Abstract
Aims: To investigate the situations of abnormal glucose metabolism and dysfunction of pancreatic islet beta cells in subjects of chronic hepatitis B (CHB) with cirrhosis. Methods: 106 hepatitis B virus (HBV) positive subjects with liver cirrhosis as well as with different grade of Child-Pugh and 37 healthy subjects were included in this study. The oral glucose tolerance test (OGTT), C-peptide and insulin release test were detected. Plasma glucose and insulin levels were analyzed periodically for 2 h after oral glucose loading. Results: There was no significant difference in the level of fasting plasma glucose and C-peptide between cirrhosis group and control group (P>0.05). The levels of OGTT 2 h glucose, insulin and C peptide were significantly higher in cirrhosis group than control group (P<0.01). Peak plasma glucose levels were obtained at 60 min in normal group and cirrhosis group. The peak insulin and C-peptide response occurred at 60 min in normal group, whereas it was delayed to 120 min in cirrhosis group. There was a significant difference between two groups in the pattern of plasma glucose levels at corresponding time points (P<0.05). The OGTT 2 h glucose and insulin levels were positively correlated with Child-Pugh Score (r1 = 0.389, r2 = 0.508, P<0.01). Conclusion: These findings implied that there was a certain degree of insulin resistance and abnormal glucose metabolism in the patients with liver cirrhosis.
Keywords: Glucose metabolism, insulin secretion, hepatitis virus, liver cirrhosis, oral glucose tolerance test
Introduction
The liver is the vital organ of body to maintain a relatively stability of the blood sugar levels, which has the capability of glycogen synthesis and decomposition. There is abnormal glucose metabolism when the liver cell damage occurs in the patients with cirrhosis. Hepatitis virus B (HBV) is the more common one of the causes of chronic liver diseases worldwide [1]. Chronic hepatitis B is an insidiously progressive form of liver disease, and it does not relentlessly but silently to cause cirrhosis and/or hepatocellular carcinoma over a period of 10-30 years. Recently, it have been reported that cirrhotic patients had a high prevalence of DM, in which most were associated with HBV/HCV infection. And the chronic HBV infection was associated with type 2 diabetes mellitus (T2DM) [2]. In cirrhotic patients with DM, the main cause of death is hepatic failure rather than the complications of DM. Moreover, it has been suggested that T2DM could promote the development of HCC and poor prognosis of liver transplantation. Thus, early intervention was necessary to prevent or improve T2DM to get good prognosis in HBV patients [3].
The 20% to 50% patients with liver cirrhosis showed impaired glucose tolerance and diabetes mellitus (DM). It has been suggested that the presence of DM had relationship with a poor prognosis in cirrhotic patients over a long follow up period [4]. It was still uncertain whether there is the prognostic significance of the presence of DM in cirrhosis. While strictly controlling blood glucose was crucial to the prognosis of diabetic patients [5]. However, glycaemic control is difficult in cirrhotic patients due to the malnutrition and their short life expectancy. Besides, several reports but no clinical data described the relationship between glycaemic control and the prognosis of cirrhotic patients.
On the basis, the aim of this study is to investigate the glucose metabolism and insulin secretion in subjects of chronic hepatitis B with cirrhosis. For this purpose, the level of fasting plasma glucose, C-peptide, HOMA-IR, insulin secretion, OGTT, OGTT 2 h glucose, insulin, C peptide were compared between cirrhosis group and the control group. The strict blood glucose control and infection control might be very important in prolonging the survival in compensated cirrhotic patients with DM.
Materials and methods
Subjects
106 HBV-infected individuals with cirrhosis and 37 healthy donors were enrolled in this study. The HBV-infected patients with cirrhosis were diagnosed with liver cirrhosis by laparoscopic and/or histological findings from December 2007 to January 2012 in the Department of Jiangyin Hospital Affiliated to Southeast University. There are 35 cases Child-Puge A, 30 cases Child-Puge B, 41 cases Child-Puge C in patients with cirrhosis. 25 of 41 cases liver cirrhosis with Child-Puge C conducted a second OGTT test after their condition improved (interval of at least half year ago). We obtained the consent of the blood donors or their guardians in a manner consistent with the policies of the appropriate local institutions. HBV-negative controls were healthy volunteers who were age-, sex-, body mass index, and ethnically-matched with cirrhosis group. A short history was obtained from all normal donors to ensure that they had no infectious disease in the past 3 months.
