Abstract
We evaluated the association of GSTM1 null/present, GSTT1 null/present, and GSTP1 IIe105Val polymorphisms with the clinical response to chemotherapy and treatment outcome of NSCLC. Between October 2009 and October 2012, a total of 282 patients with advanced NSCLC were enrolled into our study, and they were followed up until October 2014. The genotypes of GSTM1, GSTT1, and GSTP1 IIe105Val were performed by polymerase chain reaction (PCR) coupled with restriction fragment length polymorphism (RFLP). By logistic regression analysis, our study found that the Val/Val genotype of GSTP1 IIe105Val was associated with more CR+PR response to chemotherapy when compared with the IIe/IIe genotype, and the OR (95% CI) was 2.18 (1.16-4.12). By multivariate Cox proportional hazards regression analysis, we found the Val/Val genotype of GSTP1 was correlated with lower risk of death in advanced NSCLC (HR, 0.48; 95% CI, 0.25-0.93). However, no association was found between GSTT1 and GSTM1 polymorphisms and response to chemotherapy and overall survival of advanced NSCLC. Moreover, the IIe/Val + Val/Val genotypes of GSTP1 were associated with lower risk of death in never smokers, and the adjusted HR (95% CI) was 0.34 (0.12-0.93). In conclusion, we found that the GSTP1 polymorphism was correlated with better response to chemotherapy and lower risk of death in advanced NSCLC patients.
Keywords: GSTM1, GSTT1, GSTP1, polymorphism, NSCLC, chemotherapy
Introduction
Worldwide, lung cancer is the most common cancer among men in terms of both incidence and mortality, and among women it has the third highest incidence and the second mortality rate [1]. In 2012, lung cancer is the number one cause of death among people with malignant tumors in China, and there are 652,842 new cases with lung cancer [1]. Non-small cell lung cancer (NSCLC) is the most common type of lung cancer. Although both individually and in combination of treatment, such as surgery, radiotherapy and chemotherapy, have improved survival patients with of NSCLC in recent years, the long-term survival still has room to improve. TNM classification is the basis for prognostic management of NSCLC; however, it does not provide sufficient information about biological tumor progression [2]. There is still demanding for revealing biomarkers for patients’ survival. Increasing evidences have shown that drug-metabolizing enzyme (DME) have a role in determining inter-individual variations in therapeutic response [3,4].
The human glutathione S-transferases (GSTs), a superfamily of dimeric phase II metabolic enzymes, play an important role in the cellular defense system [5]. The GSTs enzymes detoxify chemotherapeutic drugs or their metabolites by catalyzing the reduction of these compounds through its conjugation with glutathione. It has been suggested that genetic polymorphisms in GSTs genes could reduce the effectiveness of detoxifying cytotoxins generated by chemotherapeutic agents in the treatment of NSCLC [3,4]. The GSTs family consists of six dimeric isoenzymes: GSTs alpha (α), mu (μ), pi (π), theta (τ), zeta (ζ), and omega (ω). Allelic deletions in GSTM1 and GSTT1 genotypes result in polymorphism, and complete deletion of both the alleles (null-type) is associated with reduced enzyme activity [6]. The rs1695 of GSTP1 results in an amino acid change at codon 105 (Ile/Val), which is correlated with lower substrate specific catalytic activity. Polymorphisms of GSTs could alter the metabolism of chemotherapeutic drugs and modify the effectiveness of therapy, as suggested by reports that GSTs polymorphisms predict differences in outcomes of treatment for NSCLC [7,8]. Analysis of the GSTs polymorphisms could be helpful in optimizing chemotherapy treatment.
Only two studies reported the association between GSTs gene polymorphisms and treatment outcome of NSCLC, and no study reported their association in Chinese population [7,8]. We conducted a study to evaluate the clinical response to chemotherapy and treatment outcome of advanced NSCLC in the presence of GSTM1 null/present, GSTT1 null/present, and GSTP1 IIe105Val polymorphisms.
