doi: 10.1016/j.arcped.2011.01.025. [DOI] [PubMed] [Google Scholar]
- 10.Varoczy L, Payer E, Kadar Z, Gergely L, Miltényi Z, Magyari F, Szodoray P, Illés A. Malignant lymphomas and autoimmunity-a single center experience from Hungary. Clin Rheumatol. 2012;31:219–224. doi: 10.1007/s10067-011-1807-1. [DOI] [PubMed] [Google Scholar]
- 11.Alvaro-Naranjo T, Lejeune M, Salvado-Usach MT, Bosch-Príncep R, Reverter-Branchat G, Jaén-Martínez J, Pons-Ferré LE. Tumor-infiltrating cells as a prognostic factor in Hodgkin’s lymphoma: a quantitative tissue microarray study in a large retrospective cohort of 267 patients. Leuk Lymphoma. 2005;46:1581–1591. doi: 10.1080/10428190500220654. [DOI] [PubMed] [Google Scholar]
- 12.Oudejans JJ, Jiwa NM, Kummer JA, Ossenkoppele GJ, van Heerde P, Baars JW, Kluin PM, Kluin-Nelemans JC, van Diest PJ, Middeldorp JM, Meijer CJ. Activated cytotoxic T cells as prognostic marker in Hodgkin’s disease. Blood. 1997;89:1376–1382. [PubMed] [Google Scholar]
- 13.Kelley TW, Pohlman B, Elson P, Hsi ED. The ratio of FOXP3+ regulatory T cells to granzyme B+ cytotoxic T/NK cells predicts prognosis in classical Hodgkin lymphoma and is independent of bcl-2 and MAL expression. Am J Clin Pathol. 2007;128:958–965. doi: 10.1309/NB3947K383DJ0LQ2. [DOI] [PubMed] [Google Scholar]
- 14.Alvaro T, Lejeune M, Salvado MT, Bosch R, García JF, Jaén J, Banham AH, Roncador G, Montalbán C, Piris MA. Outcome in Hodgkin’s lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res. 2005;11:1467–1473. doi: 10.1158/1078-0432.CCR-04-1869. [DOI] [PubMed] [Google Scholar]
- 15.Tzankov A, Meier C, Hirschmann P, Went P, Pileri SA, Dirnhofer S. Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin’s lymphoma. Haematologica. 2008;93:193–200. doi: 10.3324/haematol.11702. [DOI] [PubMed] [Google Scholar]
- 16.Beier UH, Akimova T, Liu Y, Wang L, Hancock WW. Histone/protein deacetylases control Foxp3 expression and the heat shock response of T-regulatory cells. Curr Opin Immunol. 2011;23:670–678. doi: 10.1016/j.coi.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beier UH, Wang L, Bhatti TR, Liu Y, Han R, Ge G, Hancock WW. Sirtuin-1 targeting promotes Foxp3+ T-regulatory cell function and prolongs allograft survival. Mol Cell Biol. 2011;31:1022–1029. doi: 10.1128/MCB.01206-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beier UH, Wang L, Han R, Akimova T, Liu Y, Hancock WW. Histone deacetylases 6 and 9 and sirtuin-1 control Foxp3+ regulatory T cell function through shared and isoform-specific mechanisms. Sci Signal. 2012;5:ra45. doi: 10.1126/scisignal.2002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon HS, Lim HW, Wu J, Schnolzer M, Verdin E, Ott M. Three novel acetylation sites in the Foxp3 transcription factor regulate the suppressive activity of regulatory T cells. J Immunol. 2012;188:2712–2721. doi: 10.4049/jimmunol.1100903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Loosdregt J, Vercoulen Y, Guichelaar T, Gent YY, Beekman JM, van Beekum O, Brenkman AB, Hijnen DJ, Mutis T, Kalkhoven E, Prakken BJ, Coffer PJ. Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Blood. 2010;115:965–974. doi: 10.1182/blood-2009-02-207118. [DOI] [PubMed] [Google Scholar]
