Abstract
Endometrial implantation is the major cause of endometriosis (EMS). Matrix metalloproteinase (MMPs) can degrade multiple extracellular matrix and has been postulated to be related with EMC occurrence. This study thus investigated serum and ascites levels of MMP-9 in EMS patients, in an attempt to discuss the correlation between MMP-9 and EMS. A total of 100 EMS patients, including eutopic endometrium and ectopic endometrium, were recruited in this study along with hysteromyoma patients as the control group. Peripheral blood and ascites samples were collected and tested for MMP-9 levels using gelatin zymogram and enzyme-linked immunosorbent assay (ELISA). In EMS patients, MMP-9 levels in serum and ascites were 6.24±0.53 mM and 38.57±4.93 mM, respectively. Both of them were significantly higher than those in control group (P<0.05). Eutopic endometrium group had higher MMP-9 levels compared to those in ectopic endometrium ones (P<0.05). With advancement of disease stage, EMS patients had progressively elevated MMP-9 levels (P<0.05). Patients at proliferative stage had higher MMP-9 secretion (P<0.05). In summary, site of endometrium, clinical stage and proliferative cycle were independent risk factors for EMS. The elevation of serum and ascites MMP-9 existed in EMS patients, of which those had ectopic endometrium, advanced stage and at proliferative stage had higher MMP-9 expression.
Keywords: Endometriosis, serum, ascites, matrix metalloproteinase
Introduction
Endometriosis (EMS) is a common gynecologic disease. It is caused by the hyperplasia of proliferative endometrium beyond uterus body, and is manifested with abdominal pain, infertility and other symptoms [1-3]. The incidence of EMS has reached more than 20% in adult women and almost 30% in all patients under gynecologic surgery [4]. Although having certain malignancies including angiogenesis, ectopic invasion and distal metastasis under certain tumor metastasis related genes, EMS is still of benign nature [5]. Evidences have shown the correlation between matrix metalloproteinase (MMPs) and cell invasion or metastasis. MMP-9 was known to participate in both invasion and metastasis of various tumors, and has been suggested to be related with EMS pathogenesis [6]. This study thus quantified the expression level of MP-9 in both serum and ascites from EMS patients with various disease degrees, in order to analyze their correlations.
Materials and methods
Patient information
A total of 100 EMS patients (24~48 years old, average age =34.6 years) were recruited in this study between January 2014 and January 2015 in our hospital. Laparoscopic surgery was used to confirm the diagnosis of all patients, which were divided into eutopic endometrium (50 patients, aging between 25 and 45 years, average age =35.2 years, including 31 proliferative stage cases and 19 secretion stage) and ectopic endometrium group (50 patients, aging between 24 and 48 years, average age =36.1 years, including 30 proliferative and 20 secretion cases). Meanwhile another 50 cases of hysteromyoma patients (aging between 24 and 46 years, average age =34.6 years) were also recruited as the control group, which included 25 proliferative and 25 secretion stage cases. Based on the staging criteria of EMS as stipulated by American Fertility Society revised (AFS-r), there were 25, 35 and 40 cases of stage II, III and IV, respectively. All patients had no significant difference regarding age or disease type across groups (P>0.05) and were thus comparable.
Inclusive criteria
(1) Regular menstrual cycles; (2) No hormone medicines within recent three months; (3) No other benign or malignant lesion in ovary or uterus; (4) No severe liver/kidney dysfunction or autoimmune disease.
Exclusive criteria
(1) Irregular menstrual cycles; (2) At pregnancy or gestation; (3) Hyperplasia of endometrium or inflammatory lesion of uterus/cervical or other complicated gynecologic diseases; (4) Long-term taking of hormonal medicines; (5) Malignant diseases in ovary, oviduct or uterus.
Sample collection
All patients received elbow vein puncture for collecting peripheral blood samples (5 mL) before surgery. After centrifugation at 1,000 g for 10 min, serum was saved from the supernatant and stored.