Clinical evaluation
All patients received comprehensive clinical and laboratory assessment. A 12-h over night fasting blood sample was collected at the time of biopsy to determine serum levels of ALT, total cholesterol, triglycerides and plasma glucose concentration. HBV-DNA was quantified by bDNA test. (Versant HBV3.0, Siemens Medical Solutions Diagnostics Europe, Dublin, Ireland; range 357 (R) C17857000 IU/ml).
The fasting plasma glucose, plasma insulin and C-peptide of blood samples were detected. Serum glucoses were determined by standard laboratory techniques in clinical. Serum insulin and C-peptide was determined using two-site Enzyme ELISA (Mercodia Sweden).
Insulin resistance was determined by the homeostasis model assessment (HOMA-IR) according to the formula: HOMA-IR [fasting glucose (millimoles per liter) × fasting insulin (milliunits per liter)]/22.5. Insulin secretion was assessed using the C-peptide/insulin ratio. Both models have been previously validated against clamp measurements [6,7].
Blood samples were collected at 0, 30, 60, 90 and 120 min for OGTT with 75 g of glucose; hepatic insulin extraction was assessed by calculating the molar ratio between C-peptide and insulin in the fasting state and at 2 h after OGTT and by the molar ratio of the incremental areas over 2 h between C-peptide and insulin. Total insulin and C-peptide secretion were calculated as the total area under the response curve for 2 h after OGTT.
Statistical analysis
The test results were expressed as mean ± standard deviation. The single-factor analysis of variance and linear correlation analysis was conducted using SPSS 10.0 statistical software, and P<0.05 was considered statistically significant.
Results
The clinical and bio-chemical features of patients in the study were shown in Table 1. Both groups were well-matched for age, sex, BMI, log10 HBV-DNA, alanine aminotransferase, cholesterol and triglyceride levels. There was no significant difference in these different characteristics among patients with chronic hepatitis B.
Table 1.
Demographical, laboratory, metabolic, virological and histological features of 106 patients with chronic hepatitis B
| Variable | CHB with cirrhosis | Child-Pugh A | Child-Pugh B | Child-Pugh C | Control | P value |
|---|---|---|---|---|---|---|
| Mean age (years) | 47.5±16.8 | 44.9±15.4 | 47.1±16.5 | 49.2±17.3 | 46.7±14.9 | 0.12 |
| Male/female | 81/25 | 27/8 | 23/7 | 31/10 | 29/8 | 0.45 |
| Log10HBV-DNA | 5.8±1.2 | 6.1±1.4 | 5.7±1.6 | 5.8±1.7 | - | - |
| Mean body mass index (kg/m2) | 22.5±2.1 | 21.3±1.9 | 20.9±2.3 | 23.1±2.3 | 0.30 | |
| Alanine aminotransferase | 56.3±25.2 | 72.6±30.0 | 96.4±55.8 | 25.1±13.6 | 0.36 | |
| Cholesterol (mmol/L) | 3.3±2.4 | 3.1±2.6 | 2.9±2.1 | 3.8±2.9 | 0.18 | |
| Triglycerides (mmol/L) | 1.3±0.4 | 1.2±0.5 | 1.1±0.7 | 1.3±0.6 | 0.13 |
Data are means ± SD.
Old life expectancy. Besides, the results of fasting plasma glucose, plasma insulin, C-peptide in different classification of Child-Pugh liver cirrhosis were shown in Table 2. There was no significant difference in the level of fasting plasma glucose and C-peptides between cirrhosis group and normal group. While there was a statistical significance in fasting plasma insulin between cirrhosis group and normal group (P<0.05). Besides, the cirrhosis group had statistically significant differences in HOMA-IR and insulin secretion comparing with normal group (P<0.05).
Table 2.
The level of fasting plasma glucose, plasma insulin, C-peptide in different classification of Child-Pugh liver cirrhosis
| Groups | n | Glucose (mmol/L) | Insulin (mU/L) | C-Peptides (pmol/L) | HOMA-IR | Insulin secretion |
|---|---|---|---|---|---|---|
| Cirrhosis | 106 | 4.65±1.74 | 19.27±6.21## | 490±146 | 3.98±1.89## | 25.43±10.55## |
| Child-Pugh A | 35 | 4.37±1.76 | 15.59±5.37# | 475±142 | 3.03±1.45# | 30.47±12.34# |
| Child-Pugh B | 30 | 4.50±1.69 | 16.71±5.75# | 483±137 | 3.25±1.58# | 28.9±10.68# |
| Child-Pugh C | 41 | 4.90±1.88 | 22.59±7.16## | 511±149 | 4.92±2.35## | 22.62±8.73## |
| Normal | 37 | 4.29±1.68 | 10.81±4.46 | 448±131 | 2.06±0.98 | 41.44±16.37 |
Data are means ± SD;
P<0.05 (compare with the normal group).