- 21.Lemoine M, Derenzini E, Buglio D, Medeiros LJ, Davis RE, Zhang J, Ji Y, Younes A. The pan-deacetylase inhibitor panobinostat induces cell death and synergizes with everolimus in Hodgkin lymphoma cell lines. Blood. 2012;119:4017–4025. doi: 10.1182/blood-2011-01-331421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amengual JE, Clark-Garvey S, Kalac M, Scotto L, Marchi E, Neylon E, Johannet P, Wei Y, Zain J, O’Connor OA. Sirtuin and pan-class I/II deacetylase (DAC) inhibition is synergistic in preclinical models and clinical studies of lymphoma. Blood. 2013;122:2104–2113. doi: 10.1182/blood-2013-02-485441. [DOI] [PubMed] [Google Scholar]
- 23.Brown RE. Morphoproteomics: exposing protein circuitries in tumors to identify potential therapeutic targets in cancer patients. Expert Rev Proteomics. 2005;2:337–348. doi: 10.1586/14789450.2.3.337. [DOI] [PubMed] [Google Scholar]
- 24.Brown RE. Morphogenomics and morphoproteomics: a role for anatomic pathology in personalized medicine. Arch Pathol Lab Med. 2009;133:568–579. doi: 10.5858/133.4.568. [DOI] [PubMed] [Google Scholar]
- 25.Frazzi R, Valli R, Tamagnini I, Casali B, Latruffe N, Merli F. Resveratrol-mediated apoptosis of hodgkin lymphoma cells involves SIRT1 inhibition and FOXO3a hyperacetylation. Int J Cancer. 2013;132:1013–1021. doi: 10.1002/ijc.27748. [DOI] [PubMed] [Google Scholar]
- 26.Quesada AE, Nguyen ND, Rios A, Brown RE. Morphoproteomics identifies constitutive activation of the mTORC2/Akt and NF-kappaB pathways and expressions of IGF-1R, Sirt1, COX-2, and FASN in peripheral T-cell lymphomas: pathogenetic implications and therapeutic options. Int J Clin Exp Pathol. 2014;7:8732–8739. [PMC free article] [PubMed] [Google Scholar]
- 27.Tanijiri T, Shimizu T, Uehira K, Yokoi T, Amuro H, Sugimoto H, Torii Y, Tajima K, Ito T, Amakawa R, Fukuhara S. Hodgkin’s reed-sternberg cell line (KM-H2) promotes a bidirectional differentiation of CD4+CD25+Foxp3+ T cells and CD4+ cytotoxic T lymphocytes from CD4+ naive T cells. J Leukoc Biol. 2007;82:576–584. doi: 10.1189/jlb.0906565. [DOI] [PubMed] [Google Scholar]
- 28.Eades G, Yao Y, Yang M, Zhang Y, Chumsri S, Zhou Q. miR-200a regulates SIRT1 expression and epithelial to mesenchymal transition (EMT)-like transformation in mammary epithelial cells. J Biol Chem. 2011;286:25992–26002. doi: 10.1074/jbc.M111.229401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu Z, Jiang W, McGinley JN, Thompson HJ. Defining the role of histone deacetylases in the inhibition of mammary carcinogenesis by dietary energy restriction (DER): effects of suberoylanilide hydroxamic acid (SAHA) and DER in a rat model. Cancer Prev Res (Phila) 2013;6:290–298. doi: 10.1158/1940-6207.CAPR-12-0449-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kyrylenko S, Kyrylenko O, Suuronen T, Salminen A. Differential regulation of the Sir2 histone deacetylase gene family by inhibitors of class I and II histone deacetylases. Cell Mol Life Sci. 2003;60:1990–1997. doi: 10.1007/s00018-003-3090-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Subbiah V, Brown RE, McGuire MF, Buryanek J, Janku F, Younes A, Hong D. A novel immunomodulatory molecularly targeted strategy for refractory Hodgkin’s lymphoma. Oncotarget. 2014;5:95–102. doi: 10.18632/oncotarget.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Janku F, Oki Y, Falchook GS, Subbiah V, Naing A, Bravo VMV, Hong DS, Westin JR, Nunez C, Fayad L, Neelapu SS, Kwak LW, Shpall EJ, Wheler JJ, Barnes T, Liang WS, Salhia B, Meric-Bernstam F, Kurzrock R, Fanale MA. Activity of the mTOR inhibitor sirolimus and HDAC inhibitor vorinostat in heavily pretreated refractory Hodgkin lymphoma patients. J Clin Oncol. 2014;32(Suppl):5s. abstr 8508. [Google Scholar]