Abstract
Osteoporosis is one common disease in postmenopausal women due to depressed estrogen level. It has been known that inflammatory factors are involved in osteoporosis pathogenesis. One regulator of inflammatory cascade reaction, a7-nicotinic acetylcholine receptor (a7nAChR), therefore, may exert certain role in osteoporosis. This study thus investigated this question on an osteoporosis rat model after castration. Rats were firstly castrated to induce osteoporosis, and then received a7nAChR agonist (PNU-282987), diethylstilbestrol or saline via intraperitoneal injection. After 6 or 12 weeks, bone samples were collected for counting osteoblast number, bone density and estrogen receptor (ERα and ERβ) expression, in addition to the serum laboratory of inflammatory factors. Bone density, osteoclast number, ERα and ERβ expression level were significantly depressed in model group, and were remarkable potentiated in the drug treatment group (P<0.05). The levels of BGP and PTH in drug treatment group were decreased compared to diethylstilbestrol group, while E2 and IGF-1 showed up-regulation. Agonist of a7nAChR can up-regulate estrogen receptor expression and may prevent the occurrence and development of osteoporosis.
Keywords: a7nAChR agonist, osteoporosis, estrogen receptor
Introduction
With population aging, osteoporosis has become a severe public health issue to draw the interests of researchers and clinicians worldwide. As one bone disease, osteoporosis is typically manifested with compromised bone strength and elevated bone fracture risk. Estrogen has been known to work as one critical hormone during bone formation and growth, as it can accelerate the maturation of bones and maintain normal bone volume. The deficient of estrogen thus disrupt the dynamic balance between bone formation and reabsorption, causing higher incidence of osteoporosis. One factor mediating inflammatory cascade reaction, a7nAChR, may prevent osteoporosis via the binding with acetylcholine. This study thus generated an animal model with osteoporosis and targeted estrogen receptor, in an attempt to identify the potential effect of a7nAChR agonist in osteoporosis.
Materials and methods
Animals and treatment
A total of 40 female SD rats (4 months old, body weight: 220~250 g) were provided by Laboratory Animal Center of Ningxia Medical University. All animals were randomly divided into 4 groups. After intraperitoneal injection of 2% pentobarbital sodium (0.2 mL/100 g) for general anesthesia, the peritoneal cavity was exposed by a midline incision. Bilateral ovaries were then removed. The control group only underwent the surgical procedure but without the ovary removal. Penicillin sodium (0.5 mL per animal) was intraperitoneal injected before suture. Two days after the surgery, penicillin sodium was given daily. Rats were kept in cages with food and water ad libitum.
Rats were used for all experiments, and all procedures were approved by the Animal Ethics Committee of Ningxia Medical University.
The successful generation of osteoporosis model was deduced by bone density quantification after 2 month. At 10 weeks after the surgery, animals received a7nAChR agonist (PNU-282987, Santa Cruz, US) at 2.4 mg/kg daily via intraperitoneal injection, or diethylstilbestrol (0.05 mg/kg daily, Jinyao, China) via subcutaneous injection or equal volumes of saline by intraperitoneal injection. The drug intervention lasted for 12 weeks.
Sample collection
At 6 weeks or 12 weeks of drug treatment, rats were anesthetized. Bone density of the whole body was firstly quantified by X-ray bone densitometry, with a target area at 0.05 cm2 in triplicates. Blood samples were then collected from posterior femoral artery. After incubated for 1 hour, blood was centrifuged at 12000 g for 10 min to collect serum at the supernatants.
Under sterilized condition, bone samples were removed and cut into small pieces. After rinsing in penicillin-streptomycin, bone tissues were digested by 0.1% type I collagenase. Hank’s solution was then used to wash isolated cells, which were cultured in DMEM containing 10% fetal bovine serum (DMEM) at 104~105 per mL density. After incubation at 37°C with 5% CO2 perfusion, fibroblasts were removed. Osteoblasts were collected from the supernatants and quantified under a microscope.