P<0.01 (compare with the normal group).
Results of oral glucose tolerance test were shown in Figure 1. Peak plasma glucose levels were obtained at 60 min in two groups (normal group and cirrhosis group). The peak insulin response occurred at 60 min in normal group, whereas it was delayed to 120 min in cirrhosis group. And the response pattern of C-peptide was similar to insulin, in which the curve reached the peak levels at 60 min in normal group and at 120 min in cirrhosis group. There was a significant difference between two groups in the pattern of plasma glucose levels at corresponding time points (P<0.05).
Figure 1.
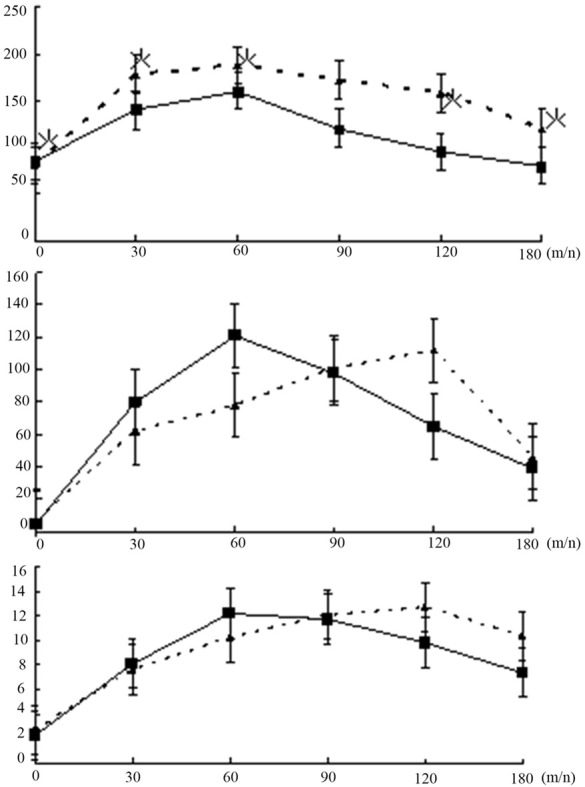
Plasma glucose (top panel), insulin (middle panel) and C-peptide (bottom panel) levels after an oral load of 75 g glucose in cirrhosis group (▲) and normal group (■). Data are mean ± standard deviation. *P<0.05; cirrhosis group vs. normal group.
The levels of OGTT 2 h glucose, insulin, C peptide were significantly higher in cirrhosis group than the control group (P<0.01), which were shown in Table 3.
Table 3.
The level of 2 h OGTT plasma glucose, plasma insulin, C-peptide in different classification of Child-Pugh liver cirrhosis
| Groups | n | Glucose (mmol/L) | Insulin (mU/L) | C-Peptides (pmol/L) |
|---|---|---|---|---|
| Cirrhosis | 106 | 8.09±2.71## | 71.87±27.51## | 2661±853## |
| Child-Pugh A | 35 | 6.91±2.17# | 60.13±21.84## | 2621±827## |
| Child-Pugh B | 30 | 7.68±2.35# | 63.89±23.21## | 2597±815## |
| Child-Puge C | 41 | 9.69±3.15## | 82.97±33.64## | 2759±902## |
| Normal | 37 | 5.79±2.01 | 40.11±15.65 | 1347±501 |
Data are means ± SD;
P<0.05 (compare with the normal group).
P<0.01 (compare with the normal group).
There was no significant difference in the plasma glucose, plasma insulin and C-peptide among different classifications of Child-Pugh and different time (P>0.05), and results were shown in Table 4. While, there were significant differences between Child-Pugh B/A and Child-Pugh C in fasting insulin, OGTT 2 h glucose and insulin (P<0.05). Furthermore, the OGTT 2h plasma glucose and insulin were positively correlated with the Child-Pugh Score (r1 = 0.389, r2 = 0.508, P<0.01).
Table 4.
Comparison of plasma glucose, plasma insulin, C-peptide on different classification of Child-Pugh and different time in 25 cases liver cirrhosis
| Child-Pugh | Glucose (mmol/L) | Insulin (mU/L) | C-Peptides (pmol/L) |
|---|---|---|---|
| The level of fasting | |||
| Child-Pugh C | 4.83±1.79 | 21.97±7.23 | 499±153 |
| Child-Pugh B/A | 4.47±1.69 | 17.13±6.47# | 481±137 |
| The level of 2 h OGTT | |||
| Child-Pugh C | 9.73±3.26 | 83.58±32.47 | 2803±887 |
| Child-Pugh B/A | 8.12±2.73# | 69.45±26.42# | 2672±867 |
Data are means ± SD;
P<0.05 (self-comparison).