Material and methods
Study subjects
Between October 2009 and October 2012, total of 318 patients who were histopathologically confirmed, without previous chemotherapy or radiotherapy, and underwent lung cancer surgery were enrolled from the Sichuan Cancer Hospital. All the NSCLC patients were inoperable stage IIIA, IIIB and IV NSCLC. The diagnosis of NSCLC were confirmed by bronchoscopic or needle aspiration biopsies. Patients who had any serious concomitant systemic disorder unable to receive chemotherapy, concurrent chemo-radiotherapy, brain metastasis with symptoms which may affect the safety of patients or the evaluation of results were excluded from our study. Finally, 282 patients were included into our study, and the participation rate was 88.68%.
Patients with NSCLC were investigated by trained nurses to collect the demographic and clinical characteristics, such as sex, age, tobacco smoking, alcohol drinking, and family history of cancer, histological classification, TNM stage, and response to chemotherapy. The TNM stage was defined according to the 7th edition of the TNM staging system of the International Union Against Cancer (UICC)/American Joint Committee on Cancer (AJCC).
All participants received cisplatin-based combination chemotherapy regimen. The chemotherapy was repeated at three-weekly intervals for up to six cycles unless unacceptable toxicity, disease progression or patients’ refusal to continue treatment. The patients were required to complete two cycles of chemotherapy at least to evaluate treatment response according to RECIST 1.1. Patients who showed complete response (CR) or partial remission (PR) were considered as good responder, and patients who presented stable disease (SD) and progressive disease (PD) were regarded as poor responder. For long-term survival, overall survival (OS) was defined as the period between the date of chemotherapy and the data of death from any cause. All the patients with advanced NSCLC were followed up until the date of October 2014. All patients with advanced NSCLC signed written informed consents before enrolling into our study. The protocol of this study was approved by the ethics committee of our hospital.
DNA extraction and genotyping
5 ml peripheral blood sample was drawn from each patient with advanced NSCLC, and the peripheral blood samples were kept in -20°C until use. Genomic DNA was isolated from peripheral blood lymphocytes using Qiagen blood mini kit (Qiagen, Germany) by the manufacturer’s protocol. The genotypes of GSTM1, GSTT1, and GSTP1 IIe105Val were performed by polymerase chain reaction (PCR) coupled with restriction fragment length polymorphism (RFLP). Primer sequences of GSTM1, GSTT1, and GSTP1 IIe105Val were designed using Sequenom Assay Design 3.1 software. The forward and reverse primers for GSTT1 were 5’-GAACTTGAACCGCTAAGC-3’ and 5’-GTTCCTCGGTATACGGTGG-3’, respectively. The forward and reverse primers for GSTM1 were 5’-CCTTACTGGCTTCTACATCTC-3’ and 5’-TCACCGGATCAGCCGAGCA-3’, respectively. The forward and reverse primers for GSTP1 IIe105Val were 5’-ACCAGGGCTCTATGGCCAA-3’ and 5’-TGACCCGAGAAGAACGGGT-3’, respectively. The reaction conditions were as follows: 94°C for 4 min; 94°C for 30 sec, 60°C for 30 sec, 72°C for 30 sec with 35 cycles; 72°C for 7 min. The PCR products were analyzed by electrophoresis in a 2% agarose gel stained with ethidium bromide and visualized under UV light.
Statistical analysis
Continuous variables were shown as the mean ± standard deviation (SD) and categorical variables were expressed as number (N) and percentage (%). Homozygote for the most frequent genotype was regarded as the reference group. The associations of GSTM1 null/present, GSTT1 null/present, and GSTP1 IIe105Val polymorphisms with the response to chemotherapy in advanced NSCLC were analyzed by logistic regression analysis, and the results were expressed by odds ratio (ORs) and 95% confidence interval (CI). The prognostic values of GSTM1 null/present, GSTT1 null/present, and GSTP1 IIe105Val polymorphisms for the OS were estimated using the multivariate Cox proportional hazards regression analysis and the results were illustrated using the hazard ratio (HR) and 95% CI. Survival distributions were estimated using the Kaplan-Meier method. Baseline characteristics, such as age, sex, smoking history, histological types and TNM stage at entry, were adjusted in order to avoid potential confounding effects. P values < 0.05 with two-sided were considered statistical differences. Statistical analyses were conducted with SAS software (version 9.1.3; SAS Institute, Cary, NC, USA).