Western blotting
Osteoblasts were collected for extracting total proteins. After quantification, proteins were separated under 8% SDS-PAGE, and were transferred to PVDF membrane. Blocking was performed under room temperature for 1 hour. Primary antibody, including rabbit anti-ERα (1:200, Santa Cruz, US), goat anti-ERβ (1:200, Santa Cruz, US) and goat anti-actin (1:500, Santa Cruz) were applied for overnight incubation at 4°C. After rinsing in TBST, anti-rabbit IgG or anti-goat IgG secondary antibody (1:2 000) was applied for 1-hour incubation at room temperature. Chromogenic substrates (Bio-Rad, US) were then added to develop the color, which was exposed and analyzed for optical density (OD) values.
Enzyme-linked immunosorbent assay (ELISA)
Serum samples were added into 96-well plate along with serially diluted standards. Serum level of BGP, estradiol (E2), parathyroid hormone (PTH) and insulin-like growth factor-1 (IGF-1) was quantified by ELISA kits following the manual instruction. Absorbance values at 450 nm were quantified by a microplate reader. The linear regression based on standard samples was used to deduce the sample concentration.
Statistical analysis
SPSS 17.0 software was used to process all collected data, which were presented as mean ± standard deviation (SD). Enumeration data were analyzed by chi-square test, while measurement data were compared by student t-test. A statistical significance was defined when P<0.05.
Results
Bone density
The osteoporosis model was successfully generated as the bone density of saline group at 6 week and 12 week was significantly lower than that in other two groups (P<0.05, Table 1). The drug treatment, however, remarkable increased bone density compared to those undergone diethylstilbestrol or saline treatment (P<0.05, Table 1).
Table 1.
Bone density of all rats
| Group | N | Bone density | |||
|---|---|---|---|---|---|
|
| |||||
| At week 6 | N | At week 12 | N | ||
| PNU-282987 | 10 | 0.21±0.12*,#,& | 5 | 0.18±0.07*,#,& | 5 |
| Diethylstilbestrol | 10 | 0.17±0.09*,& | 5 | 0.15±0.05*,& | 5 |
| Saline | 10 | 0.12±0.05& | 5 | 0.09±0.02& | 5 |
| Control | 10 | 0.31±0.17 | 5 | 0.31±0.16 | 5 |
P<0.05 compared to saline group;
P<0.05 compared to diethylstilbestrol group;
P<0.05 compared to control group.
Number of osteoblasts
The removal of ovaries significantly suppressed the number of osteoblasts. Drug treatment, however, significantly elevated number of osteoblasts when compared to diethylstilbestrol or saline group (P<0.05, Table 2).
Table 2.
Osteoblast number of all rats
| Group | N | Osteoblast number | |
|---|---|---|---|
|
| |||
| At week 6 | At week 12 | ||
| PNU-282987 | 10 | 29.11±3.11*,#,& | 27.55±2.37*,#,& |
| Diethylstilbestrol | 10 | 22.03±3.02*,& | 20.56±2.12*,& |
| Saline | 10 | 21.12±3.08& | 19.09±2.01& |
| Control | 10 | 33.56±4.58 | 33.57±4.66 |
P<0.05 compared to saline group;
P<0.05 compared to diethylstilbestrol group;
P<0.05 compared to control group.
Estrogen receptor expression level in osteoblasts
Using Western blot to quantify ERα and ERβ expression levels in osteoblasts from all rats, we found significantly elevated expression of estrogen receptors in drug intervention group compared to the other two groups, but was still lower than the control group (Table 3; Figure 1).
Table 3.
Estrogen receptor expression level in rat osteoblasts
| Group | N | At week 6 | At week 12 | ||
|---|---|---|---|---|---|
|
| |||||
| EGα | EGβ | EGα | EGβ | ||
| Saline | 10 | 133.71±4.28& | 129.82±4.12& | 124.32±4.26& | 120.12±4.05& |
| Control | 10 | 191.53±6.43 | 190.24±5.98 | 190.97±6.12 | 188.76±5.88 |
| PNU-282987 | 10 | 172.57±5.60*,#,& | 171.86±5.47*,#,& | 165.24±5.12*,#,& | 163.82±5.23*,#,& |
| Diethylstilbestrol | 10 | 141.83±4.12*,& | 134.75±4.24*,& | 139.26±4.33*,& | 133.72±4.11*,& |
P<0.05 compared to saline group;
P<0.05 compared to diethylstilbestrol group;
P<0.05 compared to control group.
