Abstract
The receptor tyrosine kinase of EphA2 has been shown frequently overexpressed in various types of human carcinomas, which implicated that it plays important roles in carcinogenesis. Although EphA2 protein expression has been investigated in many types of human carcinomas, the relationship between the expression of EphA2 protein in clear cell renal cell carcinoma was not well documented. In the present study, using specific anit-EphA2 polyclonal antibody and immunohistochemistry, we evaluated EphA2 protein expression levels in clear cell RCC specimens surgically resected from 90 patients. Our results shows that EphA2 protein was positively expressed in all normal renal tubes of 90 samples (100%, 3+), which was expressed at low levels in renal cortex but high levels in the collecting ducts of the renal medulla and papilla. EphA2 was negatively or weakly expressed in 30 out of 90 samples (33.3%, 0/1+), moderately expressed in 24 samples (26.7%, 2+) and strongly expressed in 36 samples (40%, 3+). Expression of EphA2 was positively associated with age (P=0.029), tumor diameters (P<0.001) and Fuhrman nuclear grade (P<0.001). Our results indicate that EphA2 variably expressed in clear cell renal cell carcinomas. High expression of EphA2 was more often found in big size and high nuclear grade tumors, which indicated EphA2 protein may be used as a new marker for the prognosis of clear cell renal cell carcinoma.
Keywords: EphA2, clear cell renal cell carcinoma, Fuhrman nuclear grade
Introduction
Renal cell carcinoma (RCC) is the most common neoplasm in the kidney, which comprises several histological subtypes according to the differences in genetics, biology and behavior. RCC consists of clear cell RCC, papillary RCC, chromophobe RCC, collecting duct RCC, renal medullary carcinoma, Xp11 translocation RCC. The major histologic subtype of RCC is clear cell RCC also called conventional RCC, which accounted for 75% in RCC [1]. RCC is thought to arise from a variety of specialized cells located along the length of the nephron. The clear cell RCC is thought to arise from the epithelium of the proximal tubule. Clear cell RCC shows a less favorable prognosis than other common subtypes, such as chromophobe and papillary RCC.
Recent advances in the molecular pathogenesis of clear cell RCC revealed several genetic alterations. The Von Hippel-Lindau (VHL) mutation is the most common genetic alteration. Chromosome 3p deletion and inactivation of the VHL suppressor gene is the most common genetic alteration [2-4]. Almost all familiar clear cell RCC arise from an inherited mutation in VHL tumor suppressor gene. The second allele of VHL has been shown to be inactivated by deletion and by promoter hypermethylation or rearrangement in the RCC. The VHL tumor suppressor gene stabilizes hypoxia-inducible factors (HIF-1 and HIF-2), and PBRM1 has a role in chromatin remodeling. As mutations in these 2 genes have the main pathogenic role, oncogenic metabolic shift and epigenetic alteration have been regarded as a major pathogenesis of clear cell RCC.
Receptor tyrosine kinases of the Eph family and Ephrin ligands play important roles in vascular development, tissue-border formation, cell migration, axon guidance, and angiogenesis. Abnormal expression of Eph receptor tyrosine kinases in cancers is related to malignant transformation, tumor metastasis, tumor differentiation, and outcome. The family is subdivided into class A and class B based on homology and binding affinity for 2 distinct types of membrane-anchored ephrin ligands. Class B receptors generally bind to class B ephrin that are attached to the cell membrane by a transmembrane-spanning domain, which A class receptor normally interact with glycosyl-phosphatidylinositol-linked class A ephrins, although interclass binding does occur among certain family members. Increasing evidence suggests that EphA2 expression may be causally related to neoplasia. EphA2 overexpression has been observed in several models of cancer, including primary and transplanted rodent tumors, human tumor xenografts, and primary human tumor biopsies. Overexpression of EphA2 has been observed in numerous cancer types, including melanoma [5], breast carcinoma [6,7], lung cancer [8-11], and prostate carcinoma [12-16]. However, the expression of EphA2 in RCC has not been well investigated. In this study, we investigated the expression levels of EphA2 protein in a set of clear cell RCC samples, and determined if its expression is associated with clinicopathological parameters.