Discussion
Steatosis and insulin resistance (IR) are the key conditions in patients with nonalcoholic fatty liver disease (NAFLD). Hepatic fat accumulation increases oxidative stress resulting in inflammation and fibrosis [8-10]. Large community-based studies have suggested that the prevalence of DM is much higher in HCV-infected patients than the general population as well as patients with other chronic liver diseases such as HBV and alcoholic liver disease [11]. Liver cirrhosis is a chronic disease based extensive damage, and easy to lead to abnormal glucose metabolism [12]. Previous studies showed that 60-80% of patients with cirrhosis occurred impaired glucose tolerance; about 10-15% of them might eventually develop into diabetes [13]. In our study, 67 cases of liver cirrhosis patients showed abnormal glucose tolerance, and 14 patients were diagnosed with diabetes. We also found that the abnormal glucose tolerance level was significantly higher in Child-Pugh C cirrhosis patients than Child-Pugh A grade and B grade group. Child-Pugh score was positively correlated with the OGTT 2 h glucose level.
In conclusion, DM was considered as a comorbid disease in HBV-related cirrhosis, and strict control of blood glucose levels could improve the survival rate of patients with HBV-related cirrhosis [14,15]. We suggested that individualized assessment of therapeutic benefits should be considered for patients with cirrhosis.
We also found that the Child-Pugh score were positively correlated with insulin levels in the patients with cirrhosis (P<0.01), and prompted Child classification was associated with insulin resistance. Insulin resistance was more obvious in patients with severe cirrhosis and liver damage. The possible mechanisms of insulin resistance in cirrhosis were as follow: (1) hyperinsulinemia: liver dysfunction in cirrhosis patients with insulin degradation could reduce portal blood flow of the liver cells, and reduce the uptake of insulin further then result in hyperinsulinemia. Data showed that shunt might improve glucose tolerance in patients after blocking. (2) insulin receptor abnormalities: impaired glucose tolerance have been reported in the cirrhosis patients. The number and affinity of insulin receptors of liver cells, muscle cells, fat cells decreased, which weakened the effects of peripheral insulin and caused the formation of insulin resistance; In addition, it was reported that serum soluble tumor necrosis factor receptor levels was higher in liver cirrhosis patients with insulin resistance, which suggested that tumor necrosis factor system might play an important role in the regulation of insulin activity.
C-peptide could directly reflect the islet β cell insulin production and secretion capacity, and it was barely affected by the liver and kidney function. Some researchers held that cirrhosis was a systemic disease, and hepatitis B virus might invade the pancreas, but there was no direct evidence that HBV could induce insulin secretion disorders. The results indicated that there was no significant difference in fasting C-peptide levels between patients with cirrhosis and control group. But glucose and C-peptide levels were significantly higher in cirrhosis group than normal group. Those suggested that insulin islet β-cell function had no significant obstruction, and viral factor was not an important factor of glucose metabolism disorders in cirrhosis patients.
In addition, the study found that there was no significant difference in the level of fasting glucose between cirrhosis group and control group, which reason might be that it was related with the decreased of hepatic glucose output. Therefore, patients with cirrhosis should be checked their fasting glucose and given OGTT test routinely to find glucose tolerance or diabetes early.
Besides, we also found that the peak insulin and C-peptide response occurred at 60 min in normal group, whereas it was delayed to 120 min in cirrhosis group. And there was a significant difference among two groups in the pattern of plasma glucose levels at corresponding time points (P<0.05) (Figure 1). And HOMA-IR in patients with chronic hepatitis B and cirrhosis was significantly higher, while their insulin secretion was higher than normal group. Insulin resistance increased with the development of glucose intolerance and the increase of cirrhosis degree (Table 2). These findings indicated that the glucose metabolism was abnormal in the patients with cirrhosis. And insulin resistance might play an important role in determining the magnitude of the pathogenesis of glucose intolerance.
In conclusion, there is a certain degree of insulin resistance and abnormal glucose metabolism and aggravation of liver damage in patients with liver cirrhosis. Therefore, it is very important to protect liver function early and effectively to prevent abnormal hepatic glucose metabolism.
Disclosure of conflict of interest
None.