Results
The median ages of included patients with advanced NSCLC were 59.15±10.50 years. There were 101 (35.82%) females and 181 (64.18%) males in our study. Of 282 patients with NSCLC, 193 (68.44%) patients were ever smokers, 83 (29.43%) were drinkers, 29 (10.28%) showed family history of cancer in the first relatives, 120 (42.55%) were squamous cell carcinoma, 162 (57.45%) were adenocarcinoma, 204 (72.34%) were at IIIA and IIIB TNM stage, 78 (27.66%) were at IV TNM stage, and 123 (43.62%) showed CR+PR response to chemotherapy in advanced NSCLC (Table 1).
Table 1.
Characteristics of patients with advanced NSCLC
| Variables | Patients | % |
|---|---|---|
| Age, years | ||
| < 55 | 117 | 41.49 |
| ≥ 55 | 165 | 58.51 |
| Sex | ||
| Female | 101 | 35.82 |
| Male | 181 | 64.18 |
| Tobacco smoking | ||
| Never | 89 | 31.56 |
| Ever | 193 | 68.44 |
| Alcohol drinking | ||
| Never | 199 | 70.57 |
| Ever | 83 | 29.43 |
| Family history of cancer | ||
| No | 253 | 89.72 |
| Yes | 29 | 10.28 |
| Histological types | ||
| Squamous Carcinoma | 120 | 42.55 |
| Adenocarcinoma | 162 | 57.45 |
| TNM stage | ||
| IIIA and IIIB | 204 | 72.34 |
| IV | 78 | 27.66 |
| Response to chemotherpy | ||
| CR+PR response | 123 | 43.62 |
| SD+PD response | 159 | 56.38 |
Association between the GSTM1 null/present, GSTT1 null/present, and GSTP1 IIe105Val polymorphisms and response to chemotherapy in advanced NSCLC was shown in Table 2. Of 282 patients with advanced NSCLC, the good response rate was 43.62%. By logistic regression analysis, our study found that the Val/Val genotype of GSTP1 IIe105Val was associated with more CR+PR response to chemotherapy when compared with the IIe/IIe genotype, and the OR (95% CI) was 2.18 (1.16-4.12). However, the GSTT1 and GSTM1 polymorphisms were not correlated with response to chemotherapy in advanced NSCLC.
Table 2.
Association between GSTM1 null/present, GSTT1 null/present, and GSTP1 IIe105Val polymorphisms and response to chemotherapy in advanced NSCLC
| SNPs | Patients | % | Good response | % | Poor response | % | OR (95% CI)1 | P value |
|---|---|---|---|---|---|---|---|---|
| GSTM1 | ||||||||
| Present | 168 | 59.57 | 68 | 55.28 | 100 | 62.89 | 1.0 (Ref.) | - |
| Null | 114 | 40.43 | 55 | 44.72 | 59 | 37.11 | 1.37 (0.82-2.28) | 0.2 |
| GSTT1 | ||||||||
| Present | 161 | 57.09 | 69 | 56.10 | 92 | 57.86 | 1.0 (Ref.) | - |
| Null | 121 | 42.91 | 54 | 43.90 | 67 | 42.14 | 1.07 (0.65-1.78) | 0.77 |
| GSTP1 | ||||||||
| IIe/IIe | 120 | 42.55 | 42 | 34.15 | 78 | 49.06 | 1.0 (Ref.) | - |
| IIe/Val | 89 | 31.56 | 41 | 33.33 | 48 | 30.19 | 1.59 (0.87-2.89) | 0.11 |
| Val/Val | 74 | 26.24 | 40 | 32.52 | 34 | 21.38 | 2.18 (1.16-4.12) | 0.009 |
Adjusted for age, sex, tobacco smoking, alcohol drinking, family history of cancer in the first relatives and histological types.