Figure 1.
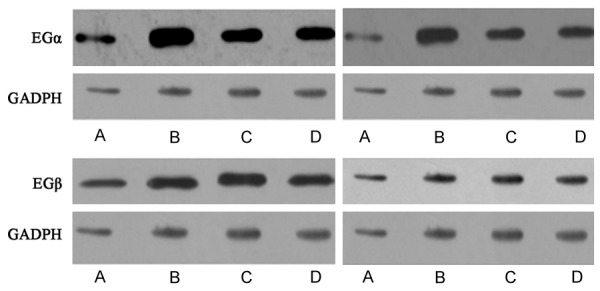
Estrogen receptor ERα (upper panels) and ERβ (low panels) expression level in rat osteoblasts. Left, 6 weeks; Right, 12 weeks. Lane A, saline group; Lane B, control group; Lane C, PNU-282987 group; Lane D, diethylstilbestrol group.
Serum BGP, E2, PTH and IGF-1 levels
Using ELISA to quantify serum levels of BGP, E2, PTH and IGF-1 in all rats, we found that BGP and PTH contents in drug treated group were significantly lower than those in diethylstilbestrol group, while E2 and IGF-1 levels were elevated (P<0.05, Table 4). All those indexes were higher in drug treatment group compared to control group.
Table 4.
Serum indexes
| Index | PNU-282987 (mmol/l) | Diethylstilbestrol (mmol/l) | Saline (mmol/l) | Control (mmol/l) |
|---|---|---|---|---|
| At week 6 | ||||
| BGP | 0.74±0.13*,#,& | 0.98±0.12*,& | 0.58±0.08& | 0.63±0.15 |
| E2 | 0.80±0.16*,#,& | 0.67±0.12*,& | 0.55±0.02& | 0.56±0.11 |
| PTH | 0.68±0.11*,#,& | 0.82±0.08*,& | 0.52±0.06& | 0.58±0.09 |
| IGF-1 | 0.76±0.02*,#,& | 0.61±0.04*,& | 0.48±0.02& | 0.56±0.06 |
| At week 12 | ||||
| BGP | 0.65±0.11*,#,& | 0.91±0.08*,& | 0.48±0.06& | 0.52±0.09 |
| E2 | 1.29±0.17*,#,& | 0.98±0.17*,& | 0.75±0.13& | 0.93±0.15 |
| PTH | 0.61±0.02*,#,& | 0.72±0.06*,& | 0.46±0.02& | 0.50±0.03 |
| IGF-1 | 1.12±0.05*,#,& | 0.97±0.08*,& | 0.81±0.09& | 0.98±0.07 |
P<0.05 compared to saline group;
P<0.05 compared to diethylstilbestrol group;
P<0.05 compared to control group.
Discussion
Osteoporosis has now become a worldwide healthy issue due to the aging of population [1,2]. As one systematic bone disease, osteoporosis is usually manifested with decreased bone volume and density, compromised bone strength, along with higher risk of bone fracture [3]. The occurrence of osteoporosis involves multiple factors. The definition of osteon has been suggested to include both osteoblasts and osteoclasts involving in the bone reformation [4]. Osteon accomplished the bone reformation cycle via bone transformation, which consists of cell activation, bone absorption and formation. As one important component of osteon, osteoblast plays a vital role in bone formation, modulating bone reabsorption, inhibiting differentiation of osteoclasts and accelerate their apoptosis [5]. Estrogen plays an important role in the development and maturation of bones in addition to modulating reproductive functions. For postmenopausal and ovary removal women, the rapid depressed on body’s estrogen level may lead to imbalance of bone metabolism, causing the degradation of bone structure, decreased bone volume and bone density, thus making higher incidence of bone fracture [6].