Before surgery, peritoneal puncture was performed to collect 5 mL ascites fluid samples from retrouterine excavation and bladder-uterine excavation. Samples were then centrifuged for 10 min at 1,000 g, followed by the saving of supernatants.
During the surgery, endometrium tissues samples were collected. Samples were then fixed in 10% formalin and embedded in paraffin.
Gelatin zymography analysis
To determine the contents of MMP-9 in serum and ascites samples, gelatin zymography was performed. In brief, 10% SDS-PAGA was mixed with 1 mg/mL gelatin and added into the electrophoresis tank. Samples were diluted in 5% SDS-PAGA and buffer (0.4 M Tris-Cl, 8% SDS, 40% glycerol and 0.08% bromophenol blue). At 4°C, electrical fields (90 V for stacking gel and 120 V for separating gel) were applied. After electrophoresis, gel was rinsed, incubated for 1 hour, and digested at 37°C water-bath for 24 hours. coomassie brilliant blue was used to stain the band. Optical density (OD) values of each band were analyzed by Gel Doc system.
Enzyme-linked immunosorbent assay (ELISA)
ELISA was used to quantify MMP-9 levels. Serial diluted standards and samples were seeded into 96-well plate. After adding reagents, washing, chromogenic development and quenching as described in the manual instruction of test kit. The absorbance value at 450 nm was measured for each well. A linear regression was performed based on the standards for further deducing the concentration of test samples.
Statistical analysis
SPSS 17.0 software package was used to process all data, which were presented as mean ± standard deviation (SD). Two-sample comparison of means was performed by student t-test. Analysis of variance (ANOVA) was used to compare means across multiple groups, followed by SNK test. A statistical analysis was defined when P<0.05.
Results
MMP-9 levels by gelatin zymography
In gelatin zymography assay, we found weak MMP-9 bands (~92 KDa) in both serum and ascites samples. Compared to the control group, EMS patients had significantly elevated MMP-9 levels in both samples (P<0.05, Table 1; Figure 1).
Table 1.
Serum and ascites MMP-9 level
| Group | N | MMP-9 (mM) | |
|---|---|---|---|
|
| |||
| Serum | Ascites | ||
| EMS | 100 | 6.42±0.53* | 38.57±4.93* |
| Control | 50 | 1.21±0.51 | 19.98±3.47 |
| t value | 2.375 | 1.782 | |
| p value | 0.026 | 0.017 | |
P<0.05 compared to the control group.
Figure 1.
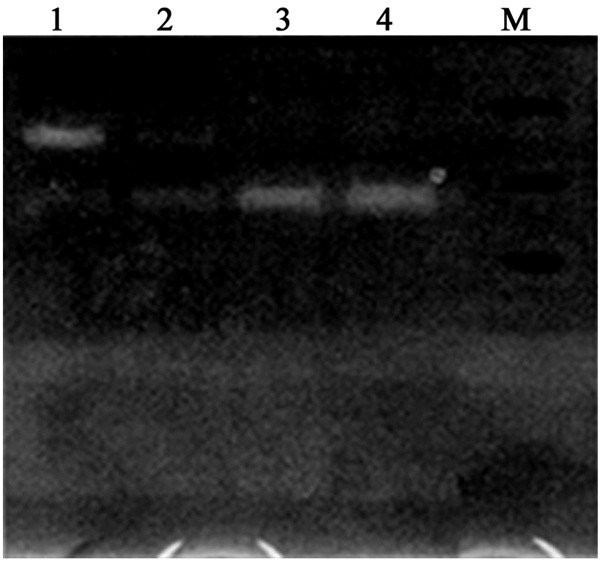
Gelatin zymography images of MMP-9. Lane 1, ascites of EMS group; Lane 2, ascites of control group; Lane 3, serum of EMS group; Lane 4, serum of control group. M, molecular weight markers.