Materials and methods
Tissue samples
The RCC tissue samples in our study were collected from 90 patients (57 males, 33 females, average age=56.3 years; range=35-81 years old at the time of resection) with RCC as part of a study approved by the Research Ethics Board of Jinling Hospital, China. All patients were treated by radical or partial nephrectomy and rendered disease-free. Formalin-fixed and paraffin-embedded tissues were sectioned into slices 4 m thick and stained with hematoxylin and eosin for pathological identification.
Immunohistochemical staining
Sections from surgical specimens fixed in 10% formalin and embedded in paraffin were used for immunohistochemical staining according to the standard method. Briefly, each 4-m tissue section was deparaffinized and rehydrated. After rehydration through a graded ethanol series, the sections were autoclaved in 10 mM citrate buffer (pH 6.0) at 120°C for 2 min for antigen retrieval, then cooled to 30°C and washed with phosphate-buffered saline (PBS, pH 7.3). After non-specific sites had been blocked in 10% normal calf serum in PBS for 10 min, the sections were incubated at 4°C overnight with an anti-EphA1 polyclonal antibody (Abgent, San Diego, CA, USA) at a 1:500 dilution in antibody diluent solution (Zymed, Invitrogen), and then washed with PBS. The specificity of EphA2 antibody was previously investigated in colorectal cancer sections using blocking peptide. Next, the sections were incubated with secondary antibody (Dako REAL EnVision Detection System, Dako, UK) for 30 min at room temperature. Color development was performed with 3,3’-diaminobenzidine (DAB). Nuclei were lightly counterstained with hematoxylin. Two pathologists independently assessed the immunostained slides. Any difference in immunohistochemical scores was resolved by a consensus. Immunohistochemical staining of both normal and cancer cells was assessed according to the intensity of stained cells. Staining intensity was evaluated as: 0=negative, 1=weak, 2=moderate, 3=strong.
Statistical analysis
The statistical significance of intergroup differences was evaluated by a chi-square test. All statistical analyses were performed using SPSS software (SPSS 11.5, Chicago, IL). A two-sided P value less than 0.05 was considered statistically significant.`
Results
Expression of EphA2 protein in normal renal tubes
The subcellular location of EphA1 protein was in cytoplasm (Figure 1). The EphA2 protein was positively expressed in normal renal tubes of 90 samples (100%, 2+ or 3+), which was expressed at low levels in renal cortex but high levels in the collecting ducts of the renal medulla and papilla, but not expressed in renal glomerulus.
Figure 1.
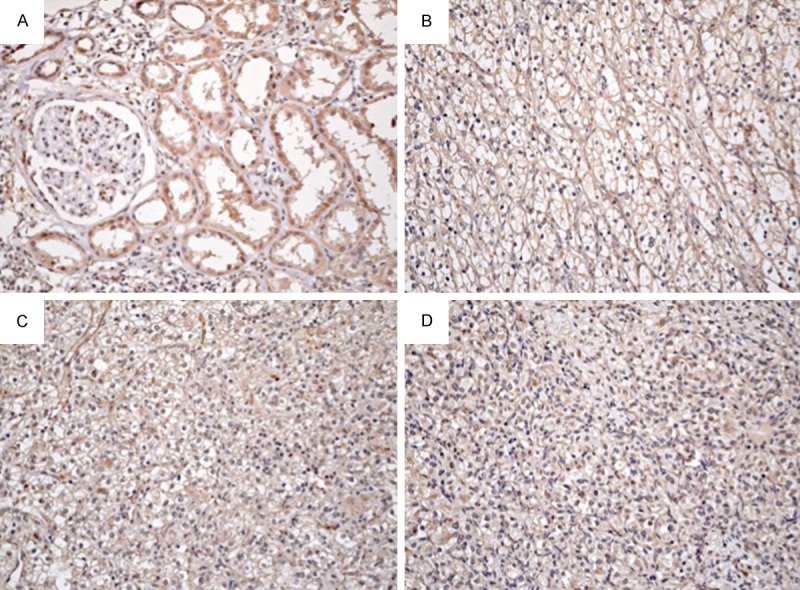
Expression of EphA2 protein in clear cell renal cell carcinoma and normal renal tube. (A) High expression of EphA2 in normal renal tubes and negative in renal glomerulus. Representative samples of high expression of EphA2 Fuhrman nuclear grade I (B), II (C) and III (D) clear cell renal cell carcinoma. Original magnification, ×200.