In this study, we generated an osteoporosis rat model by artificial castration. Specific agonist of a7nAChR, PNU-282927, was applied for intervention. We found significantly depressed bone density and osteoblast number in model group, suggesting the occurrence of osteoporosis at 6 weeks. The drug intervention significantly improved the bone density, and elevated ERα and ERβ expression levels when compared to those undergone saline or diethylstilbestrol treatment. These results suggested the hypothalamic inhibition of estrogen receptor expression by castration, while a7nAChR agonist can increase estrogen receptor expression, thus exerting its anti-osteoporosis role. Serum laboratory results showed elevated BGP and PTH, in addition to depressed E2 and IGF-1 levels in model group. These abnormalities, however, can be alleviated by PNU-282927 treatment. Previous studies have suggested the correlation between inflammatory factors and osteoporosis [7-10]. The nervous stimulation of certain components in cholinergic anti-inflammatory pathway thus can inhibit the production of pro-inflammatory cytokines without adverse effects of central nervous system [11-13]. Inflammation is related with vagus nerve, which consists of afferent and efferent nerves and can modulate body inflammation by hypothalamic-pituitary-adrenal gland axis [14,15]. The efferent cholinergic activity exerts certain modulatory effects on immune functions [16-18]. Among all agonists of a7nAChR, acetylcholine can be hydrolyzed by acetylcholine lipase to generate toxic nicotine, making it unfavorable as the drug candidate in clinics. PNU-282927 is one novel high-selective a7nAChR agonist [19,20]. This study illustrated the possible correlation between osteoporosis and decreased estrogen level. The agonist of a7nAChR has been shown to increase both bone density and osteoblast number, increase estrogen receptor expression, decrease pro-inflammatory cytokine levels and inhibit over-reactive inflammation, thus exerting certain role in preventing and/or treating osteoporosis.
In summary, the occurrence of osteoporosis is correlated with decreased estrogen level. The agonist o a7nAChR can increase estrogen receptor expression, suppress body’s inflammatory factor level, thus may be a candidate for preventing/treating osteoporosis. Results from this study may help us to explore novel ways of non-medication prevention and treatment of osteoporosis.
Acknowledgements
Ningxia Natural Science Foundation (NZ13188).
Disclosure of conflict of interest
None.
References
- 1.Kim MH, Choi YY, Han JM, Lee HS, Hong SB, Lee SG, Yang WM. Ameliorative effects of Schizandra chinensis on osteoporosis via activation of estrogen receptor (ER)-alpha/-beta. Food Funct. 2014;5:1594–601. doi: 10.1039/c4fo00133h. [DOI] [PubMed] [Google Scholar]
- 2.Soubhye J, Alard IC, van Antwerpen P, Dufrasne F. Type 2 17-beta hydroxysteroid dehydrogenase as a novel target for the treatment of osteoporosis. Future Med Chem. 2015;7:1431–56. doi: 10.4155/fmc.15.74. [DOI] [PubMed] [Google Scholar]
- 3.Ashraf A, Roshanzamir S, Bemana G, Mohammadi A, Jahani N, Naseri M. Sympathetic Skin Response and Vasomotor Symptoms in Postmenopausal Osteoporotic Women. Int J Community Based Nurs Midwifery. 2015;3:227–33. [PMC free article] [PubMed] [Google Scholar]
- 4.Miyakoshi N, Hongo M, Kobayashi T, Abe T, Abe E, Shimada Y. Improvement of spinal alignment and quality of life after corrective surgery for spinal kyphosis in patients with osteoporosis: a comparative study with non-operated patients. Osteoporos Int. 2015;26:2657–64. doi: 10.1007/s00198-015-3163-5. [DOI] [PubMed] [Google Scholar]