By the end of follow-up, we found that the Val/Val genotype of GSTP1 was associated with longer overall survival time of advanced NSCLC when compared with the IIe/IIe genotype (17.45 months vs. 15.30 months) (Figure 1). By multivariate Cox proportional hazards regression analysis, we found the Val/Val genotype of GSTP1 was correlated with lower risk of death in advanced NSCLC (HR, 0.48; 95% CI, 0.25-0.93) (Table 3). However, the GSTT1 and GSTM1 polymorphisms did not contribute to the overall survival of advanced NSCLC.
Figure 1.
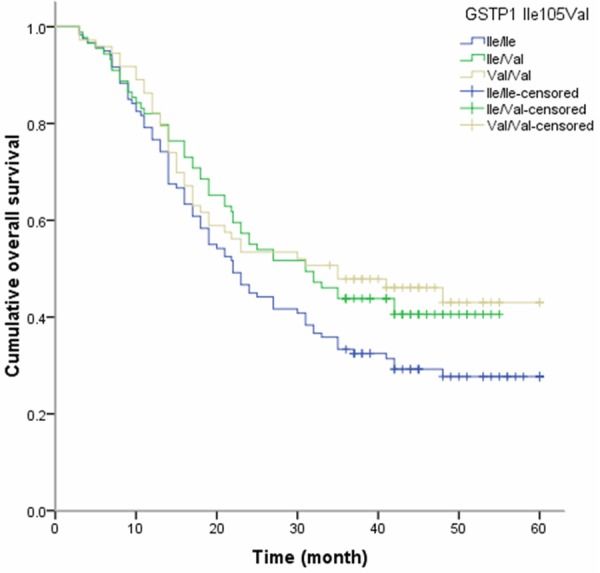
Kaplan-Meier survival curves by GSTP1 IIe105Val polymorphisms in patients with advanced NSCLC.
Table 3.
Association between GSTM1 null/present, GSTT1 null/present, and GSTP1 IIe105Val polymorphisms and overall survival of NSCLC
| Genotypes | Patients | % | Death | % | Alive | % | HR (95% CI)1 | P value |
|---|---|---|---|---|---|---|---|---|
| GSTM1 | ||||||||
| Present | 168 | 59.57 | 102 | 57.63 | 66 | 62.86 | 1.0 (Ref.) | - |
| Null | 114 | 40.43 | 75 | 42.37 | 39 | 37.14 | 1.24 (0.74-2.11) | 0.39 |
| GSTT1 | ||||||||
| Present | 161 | 57.09 | 105 | 59.32 | 56 | 53.33 | 1.0 (Ref.) | - |
| Null | 121 | 42.91 | 72 | 40.68 | 49 | 46.67 | 0.78 (0.47-1.31) | 0.33 |
| GSTP1 | ||||||||
| IIe/IIe | 120 | 42.55 | 85 | 48.02 | 35 | 33.33 | 1.0 (Ref.) | - |
| IIe/Val | 89 | 31.56 | 52 | 29.38 | 37 | 35.24 | 0.58 (0.31-1.07) | 0.06 |
| Val/Val | 74 | 26.24 | 40 | 22.60 | 34 | 32.38 | 0.48 (0.25-0.93) | 0.02 |
Adjusted for age, sex, tobacco smoking, alcohol drinking, family history of cancer in the first relatives and histological types.
By stratification analysis, we found that the IIe/Val + Val/Val genotype of GSTP1 was associated with lower risk of death in never smokers, and the adjusted HR (95% CI) was 0.34 (0.12-0.93) (Table 4). However, no correlation was found between the GSTP1 polymorphism and alcohol drinking, histological types and TNM stage in the overall survival of advanced NSCLC.
Table 4.