ELISA assay for MMP-9 contents
Using ELISA for detecting ascites MMP-9 levels in all patients, we found significantly elevated expression in disease group compared to control group (P<0.05, Table 2). Compared to eutopic endometrium patients, ectopic ones had remarkably increased MMP-9 contents (P<0.05).
Table 2.
MMP-9 level by ELISA
| Group | N | MMP-9 (mM) | |
|---|---|---|---|
|
| |||
| Serum | Ascites | ||
| EMS | 100 | ||
| Ectopic | 50 | 2.51±0.53*,# | 38.65±5.39*,# |
| Eutopic | 50 | 2.12±0.32* | 34.12±4.22* |
| Control | 50 | 1.87±0.23 | 20.87±3.63 |
P<0.05 compared to the control group.
P<0.05 compared to eutopic endometrium patients.
MMP-9 level and differential stages of EMS
We further analyzed the MMP-9 level (by ELISA) in EMS patients with different clinical stages. It was found that besides the intra-subtype difference, patients with advanced stages had even higher MMP-9 levels compared to those at relatively primary stages, even in the same sub-type (P<0.05, Table 3).
Table 3.
MMP-9 levels in EMS patients at different clinical stages
| Stage | N | Ectopic endometrium MMP-9 (mM) | Eutopic endometrium MMP-9 (mM) | Control MMP-9 (mM) | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Serum | Ascites | Serum | Ascites | Serum | Ascites | ||
| I-II | 25 | 3.15±0.02* | 34.15±4.02* | 2.76±0.02 | 32.76±2.72* | 1.65±0.19 | 19.65±2.12 |
| III | 35 | 4.23±0.31* | 37.23±4.31* | 3.13±0.04* | 35.13±3.04* | 1.62±0.65 | 20.12±3.63 |
| IV | 40 | 5.98±0.78*,#,& | 39.98±5.78*,#,& | 4.22±0.47*,#,& | 37.22±3.87*,#,& | 1.74±0.37 | 21.24±3.02 |
P<0.05 compared to control group.
P<0.05 compared to eutopic endometrium patients.
P<0.05 compared to stage II and stage III ones in the same group.
MMP-9 contents during different proliferative cycles of EMS
As EMS patients can be at either proliferative stage or secretory stage, we compared MMP-9 levels. Results found significantly higher MMP-9 expression in both serum and ascites from patients at proliferative stage of ectopic endometrium (P<0.05, Table 4). Patients with eutopic endometrium, however, had similar MMP-9 levels at both proliferative and secretory stages.
Table 4.
MMP-9 levels across different proliferative stages of EMS patients
| Control | Serum MMP-9 (mM) | Ascites MMP-9 (mM) | ||
|---|---|---|---|---|
|
| ||||
| EMS | Proliferative stage | Secretory stage | Proliferative stage | Secretory stage |
| Ectopic | 3.85±0.36*,# | 3.71±0.26* | 36.46±3.25* | 34.71±3.01* |
| Eutopic | 3.34±0.25* | 3.43±0.21* | 33.21±3.12* | 33.13±3.21* |
| Control | 2.76±0.21 | 2.77±0.27 | 18.37±2.57 | 16.56±2.34 |
P<0.05 compared to control group.
P<0.05 compared to proliferative stage in the same sub-group.
Correlation between MMP-9 and EMS
We further analyzed the correlation between MMP-9 level and information of EMS patients including age, endometrium site, lesion range, clinical stage and proliferative cycles. Results showed significant correlation between MMP-9 and endometrium site, clinical stage and proliferative cycle (P<0.05, Table 5). Other parameters, such as age and lesion range, had no correlation with MMP-9.
Table 5.