The relationship between the expression of EphA2 protein in RCC and clinicopathologic parameters
Our results indicate that EphA2 variably expressed in clear cell renal cell carcinomas. EphA2 was negatively or weakly expressed in 30 out of 90 samples (33.3%, 0/1+), moderately expressed in 24 samples (26.7%, 2+) and strongly expressed in 36 samples (40%, 3+). Expression of EphA2 was positively associated with age (P=0.029), tumor diameters (P<0.001) and Fuhrman nuclear grade (P<0.001) (Table 1). Fuhrman (nuclear) grade criterion used in present study is as follows. Grade I: Small, round, uniform nuclei (10 microns), inconspicuous nucleoli, look like lymphocytes (very rare). Grade II: Slightly irregular nuclei, see nucleoli at 40× only, nuclear diameter 15 microns, open chromatin (40% of tumors). Grade III: See nucleoli at 10×, nuclei very irregular, diameter 20 microns, open chromatin (30-40% of tumors). Grade IV: Mitoses; bizarre, multilobated, pleomorphic cells plus grade 3 features, macronucleoli (15% of tumors).
Table 1.
Expression of EphA2 protein in ccRCC and the association to clinicopathologic parameters
| No. | EphA2 protein | P value | |||
|---|---|---|---|---|---|
|
|
|||||
| 0, 1+ | 2+ | 3+ | |||
| Sex | |||||
| Male | 57 | 24 | 12 | 21 | 0.055 |
| Female | 33 | 6 | 12 | 15 | |
| Age (years) | |||||
| <50 | 21 | 12 | 3 | 6 | 0.029 |
| 50-70 | 53 | 17 | 15 | 21 | |
| >70 | 16 | 1 | 6 | 9 | |
| Tumor diameter (cm) | |||||
| <7 | 63 | 27 | 21 | 15 | <0.001 |
| ≥7 | 27 | 3 | 3 | 21 | |
| Nuclear grade | |||||
| I | 27 | 21 | 3 | 3 | <0.001 |
| II | 41 | 6 | 20 | 15 | |
| III+IV | 22 | 3 | 1 | 18 | |
Although no significant relation between the expression of EphA2 and sex of patients was found (P=0.055), female patients were often showed high level expression of EphA2 protein than male patients (Table 1).
Discussion
Overexpression of EphA2 has been found in a wide array of solid tumors, including breast cancer [17], prostate cancer [12,18], colorectal cancer [19,20], ovarian cancer [21,22], non-small cell lung cancer [8], gastric cancer [23], squamous cervical carcinoma [24], esophageal squamous cell carcinoma [25], and melanoma [5]. Overexpression of EphA2 is significantly associated with cancer progression [26], metastasis and shorter overall survival in cancers [8,9]. EphA2 is proposed to be an oncogene and a potential target for cancer therapy [27-32].