Association between GSTP1 null/present and overall survival of NSCLC stratified by clinical characteristics
| Characteristics | IIe/IIe | IIe/Val + Val/Val | HR (95% CI)1 | P value | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Death | Alive | Death | Alive | |||
| Tobacco smoking | ||||||
| Never | 39 | 12 | 20 | 18 | 0.34 (0.12-0.93) | 0.02 |
| Ever | 46 | 23 | 72 | 52 | 0.69 (0.36-1.33) | 0.24 |
| Alcohol drinking | ||||||
| Never | 58 | 21 | 69 | 51 | 0.49 (0.25-0.94) | 0.02 |
| Ever | 27 | 14 | 23 | 19 | 0.63 (0.23-1.67) | 0.30 |
| Histological types | ||||||
| Squamous Carcinoma | 32 | 15 | 41 | 32 | 0.55 (0.24-1.23) | 0.11 |
| Adenocarcinoma | 53 | 20 | 51 | 38 | 0.49 (0.28-1.42) | 0.17 |
| TNM stage | ||||||
| IIIA and IIIB | 68 | 23 | 72 | 41 | 0.55 (0.32-1.07) | 0.08 |
| IV | 17 | 12 | 20 | 29 | 0.43 (0.16-1.17) | 0.07 |
Adjusted for age and sex.
Discussion
It is well known that genetic factors may contribute to the individualized response to chemotherapy. The GST super-family of enzymes belongs to the phase II group of enzymes, which are responsible for the metabolism of a wide range of xenobiotics and drugs including a variety of cytotoxic cancer chemotherapeutic agents [9]. Since the first evidence that glutathione S-transferases may play an important role in chemotherapy efficacy [10], the results of various subsequent studies have shown the inconsistent nature of this relationship. Although many clinicopathologic characteristics, such as patient age and clinical disease stage, have been identified as being influential factors in predicting the efficacy of chemotherapy, and accumulated evidences have reported that drug-metabolizing enzyme plays a critical role in determining inter-individual variations in therapeutic response [11,12]. In the present study, we examine the association of GSTM1, GSTT1, and GSTP1 IIe105Val polymorphisms with the treatment response to chemotherapy and treatment outcome of advanced NSCLC. We found that the Val/Val genotype of GSTP1 was associated with better response to chemotherapy and longer overall survival time when compared with the IIe/IIe genotype.
GSTP1 is a kind of phase II detoxification enzymes, which is involved in the detoxification of cisplatin [13]. Over expression of GSTP1 in NSCLC patient could decrease the sensitivity to platinum agents in vitro [3]. The polymorphism of GSTP1 IIe105Val changes the thermal stability and conjugation capacity of GSTP1, which alters the ability of GSTP1 to detoxify chemotherapeutic agents and modulates drug res-ponses. Therefore, enzyme activity with the GSTP1 IIe105Val is more effective on cisplatin than that with wild-type in vitro [14]. However, it is a controversy that GSTP1 IIe105Val polymorphism would enhance clinical response of cisplatin-based chemotherapy [15-21]. Oliveira et al. conducted a study in a Chinese population, and they found that GSTP1 rs1695 polymorphisms were associated with poor response to chemotherapy and shorter overall survival of breast cancer [20]. Goričar et al. suggested that GSTP1 polymorphism was associated with shorter overall survival of osteosarcoma [21]. Han et al. have reported that GSTP1 polymorphism was collected with a longer median survival time when compared with the wide-type genotype [17]. These inconsistency results might be due to differences in ethnicities, conditions of patients, sample size, and by chance.
Only two previous studies reported the association of GSTs gene polymorphisms with the efficacy of chemotherapy treatment and overall survival of NSCL [7,8]. Ada et al. conducted a study in a Greek population, and they found that variants of GSTP1 were associated an improved survival in the patients with advanced NSCLC [7]. Viachogeorgos et al. conducted a study with 39 patients with NSCLC, and they found that GSTP1 expression was associated inversely with response to chemotherapy and the survival of patients with advanced NSCLC [8]. In our study, we found that the null genotype of GSTP1 were correlated with better response to chemotherapy and lower risk of death in advanced NSCLC patients, which were in line with previous results. Moreover, we found that the GSTP1 polymorphism has interaction with tobacco smoking in the treatment efficacy of advanced NSCLC. Previous studies have reported an interaction between GSTP1 polymorphism and smoking in the risk of several diseases [22,23], which suggests that GSTP1 polymorphism could have interaction with tobacco smoking in the treatment outcome of chemotherapy in NSCLC.