MMP-9 level and clinical indexes of EMS
| Index/Factor | N | MMP-9 | χ2 | P value |
|---|---|---|---|---|
| Age | ||||
| ≤60 | 47 | 7 (14.89%) | ||
| >60 | 53 | 8 (15.09%) | 0.462 | >0.05 |
| Endometrium site | ||||
| Ectopic | 41 | 6 (14.63%) | <0.05 | |
| Eutopic | 32 | 5 (15.63%) | ||
| Lesion range | ||||
| Single | 62 | 6 (9.68%) | ||
| Multiple | 38 | 11 (28.95%) | 7.946 | >0.05 |
| Clinical stage | ||||
| I-II | 22 | 1 (4.55%) | ||
| III | 23 | 2 (8.69%) | ||
| IV | 55 | 13 (23.64%) | 18.243 | <0.05 |
| Proliferative cycle | ||||
| Proliferative | 5 | 2 (40%) | <0.05 | |
| Secretory | 95 | 14 (14.74%) | 21.674 |
MMP-9 and pathological parameters
A multi-variant analysis of Logistic test found that endometrium sites, clinical stage and proliferative cycle were all independent risk factors for MMP-9 expression in EMS patients (Table 6).
Table 6.
Multi-variant analysis between MMP-9 and EMS clinical features
| Observable index | Regression coefficient | P value | Relative risk |
|---|---|---|---|
| Endometrium site | 0.801 | 0.004 | 2.228 |
| Clinical stage | 1.275 | 0.001 | 2.852 |
| Proliferative cycle | 1.004 | 0.001 | 2.730 |
Discussion
The elevated incidence of EMS is one of major reasons causing female fertility nowadays. It is mainly manifested as the hyperplasia of endometrium tissues beyond normal uterus cavity, leading to a series of syndromes. About 30%~40% of EMS women suffer from infertility, thus comprising a public healthy issue [7,8]. The theory of retrograde menstruation proposed that part of detached endometrium tissues may back flow in the peritoneal cavity via oviduct, and implanted onto the surface of peritoneum for invasion of adjacent tissues accompanied with destruction and re-construction of extracellular matrix. Recent studies, however, found evidences against this theory as most adult women had retrograde menstruation but only a minor of them suffered from EMS. Even with peritoneal implantation of endometrium, the occurrence of EMS requires the de novo angiogenesis to provide sufficient blood flow for the further proliferation of the lesion [9,10].
EMS has a complex pathogenesis mechanism involving a progressively aggravation of ectopic tissue proliferation and invasion, including anti-apoptosis potency, degradation of extracellular matrix and elevation of adhesion molecules [11]. MMPs have been suggested to be involved in the ectopic implantation and invasion of endometrium tissues, as it can facilitate the degradation of extracellular matrix and help the penetration of basal membrane. As one important member of MMPs family, MMP-9 is a type of gelatin enzyme for degrading type III, type IV and type V collagen and elastin. It also exert important functions in facilitating neo-angiogenesis, which accelerates the selective expansion of vascular endothelial cells and increase blood flow, thus benefiting the invasive growth of ectopic endometrium [12-14]. In a word, MMP-9 plays a crucial role in both occurrence and progression of EMS.
In this study, we observed MMP-9 levels in both serum and ascites samples from EMS patients before the surgery, using both gelatin enzymography and ELISA approaches. Results showed significantly elevated MMP-9 contents in both ectopic and eutopic EMS patients. Further comparisons revealed higher MMP-9 levels in those patients with ectopic endometrium, and in those with advanced stages of disease. Proliferative stage patients also had higher MMP-9 in ectopic endometrium cases. The endometrium site, clinical stage and proliferative cycle were all found to be independent risk factors of MMP-9 expression. These results collectively suggested the presence of MMP-9 in both ascites fluids and serum of EMS patients. The MMP-9 synthesis is positively correlated with clinical stage. Past studies have revealed the correlation between pelvic focal inflammation and EMS, as the altered immune cell functions in peritoneal cavity [15]. MMP-9 is mainly synthesized and secreted from macrophages and neutrophil leukocytes [16]. Within peritoneal fluids, there were large amounts of macrophage-derived substances, especially in EMS patients whose macrophages are activated in the peritoneal cavity [17,18]. Therefore the elevated MMP-9 level in EMS patients’ peritoneal fluids is mainly due to the high percentage of local macrophages. The ectopic implanted endometrium, once adhesion to the peritoneum, can express large amounts of MMPs under the governing of autocrine and paracrine cytokines, thus increasing the degradation of extracellular matrix, contributing to the further growth of endometrium [19,20].