- 5.Kootala S, Ossipov D, van den Beucken JJ, Leeuwenburgh S, Hilborn J. Bisphosphonate-functionalized hyaluronic acid showing selective affinity for osteoclasts as a potential treatment for osteoporosis. Biomater Sci. 2015;3:1197–207. doi: 10.1039/c5bm00096c. [DOI] [PubMed] [Google Scholar]
- 6.Siddapur PR, Patil AB, Borde VS. Comparison of Bone Mineral Density, T-Scores and Serum Zinc between Diabetic and Non Diabetic Postmenopausal Women with Osteoporosis. J Lab Physicians. 2015;7:43–8. doi: 10.4103/0974-2727.151681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kusec V, Virdi AS, Prince R, Triffitt JT. Localization of estrogen receptor-alpha in human and rabbit skeletal tissues. J Clin Endocrinol Metab. 1998;83:2421–8. doi: 10.1210/jcem.83.7.4981. [DOI] [PubMed] [Google Scholar]
- 8.Mundy GR. Osteoporosis and inflammation. Nutr Rev. 2007;65:S147–51. doi: 10.1111/j.1753-4887.2007.tb00353.x. [DOI] [PubMed] [Google Scholar]
- 9.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 10.Pavlov VA, Tracey KJ. Controlling inflammation: the cholinergic anti-inflammatory pathway. Biochem Soc Trans. 2006;34:1037–40. doi: 10.1042/BST0341037. [DOI] [PubMed] [Google Scholar]
- 11.Rosas-Ballina M, Tracey KJ. Cholinergic control of inflammation. J Intern Med. 2009;265:663–79. doi: 10.1111/j.1365-2796.2009.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beekwilder JP, Beems T. Overview of the clinical applications of vagus nerve stimulation. J Clin Neurophysiol. 2010;27:130–8. doi: 10.1097/WNP.0b013e3181d64d8a. [DOI] [PubMed] [Google Scholar]
- 13.Huston JM, Gallowitsch-Puerta M, Ochani M, Ochani K, Yuan R, Rosas-Ballina M, Ashok M, Goldstein RS, Chavan S, Pavlov VA, Metz CN, Yang H, Czura CJ, Wang H, Tracey KJ. Transcutaneous vagus nerve stimulation reduces serum high mobility group box 1 levels and improves survival in murine sepsis. Crit Care Med. 2007;35:2762–8. doi: 10.1097/01.CCM.0000288102.15975.BA. [DOI] [PubMed] [Google Scholar]
- 14.La Marca R, Nedeljkovic M, Yuan L, Maercker A, Elhert U. Effects of auricular electrical stimulation on vagal activity in healthy men: evidence from a three-armed randomized trial. Clin Sci (Lond) 2010;118:537–46. doi: 10.1042/CS20090264. [DOI] [PubMed] [Google Scholar]
- 15.Thayer JF. Vagal tone and the inflammatory reflex. Cleve Clin J Med. 2009;76(Suppl 2):S23–6. doi: 10.3949/ccjm.76.s2.05. [DOI] [PubMed] [Google Scholar]
- 16.de Jonge WJ, van der Zanden EP, The FO, Bijlsma MF, van Westerloo DJ, Bennink RJ, Berthoud HR, Uematsu S, Akira S, van den Wijngaard RM, Boeckxstaens GE. Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat Immunol. 2005;6:844–51. doi: 10.1038/ni1229. [DOI] [PubMed] [Google Scholar]
- 17.Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623–8. doi: 10.1084/jem.20052362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghia JE, Blennerhassett P, Kumar-Ondiveeran H, Verdu EF, Collins SM. The vagus nerve: a tonic inhibitory influence associated with inflammatory bowel disease in a murine model. Gastroenterology. 2006;131:1122–30. doi: 10.1053/j.gastro.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 19.Johnston GR, Webster NR. Cytokines and the immunomodulatory function of the vagus nerve. Br J Anaesth. 2009;102:453–62. doi: 10.1093/bja/aep037. [DOI] [PubMed] [Google Scholar]
- 20.Iwasaki K, Hatip-Al-Khatib I, Egashira N, Akiyoshi Y, Arai T, Mishima K, Takagaki Y, Inui K, Fujiwara M. Ovariectomy combined with amyloid beta (1-42) impairs memory by decreasing acetylcholine release and alpha 7nAChR expression without induction of apoptosis in the hippocampus CA1 neurons of rats. Neurotox Res. 2004;6:299–309. doi: 10.1007/BF03033440. [DOI] [PubMed] [Google Scholar]