There were two limitations in our study. First, there may be selection bias may overestimate the true size of effect or lead to spurious findings. Second, the sample size of patients with advanced NSCLC is relatively small, which may limit the statistical power to find difference between groups. Therefore, the actual biological mechanism of effects of GSTs polymorphisms on the treatment outcome of advanced NSCLC required further large-scale studies in different ethnic groups and functional experiments to replicate our findings.
In conclusion, we found that the GSTP1 polymorphism was correlated with better response to chemotherapy and lower risk of death in advanced NSCLC patients. Future studies with larger sample size are greatly needed to confirm the role of GSTs polymorphisms in the clinical outcome of NSCLC.
Disclosure of conflict of interest
None.
References
- 1.International Agency for Research on Cancer. 2012. http://globocan.iarc.fr/Pages/fact_sheets_population.aspx.
- 2.Chansky K, Sculier JP, Crowley JJ, Giroux D, Van Meerbeeck J, Goldstraw P International Staging Committee and Participating Institutions. The International Association for the Study of Lung Cancer Staging Project: prognostic factors and pathologic TNM stage in surgically managed non-small cell lung cancer. J Thorac Oncol. 2009;4:792–801. doi: 10.1097/JTO.0b013e3181a7716e. [DOI] [PubMed] [Google Scholar]
- 3.Oguri T, Fujiwara Y, Katoh O, Daga H, Ishikawa N, Fujitaka K, Yamasaki M, Yokozaki M, Isobe T, Ishioka S, Yamakido M. Glutathione S-transferase-pi gene expression and platinum drug exposure in human lung cancer. Cancer Lett. 2000;156:93–99. doi: 10.1016/s0304-3835(00)00447-x. [DOI] [PubMed] [Google Scholar]
- 4.Joerger M, Burgers SA, Baas P, Smit EF, Haitjema TJ, Bard MP, Doodeman VD, Smits PH, Vincent A, Huitema AD, Beijnen JH, Schellens JH. Germline polymorphisms in patients with advanced nonsmall cell lung cancer receiving first-line platinum-gemcitabine chemotherapy: a prospective clinical study. Cancer. 2012;118:2466–2475. doi: 10.1002/cncr.26562. [DOI] [PubMed] [Google Scholar]
- 5.Strange RC, Fryer AA. The glutathione S-transferases: influence of polymorphism on cancer susceptibility. IARC Sci Publ. 1999:231–49. [PubMed] [Google Scholar]
- 6.Strange RC, Spiteri MA, Ramachandran S, Fryer AA. Glutathione-S-transferase family of enzymes. Mutat Res. 2001;482:21–26. doi: 10.1016/s0027-5107(01)00206-8. [DOI] [PubMed] [Google Scholar]
- 7.Ada AO, C Kunak S, Hancer F, Bilgen S, Suzen SH, Alpar S, Gulhan M, Kurt B, Iscan M. CYP and GST polymorphisms and survival in advanced non-small cell lung cancer patients. Neoplasma. 2010;57:512–521. doi: 10.4149/neo_2010_06_512. [DOI] [PubMed] [Google Scholar]
- 8.Vlachogeorgos GS, Manali ED, Blana E, Legaki S, Karagiannidis N, Polychronopoulos VS, Roussos C. Placental isoform glutathione S-transferase and P-glycoprotein expression in advanced nonsmall cell lung cancer: association with response to treatment and survival. Cancer. 2008;114:519–526. doi: 10.1002/cncr.23981. [DOI] [PubMed] [Google Scholar]
- 9.Tew KD. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 1994;54:4313–4320. [PubMed] [Google Scholar]
- 10.Anderer G, Schrappe M, Brechlin AM, Lauten M, Muti P, Welte K, Stanulla M. Polymorphisms within glutathione S-transferase genes and initial response to glucocorticoids in childhood acute lymphoblastic leukaemia. Pharmacogenetics. 2000;10:715–726. doi: 10.1097/00008571-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Arun BK, Granville LA, Yin G, Middleton LP, Dawood S, Kau SW, Kamal A, Hsu L, Hortobagyi GN, Sahin AA. Glutathione-s-transferase-pi expression in early breast cancer: association with outcome and response to chemotherapy. Cancer Invest. 2010;28:554–559. doi: 10.3109/07357900903286925. [DOI] [PubMed] [Google Scholar]