In summary, MMP-9 expression levels are potentiated in both serum and ascites of EMS patients, especially in those with ectopic endometrium with advanced stages or at proliferative stage. Such elevated MMP-9 level may be related to the ectopic implantation of endometrium and plays a critical role in EMS progression. Therefore, the timely detection of MMP-9 level and possible interference measures may provide novel target for EMS treatment in clinics.
Disclosure of conflict of interest
None.
References
- 1.Uzunlar O, Ozyer S, Engin-Ustun Y, Moraloglu O, Gulerman HC, Caydere M, Keskin SM, Mollamahmutoglu L. Effects of repeated propranolol administration in a rat model of surgically induced endometriosis. Eur J Obstet Gynecol Reprod Biol. 2014;182:167–71. doi: 10.1016/j.ejogrb.2014.09.025. [DOI] [PubMed] [Google Scholar]
- 2.Iarmolinskaia MI, Molotkov AS, Bezhenar’ VF, Shved NIu, Ivashchenko TE, Baranov VS. [Association of matrix metalloproteinases’ polymorphisms of MMP3 and MMP9 with development of genital endometriosis] . Genetika. 2014;50:230–5. [PubMed] [Google Scholar]
- 3.Williams KE, Miroshnychenko O, Johansen EB, Niles RK, Sundaram R, Kannan K, Albertolle M, Zhou Y, Prasad N, Drake PM, Giudice LC, Hall SC, Witkowska HE, Buck Louis GM, Fisher SJ. Urine, peritoneal fluid and omental fat proteomes of reproductive age women: Endometriosis-related changes and associations with endocrine disrupting chemicals. J Proteomics. 2015;113:194–205. doi: 10.1016/j.jprot.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antsiferova Y, Sotnikova N, Parfenyuk E. Different effects of the immunomodulatory drug GMDP immobilized onto aminopropyl modified and unmodified mesoporous silica nanoparticles upon peritoneal macrophages of women with endometriosis. Biomed Res Int. 2013;2013:924362. doi: 10.1155/2013/924362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiao L, Qi X, Lu G, Zhang Q, Zhang C, Gao J. Effect of traditional Chinese medicine (Xiaochaihu Tang) on the expression of MMP-2 and MMP-9 in rats with endometriosis. Exp Ther Med. 2013;6:1385–1389. doi: 10.3892/etm.2013.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuzaki S, Darcha C. In vitro effects of a small-molecule antagonist of the Tcf/ss-catenin complex on endometrial and endometriotic cells of patients with endometriosis. PLoS One. 2013;8:e61690. doi: 10.1371/journal.pone.0061690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han SJ, Hawkins SM, Begum K, Jung SY, Kovanci E, Qin J, Lydon JP, DeMayo FJ, O’Malley BW. A new isoform of steroid receptor coactivator-1 is crucial for pathogenic progression of endometriosis. Nat Med. 2012;18:1102–11. doi: 10.1038/nm.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malvezzi H, Aguiar VG, Paz CC, Tanus-Santos JE, Penna IA, Navarro PA. Increased circulating MMP-2 levels in infertile patients with moderate and severe pelvic endometriosis. Reprod Sci. 2013;20:557–62. doi: 10.1177/1933719112459234. [DOI] [PubMed] [Google Scholar]
- 9.Paul S, Bhattacharya P, Das Mahapatra P, Swarnakar S. Melatonin protects against endometriosis via regulation of matrix metalloproteinase-3 and an apoptotic pathway. J Pineal Res. 2010;49:156–68. doi: 10.1111/j.1600-079X.2010.00780.x. [DOI] [PubMed] [Google Scholar]