The EphA2 expression in renal cell carcinoma has been studied to a degree by several groups and summarized in Table 2. Walter J. Storkus et al reported that EphA2 is overexpressed in renal cell carcinoma cell lines and 40 patients with RCC. They found that highest levels of EphA2 are consistently expressed in the most advanced stages of the disease [33]. In another paper, Walter J. Storkus et al tested expression of EphA2 in 34 patients of RCC, including 30 conventional clear-cell RCC, 3 papillary, and 1 chormophobic RCC by using specific anit-EphA2 monoclonal antibody and immunohistochemistry [34]. They found that RCC lesions expressing higher levels of EphA2 tended to be of a higher grade and larger, more highly vascularized tumors. Most notable, they found the degree of EphA2 overexpression seemed predictive of short-term versus longer-term disease-free interval and of overall survival. Bai et al evaluated the expression of EphA2 protein in 62 patients with RCC by immunohistochemistry and analyzed the correlations between EphA2 expression levels and clinicopathological variables and outcome of patients. They found that EphA2 expression was positively related with TNM classification, size of tumor, and lymph node metastasis. The length of the survival time of the patients with high levels of EphA2 expression was significantly lower than that of the patients with low levels of EphA2 expression [35].
Table 2.
Summary of researches of EphA2 expression on renal cell carcinoma
| Authors | Date | Samples | Methods | Results |
|---|---|---|---|---|
| Walter J. Storkus et al | 2003 | Cell line, 40 patients with RCC | Western blot | The highest levels of EphA2 are consistently found in most advanced stage of disease |
| Keisuke Shirai et al | 2005 | 34 patients (30 CCRCC, 3papillary, and 1 choromophobic RCC) | IHC | RCC lesions express higher levels of EphA2. The degree of EphA2 overexpression seemed predictive of short-term interval |
| Yaling Bai et al | 2014 | 62 patients with RCC | IHC | 67.7% cases of RCC demonstrated a high expression of EphA2 protein. High EphA2 expression predicted a shorter survival |
In the present study, we subjected 90 clear cell renal cell carcinoma samples to immiunohistochemstry and analyzed the relationship between the expression of EphA2 protein and clinicopathologic parameters. To our knowledge, till now, this is the largest number of patients with clear cell renal cell carcinoma. Our results showed that EphA2 protein was positively expressed in normal renal tubes of 90 samples (100%, 2+ or 3+). This is a very interesting phenomenon. EphA2 plays a role of oncogene in tumors based on it overexpressed in tumors, correlated with poor prognosis and recurrence of patients. In this study, although EphA2 protein positively expressed in 66.7% of samples of clear cell renal cell carcinomas (2+ and 3+), its expression decreased from normal renal tube cells to tumor cells. Eph receptors and their ephrin ligands have multi-faceted functions as tumor suppressor or tumor promoters [36,37]. EphA2 has been reported as an oncogene playing roles in oncogenic transformation and tumor progression. This result indicated that EphA2 may not an oncogene in renal tissue.
In the present study, we found that expression of EphA2 was positively associated with age, tumor diameters and Fuhrman nuclear grade. Our results showed that high level of EphA2 protein was more often detected in aged patients and larger tumor. The results are partially consistent with that of other group. Fuhrman nuclear grade is an important prognosis parameter in renal cell carcinoma. We found that high level of EphA2 protein was more often detected in higher grade of tumors. This indicated that EphA2 protein may be used as a new marker for the prognosis of clear cell renal cell carcinoma.
Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (81472392, 81372741).
Disclosure of conflict of interest
None.