References
- 1.Matsumoto N, Arase Y, Seko Y, Imai N, Kawamura Y, Sezaki H, Hosaka T, Akuta N, Kobayashi M, Kobayashi M, Suzuki Y, Saito S, Suzuki F, Ikeda K, Kumada H, Aida K, Kobayashi T. Prevalence and predictive factors of diabetes in hepatitis virus positive liver cirrhosis with fasting plasma glucose levelof <126 mg/dL. Hepatol Res. 2012;42:558–563. doi: 10.1111/j.1872-034X.2011.00957.x. [DOI] [PubMed] [Google Scholar]
- 2.Chao LT, Wu CF, Sung FY, Lin CL, Liu CJ, Huang CJ, Tsai KS, Yu MW. Insulin, glucose and hepatocellular carcinoma risk in male hepatitis B carriers: results from 17-yearfollow-up of apopulation-based cohort. Carcinogenesis. 2011;32:876–881. doi: 10.1093/carcin/bgr058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petta S, Cammà C, Di Marco V, Macaluso FS, Maida M, Pizzolanti G, Belmonte B, Cabibi D, Di Stefano R, Ferraro D, Guarnotta C, Venezia G, Craxì A. Hepatic steatosis and insulin resistance are associated with severe brosi in patients with chronic hepatitis caused by HBV or HCV infection. Liver Int. 2011;31:507–515. doi: 10.1111/j.1478-3231.2011.02453.x. [DOI] [PubMed] [Google Scholar]
- 4.Hung CH, Wang JH, Hu TH, Chen CH, Chang KC, Yen YH, Kuo YH, Tsai MC, Lu SN, Lee CM. Insulin resistance is associated with hepatocellular carcinoma in chronic hepatitis C infection. World J Gastroenterol. 2010;16:2265–2271. doi: 10.3748/wjg.v16.i18.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim K, Kim KH, Cheong J. Hepatitis B Virus X Protein Impairs Hepatic Insulin Signaling Through Degradation of IRS1 and Induction of SOCS3. PLoS One. 2010;5:e8649. doi: 10.1371/journal.pone.0008649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthews DR, Hosker JP, Rudenki AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and -cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 7.Kruszynska YT, Home PD, McIntyre N. Relationship between insulin sensitivity, insulin secretion and glucose tolerance in cirrhosis. Hepatology. 1991;14:103–111. doi: 10.1002/hep.1840140117. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Compean D, Jaquez-Quintana JO, Gonzalez-Gonzalez JA, Maldonado-Garza H. Liver cirrhosis and diabetes: Risk factors, pathophysiology clinical implications and management. World J Gastroenterol. 2009;15:280–288. doi: 10.3748/wjg.15.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon SY, Kim SS, Kwon OS, Kwon KA, Chung MG, Park DK, Kim YS, Koo YS, Kim YK, Choi DJ, Kim JH. Prognostic significance of glycaemic control in patients with HBV and HCV-related cirrhosis and diabetes mellitus. Diabet Med. 2005;22:1530–5. doi: 10.1111/j.1464-5491.2005.01687.x. [DOI] [PubMed] [Google Scholar]
- 10.Sedlaczek N, Hasilik A, Neuhaus P, Schuppan D, Herbst H. Focal over expression of insulin-like growth factor 2 by hepatocytes and cholangiocytes in viral liver cirrhosis. Br J Cancer. 2003;88:733–739. doi: 10.1038/sj.bjc.6600777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Megyesi C, Samols E, Marks V. Glucose tolerance and diabetes in chronic liver disease. Lancet. 1967;2:1051–1056. doi: 10.1016/s0140-6736(67)90334-0. [DOI] [PubMed] [Google Scholar]
- 12.Muting D, Wohlgemuth D, Dorsett R. Liver cirrhosis and diabetes mellitus. Geriatrics. 1969;24:91–99. [PubMed] [Google Scholar]
- 13.Gentile S, Loguercio C, Marmo R, Carbone L, Del Vecchio Blanco C. Incidence of altered glucose tolerance in liver cirrhosis. Diabetes Res Clin Pract. 1993;22:37–44. doi: 10.1016/0168-8227(93)90130-w. [DOI] [PubMed] [Google Scholar]
- 14.Vidal J, Ferrer JP, Esmatjes E, Salmeron JM, González-Clemente JM, Gomis R, Rodés J. Diabetes mellitus in patients with liver cirrhosis. Diabetes Res Clin Pract. 1994;25:19–25. doi: 10.1016/0168-8227(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 15.Mason AL, Lau JY, Hoang N, Qian K, Alexander GJ, Xu L, Guo L, Jacob S, Regenstein FG, Zimmerman R, Everhart JE, Wasserfall C, Maclaren NK, Perrillo RP. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;29:328–333. doi: 10.1002/hep.510290235. [DOI] [PubMed] [Google Scholar]