- 12.Franco RL, Schenka NG, Schenka AA, Rezende LF, Gurgel MS. Glutathione S-transferase Pi expression in invasive breast cancer and its relation with the clinical outcome. J Buon. 2012;17:259–264. [PubMed] [Google Scholar]
- 13.Sheehan D, Meade G, Foley VM, Dowd CA. Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem J. 2001;360:1–16. doi: 10.1042/0264-6021:3600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McIlwain CC, Townsend DM, Tew KD. Glutathione S-transferase polymorphisms: cancer incidence and therapy. Oncogene. 2006;25:1639–1648. doi: 10.1038/sj.onc.1209373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khrunin AV, Moisseev A, Gorbunova V, Limborska S. Genetic polymorphisms and the efficacy and toxicity of cisplatin-based chemotherapy in ovarian cancer patients. Pharmacogenomics J. 2010;10:54–61. doi: 10.1038/tpj.2009.45. [DOI] [PubMed] [Google Scholar]
- 16.Kap EJ, Richter S, Rudolph A, Jansen L, Ulrich A, Hoffmeister M, Ulrich CM, Brenner H, Chang-Claude J. Genetic variants in the glutathione S-transferase genes and survival in colorectal cancer patients after chemotherapy and differences according to treatment with oxaliplatin. Pharmacogenet Genomics. 2014;24:340–347. doi: 10.1097/FPC.0000000000000059. [DOI] [PubMed] [Google Scholar]
- 17.Han B, Guo Z, Ma Y, Kang S, Wang Y, Wei Q, Wu X. Association of GSTP1 and XRCC1 gene polymorphisms with clinical outcome of advanced non-small cell lung cancer patients with cisplatin-based chemotherapy. Int J Clin Exp Pathol. 2015;8:4113–419. [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou L, Huang A, Zhang D, Yao J, Zhang Y, Li X. Genetic variability of glutathione S-transferases influences treatment outcome of breast cancer. Tumour Biol. 2015;36:5925–9. doi: 10.1007/s13277-015-3266-9. [DOI] [PubMed] [Google Scholar]
- 19.Liu S, Yi Z, Ling M, Shi J, Qiu Y, Yang S. Predictive potential of ABCB1, ABCC3, and GSTP1 gene polymorphisms on osteosarcoma survival after chemotherapy. Tumour Biol. 2014;35:9897–9904. doi: 10.1007/s13277-014-1917-x. [DOI] [PubMed] [Google Scholar]
- 20.Oliveira AL, Oliveira Rodrigues FF, Dos Santos RE, Rozenowicz RL, Barbosa de Melo M. GSTT1, GSTM1, and GSTP1 polymorphisms as a prognostic factor in women with breast cancer. Genet Mol Res. 2014;13:2521–2530. doi: 10.4238/2014.January.22.9. [DOI] [PubMed] [Google Scholar]
- 21.Goričar K, Kovač V, Jazbec J, Zakotnik B, Lamovec J, Dolžan V. Genetic variability of DNA repair mechanisms and glutathione-S-transferase genes influences treatment outcome in osteosarcoma. Cancer Epidemiol. 2015;39:182–188. doi: 10.1016/j.canep.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 22.Kiyohara C, Miyake Y, Koyanagi M, Fujimoto T, Shirasawa S, Tanaka K, Fukushima W, Sasaki S, Tsuboi Y, Yamada T, Oeda T, Miki T, Kawamura N, Sakae N, Fukuyama H, Hirota Y, Nagai M Fukuoka Kinki Parkinson’s Disease Study Group. GST polymorphisms, interaction with smoking and pesticide use, and risk for Parkinson’s disease in a Japanese population. Parkinsonism Relat Disord. 2010;16:447–452. doi: 10.1016/j.parkreldis.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Singh N, Sinha N, Kumar S, Pandey CM, Agrawal S. Glutathione S-transferase gene polymorphism as a susceptibility factor for acute myocardial infarction and smoking in the North Indian population. Cardiology. 2011;118:16–21. doi: 10.1159/000324066. [DOI] [PubMed] [Google Scholar]