References
- 1.Dagher J, Dugay F, Rioux-Leclercq N, Verhoest G, Oger E, Bensalah K, Cabillic F, Jouan F, Kammerer-Jacquet SF, Fergelot P, Vigneau C, Arlot-Bonnemains Y, Belaud-Rotureau MA. Cytoplasmic PAR-3 protein expression is associated with adverse prognostic factors in clear cell renal cell carcinoma and independently impacts survival. Hum Pathol. 2014;45:1639–1646. doi: 10.1016/j.humpath.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 2.Lessi F, Mazzanti CM, Tomei S, Di Cristofano C, Minervini A, Menicagli M, Apollo A, Masieri L, Collecchi P, Minervini R, Carini M, Bevilacqua G. VHL and HIF-1alpha: gene variations and prognosis in early-stage clear cell renal cell carcinoma. Med Oncol. 2014;31:840. doi: 10.1007/s12032-014-0840-8. [DOI] [PubMed] [Google Scholar]
- 3.Singh RB, Amare Kadam PS. Investigation of tumor suppressor genes apart from VHL on 3p by deletion mapping in sporadic clear cell renal cell carcinoma (cRCC) Urol Oncol. 2013;31:1333–1342. doi: 10.1016/j.urolonc.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Choueiri TK, Fay AP, Gagnon R, Lin Y, Bahamon B, Brown V, Rosenberg JE, Hutson TE, Baker-Neblett KL, Carpenter C, Liu Y, Pandite L, Signoretti S. The role of aberrant VHL/HIF pathway elements in predicting clinical outcome to pazopanib therapy in patients with metastatic clear-cell renal cell carcinoma. Clin Cancer Res. 2013;19:5218–5226. doi: 10.1158/1078-0432.CCR-13-0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Straume O, Akslen LA. Strong expression of ID1 protein is associated with decreased survival, increased expression of ephrin-A1/EPHA2, and reduced thrombospondin-1 in malignant melanoma. Br J Cancer. 2005;93:933–938. doi: 10.1038/sj.bjc.6602792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brantley-Sieders DM, Jiang A, Sarma K, Badu-Nkansah A, Walter DL, Shyr Y, Chen J. Eph/ephrin profiling in human breast cancer reveals significant associations between expression level and clinical outcome. PLoS One. 2011;6:e24426. doi: 10.1371/journal.pone.0024426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chukkapalli S, Amessou M, Dilly AK, Dekhil H, Zhao J, Liu Q, Bejna A, Thomas RD, Bandyopadhyay S, Bismar TA, Neill D, Azoulay L, Batist G, Kandouz M. Role of the EphB2 receptor in autophagy, apoptosis and invasion in human breast cancer cells. Exp Cell Res. 2014;320:233–246. doi: 10.1016/j.yexcr.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 8.Kinch MS, Moore MB, Harpole DH Jr. Predictive value of the EphA2 receptor tyrosine kinase in lung cancer recurrence and survival. Clin Cancer Res. 2003;9:613–618. [PubMed] [Google Scholar]
- 9.Brannan JM, Dong W, Prudkin L, Behrens C, Lotan R, Bekele BN, Wistuba I, Johnson FM. Expression of the receptor tyrosine kinase EphA2 is increased in smokers and predicts poor survival in non-small cell lung cancer. Clin Cancer Res. 2009;15:4423–4430. doi: 10.1158/1078-0432.CCR-09-0473. [DOI] [PubMed] [Google Scholar]
- 10.Patel AR, Chougule M, Singh M. EphA2 targeting pegylated nanocarrier drug delivery system for treatment of lung cancer. Pharm Res. 2014;31:2796–2809. doi: 10.1007/s11095-014-1377-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song W, Ma Y, Wang J, Brantley-Sieders D, Chen J. JNK signaling mediates EPHA2-dependent tumor cell proliferation, motility, and cancer stem cell-like properties in non-small cell lung cancer. Cancer Res. 2014;74:2444–2454. doi: 10.1158/0008-5472.CAN-13-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walker-Daniels J, Coffman K, Azimi M, Rhim JS, Bostwick DG, Snyder P, Kerns BJ, Waters DJ, Kinch MS. Overexpression of the EphA2 tyrosine kinase in prostate cancer. Prostate. 