- 10.Becker CM, Louis G, Exarhopoulos A, Mechsner S, Ebert AD, Zurakowski D, Moses MA. Matrix metalloproteinases are elevated in the urine of patients with endometriosis. Fertil Steril. 2010;94:2343–6. doi: 10.1016/j.fertnstert.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 11.Singh AK, Chattopadhyay R, Chakravarty B, Chaudhury K. Altered circulating levels of matrix metalloproteinases 2 and 9 and their inhibitors and effect of progesterone supplementation in women with endometriosis undergoing in vitro fertilization. Fertil Steril. 2013;100:127–34. e1. doi: 10.1016/j.fertnstert.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Aresu L, Benali S, Giannuzzi D, Mantovani R, Castagnaro M, Falomo ME. The role of inflammation and matrix metalloproteinases in equine endometriosis. J Vet Sci. 2012;13:171–7. doi: 10.4142/jvs.2012.13.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Sanctis P, Elmakky A, Farina A, Caramelli E, Seracchioli R, Mabrouk M, Mignemi G, Venturoli S, Villa G, Guerrini M, Manuzzi L, Montanari G, Valvassori L, Zucchini C. Matrix metalloproteinase-3 mRNA: A promising peripheral blood marker for diagnosis of endometriosis. Gynecol Obstet Invest. 2011;71:118–23. doi: 10.1159/000320752. [DOI] [PubMed] [Google Scholar]
- 14.Mei J, Jin LP, Ding D, Li MQ, Li DJ, Zhu XY. Inhibition of IDO1 suppresses cyclooxygenase-2 and matrix metalloproteinase-9 expression and decreases proliferation, adhesion and invasion of endometrial stromal cells. Mol Hum Reprod. 2012;18:467–76. doi: 10.1093/molehr/gas021. [DOI] [PubMed] [Google Scholar]
- 15.Swarnakar S, Paul S. Curcumin arrests endometriosis by downregulation of matrix metalloproteinase-9 activity. Indian J Biochem Biophys. 2009;46:59–65. [PubMed] [Google Scholar]
- 16.Long L, Cao Y, Tang L. Transmembrane estrogen receptor GPR30 is more frequently expressed in malignant than benign ovarian endometriotic cysts and correlates with MMP-9 expression. Int J Gynecol Cancer. 2012;22:539–45. doi: 10.1097/IGC.0b013e318247323d. [DOI] [PubMed] [Google Scholar]
- 17.Weigel MT, Krämer J, Schem C, Wenners A, Alkatout I, Jonat W, Maass N, Mundhenke C. Differential expression of MMP-2, MMP-9 and PCNA in endometriosis and endometrial carcinoma. Eur J Obstet Gynecol Reprod Biol. 2012;160:74–8. doi: 10.1016/j.ejogrb.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 18.Saare M, Lamp M, Kaart T, Karro H, Kadastik U, Metspalu A, Peters M, Salumets A. Polymorphisms in MMP-2 and MMP-9 promoter regions are associated with endometriosis. Fertil Steril. 2010;94:1560–3. doi: 10.1016/j.fertnstert.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 19.Machado DE, Berardo PT, Palmero CY, Nasciutti LE. Higher expression of vascular endothelial growth factor (VEGF) and its receptor VEGFR-2 (Flk-1) and metalloproteinase-9 (MMP-9) in a rat model of peritoneal endometriosis is similar to cancer diseases. J Exp Clin Cancer Res. 2010;29:4. doi: 10.1186/1756-9966-29-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han YJ, Kim HN, Yoon JK, Yi SY, Moon HS, Ahn JJ, Kim HL, Chung HW. Haplotype analysis of the matrix metalloproteinase-9 gene associated with advanced-stage endometriosis. Fertil Steril. 2009;91:2324–30. doi: 10.1016/j.fertnstert.2008.03.047. [DOI] [PubMed] [Google Scholar]