1999;41:275–280. doi: 10.1002/(sici)1097-0045(19991201)41:4<275::aid-pros8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 13.Petty A, Myshkin E, Qin H, Guo H, Miao H, Tochtrop GP, Hsieh JT, Page P, Liu L, Lindner DJ, Acharya C, MacKerell AD Jr, Ficker E, Song J, Wang B. A small molecule agonist of EphA2 receptor tyrosine kinase inhibits tumor cell migration in vitro and prostate cancer metastasis in vivo. PLoS One. 2012;7:e42120. doi: 10.1371/journal.pone.0042120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tawadros T, Brown MD, Hart CA, Clarke NW. Ligand-independent activation of EphA2 by arachidonic acid induces metastasis-like behaviour in prostate cancer cells. Br J Cancer. 2012;107:1737–1744. doi: 10.1038/bjc.2012.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tognolini M, Giorgio C, Hassan Mohamed I, Barocelli E, Calani L, Reynaud E, Dangles O, Borges G, Crozier A, Brighenti F, Del Rio D. Perturbation of the EphA2-EphrinA1 system in human prostate cancer cells by colonic (poly)phenol catabolites. J Agric Food Chem. 2012;60:8877–8884. doi: 10.1021/jf205305m. [DOI] [PubMed] [Google Scholar]
- 16.Chen P, Huang Y, Zhang B, Wang Q, Bai P. EphA2 enhances the proliferation and invasion ability of LNCaP prostate cancer cells. Oncol Lett. 2014;8:41–46. doi: 10.3892/ol.2014.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301–2306. [PubMed] [Google Scholar]
- 18.Zeng G, Hu Z, Kinch MS, Pan CX, Flockhart DA, Kao C, Gardner TA, Zhang S, Li L, Baldridge LA, Koch MO, Ulbright TM, Eble JN, Cheng L. High-level expression of EphA2 receptor tyrosine kinase in prostatic intraepithelial neoplasia. Am J Pathol. 2003;163:2271–2276. doi: 10.1016/S0002-9440(10)63584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kataoka H, Igarashi H, Kanamori M, Ihara M, Wang JD, Wang YJ, Li ZY, Shimamura T, Kobayashi T, Maruyama K, Nakamura T, Arai H, Kajimura M, Hanai H, Tanaka M, Sugimura H. Correlation of EPHA2 overexpression with high microvessel count in human primary colorectal cancer. Cancer Sci. 2004;95:136–141. doi: 10.1111/j.1349-7006.2004.tb03194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saito T, Masuda N, Miyazaki T, Kanoh K, Suzuki H, Shimura T, Asao T, Kuwano H. Expression of EphA2 and E-cadherin in colorectal cancer: correlation with cancer metastasis. Oncol Rep. 2004;11:605–611. [PubMed] [Google Scholar]
- 21.Thaker PH, Deavers M, Celestino J, Thornton A, Fletcher MS, Landen CN, Kinch MS, Kiener PA, Sood AK. EphA2 expression is associated with aggressive features in ovarian carcinoma. Clin Cancer Res. 2004;10:5145–5150. doi: 10.1158/1078-0432.CCR-03-0589. [DOI] [PubMed] [Google Scholar]
- 22.Han L, Dong Z, Qiao Y, Kristensen GB, Holm R, Nesland JM, Suo Z. The clinical significance of EphA2 and Ephrin A-1 in epithelial ovarian carcinomas. Gynecol Oncol. 2005;99:278–286. doi: 10.1016/j.ygyno.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura R, Kataoka H, Sato N, Kanamori M, Ihara M, Igarashi H, Ravshanov S, Wang YJ, Li ZY, Shimamura T, Kobayashi T, Konno H, Shinmura K, Tanaka M, Sugimura H. EPHA2/EFNA1 expression in human gastric cancer. Cancer Sci. 2005;96:42–47. doi: 10.1111/j.1349-7006.2005.00007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu D, Suo Z, Kristensen GB, Li S, Troen G, Holm R, Nesland JM. Prognostic value of EphA2 and EphrinA-1 in squamous cell cervical carcinoma. Gynecol Oncol. 2004;94:312–319. doi: 10.1016/j.ygyno.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 25.Miyazaki T, Kato H, Fukuchi M, Nakajima M, Kuwano H. EphA2 overexpression correlates with poor prognosis in esophageal squamous cell carcinoma. Int J Cancer. 2003;103:657–663. doi: 10.1002/ijc.10860. [DOI] [PubMed] [Google Scholar]
- 26.Straume O, Akslen LA. Importance of vascular phenotype by basic fibroblast growth factor, and influence of the angiogenic factors basic fibroblast growth factor/fibroblast growth factor receptor-1 and ephrin-A1/EphA2 on melanoma progression. Am J Pathol. 2002;160:1009–1019. doi: 10.1016/S0002-9440(10)64922-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landen CN, Kinch MS, Sood AK. EphA2 as a target for ovarian cancer therapy. Expert Opin Ther Targets. 2005;9:1179–1187. doi: 10.1517/14728222.9.6.1179. [DOI] [PubMed] [Google Scholar]
- 28.Kiewlich D, Zhang J, Gross C, Xia W, Larsen B, Cobb RR, Biroc S, Gu JM, Sato T, Light DR, Heitner T, Willuda J, Vogel D, Monteclaro F, Citkowicz A, Roffler SR, Zajchowski DA. Anti-EphA2 antibodies decrease EphA2 protein levels in murine CT26 colorectal and human MDA-231 breast tumors but do not inhibit tumor growth. Neoplasia. 2006;8:18–30. doi: 10.1593/neo.05544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsouko E, Wang J, Frigo DE, Aydogdu E, Williams C. miR-200a inhibits migration of triple-negative breast cancer cells through direct repression of the EPHA2 oncogene. Carcinogenesis. 2015;36:1051–60. doi: 10.1093/carcin/bgv087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paraiso KH, Das Thakur M, Fang B, Koomen JM, Fedorenko IV, John JK, Tsao H, Flaherty KT, Sondak VK, Messina JL, Pasquale EB, Villagra A, Rao UN, Kirkwood JM, Meier F, Sloot S, Gibney GT, Stuart D, Tawbi H, Smalley KS. Ligand-independent EPHA2 signaling drives the adoption of a targeted therapy-mediated metastatic melanoma phenotype. Cancer Discov. 2015;5:264–273. doi: 10.1158/2159-8290.CD-14-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo Z, He B, Yuan L, Dai W, Zhang H, Wang X, Wang J, Zhang X, Zhang Q. Dual targeting for metastatic breast cancer and tumor neovasculature by EphA2-mediated nanocarriers. Int J Pharm. 2015;493:380–9. doi: 10.1016/j.ijpharm.2015.05.051. [DOI] [PubMed] [Google Scholar]
- 32.Charmsaz S, Beckett K, Smith FM, Bruedigam C, Moore AS, Al-Ejeh F, Lane SW, Boyd AW. EphA2 Is a Therapy Target in EphA2-Positive Leukemias but Is Not Essential for Normal Hematopoiesis or Leukemia. PLoS One. 2015;10:e0130692. doi: 10.1371/journal.pone.0130692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tatsumi T, Herrem CJ, Olson WC, Finke JH, Bukowski RM, Kinch MS, Ranieri E, Storkus WJ. Disease stage variation in CD4+ and CD8+ T-cell reactivity to the receptor tyrosine kinase EphA2 in patients with renal cell carcinoma. Cancer Res. 2003;63:4481–4489. [PubMed] [Google Scholar]
- 34.Herrem CJ, Tatsumi T, Olson KS, Shirai K, Finke JH, Bukowski RM, Zhou M, Richmond AL, Derweesh I, Kinch MS, Storkus WJ. Expression of EphA2 is prognostic of disease-free interval and overall survival in surgically treated patients with renal cell carcinoma. Clin Cancer Res. 2005;11:226–231. [PubMed] [Google Scholar]
- 35.Xu J, Zhang J, Cui L, Zhang H, Zhang S, Bai Y. High EphA2 protein expression in renal cell carcinoma is associated with a poor disease outcome. Oncol Lett. 2014;8:687–692. doi: 10.3892/ol.2014.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaenel P, Mosimann M, Andres AC. The multifaceted roles of Eph/ephrin signaling in breast cancer. Cell Adh Migr. 2012;6:138–147. doi: 10.4161/cam.20154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J, Zhuang G, Frieden L, Debinski W. Eph receptors and Ephrins in cancer: common themes and controversies. Cancer Res. 2008;68:10031–10033. doi: 10.1158/0008-5472.CAN-08-3010. [DOI] [PMC free article] [PubMed] [Google Scholar]


