Abstract
Sinus histiocytosis with massive lymphadenopathy is also known as Rosai-Dorfman disease (RDD) and is characterized by painless bilateral cervical lymphadenopathy. In the present case report, a 67-year-old Chinese woman presented with a 3-month history of progressive voice hoarseness, progressive dyspnea on exertion and a foreign body sensation. MRI revealed a lesion involving the right side of the paraglottic space. The lesion was totally resected. Based on the histologic features and immunoreactivity for the S-100 protein and CD68, a diagnosis of RDD was made. We described an extremely unique case of RDD that was observed in the paraglottic space and discussed its clinical and histopathologic features, differential diagnoses and treatment options.
Keywords: Laryngeal, paraglottic space, Rosai-Dorfman disease, sinus histiocytosis with massive lymphadenopathy
Introduction
RDD was first described in 1965 by Destombes and identified as a distinct clinicopathologic entity in 1969 by Rosai and Dorfman [1]. RDD is a rare, benign, idiopathic, histiocytic, proliferative, self-limiting disease that seriously affects healthy young adults and adolescents. This disease process is characterized by dilated lymph node sinuses that are filled with massive lymphocytes, plasma cells, and histiocytes that are typically accompanied by fever, polyclonal gammopathy, and leukocytosis with neutrophilia [2]. Histology and immunohistochemistry aid the differentiation of RDD from malignant disorders. The majority of cases present with hidden and atypical symptoms; thus, patients are easily misdiagnosed. The imaging features of extranodal RDD are nonspecific and definitive diagnosis almost always requires pathologic confirmation. Here, we report a case of a 67-year-old woman with RDD in the paraglottic space, which should be considered in the differential diagnosis of laryngeal lesions, and review the literature to understand the clinical behavior, imaging results, clinic-pathological features and treatment protocols of RDD.
Case report
A 67-year-old Chinese woman was referred to our Otolaryngology Head and Neck Surgery clinic for progressive voice hoarseness, progressive dyspnea on exertion, and a foreign body sensation. These symptoms persisted for 3 months but were not accompanied by weight loss. Symptoms, such as larynx pain, asthma, and dysphagia, were not reported by the patient. She had previously been in good health, and her history was free of smoking and drinking.
Her hematologic tests and physical examination were unremarkable; no cervical lymphadenopathy or fever was identified. Electronic laryngoscopy revealed a right smooth ventricular band mucosal swelling that protruded toward the airway covered the right vocal cord, and caused a severe restriction of the movement of the right vocal cord and ventriculus larynges to become shallow (Figure 1A). The other otolaryngological examination findings were within normal limits. Due to her hoarseness symptom, an enhanced computed tomographic (CT) scan of the neck and magnetic resonance imaging (MRI) of the larynx were ordered. The CT revealed a hypertrophy of a right ventricular band, a narrow pharyngolaryngeal cavity, and 3.0-cm solitary submucosal mass with the density of homogeneous soft tissue involving the right side of the paraglottic space that was associated with partial destruction of the thyroid cartilage and the cricoid ring (Figure 2A). MRI demonstrated the homogeneous thickening of a destructive mass lesion in the right side of the paraglottic space with a mild increase in metabolic activity. The lesion was enhanced with gadolinium-based contrast agents, was slightly hypo-intense on T1-weighted images (Figure 2B) and exhibited strong homogeneous enhancement on T2-weighted images (Figure 2C).
Figure 1.
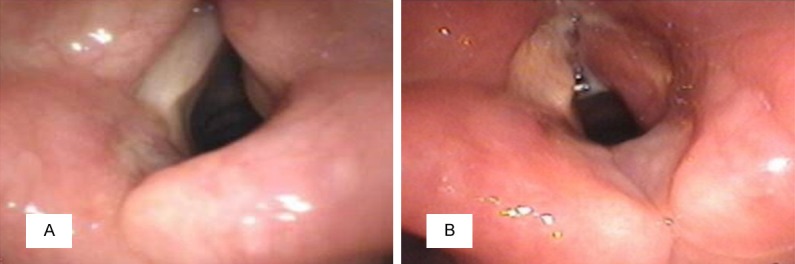
A. Electronic laryngoscopy revealed a right smooth ventricular band mucosal swelling that covered the right vocal cord, and caused a severe restriction of the movement of the right vocal cord and ventriculus larynges to become shallow. B. After operation, an electronic laryngoscopic image showed that the right vocal cord was exposed, the right vocal cord mucosal became hyperemia and swelling, bilateral ventriculus larynges size were basically identical and the movement of the right vocal cord was good.
Figure 2.
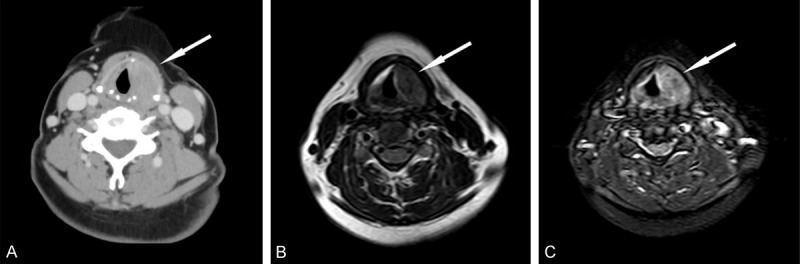
(A) CT images showed a solitary mass with the density of homogeneous soft tissue in the right paraglottic space. MRI images showed that a mass arised from the right side of the paraglottic space and exhibited a mild increase of metabolic activity, with the same level as the CT. (B) The lesion exhibited slight hypointensity on T1-weighted imaging and (C) strong homogeneous enhancement on T2-weighted imaging.
A biopsy of the mass prior to surgery failed. Due to the risk of the lesion causing significant edema that could have resulted in airway obstruction, we extracted the mass from the right paraglottic area with blunt dissection movements applied via the laryngofissure approach without damaging the laryngeal mucosal membrane after the intravenous induction of general anesthesia. The intraoperative findings revealed that the mass was located on the right side of the paraglottic space (Figure 3A), was gray-white in color and measured 3.5 cm × 3.0 cm × 3.0 cm totally (Figure 3B). Frozen sections of the mass confirmed the diagnosis of granulomatous inflammation without malignant cells.
Figure 3.
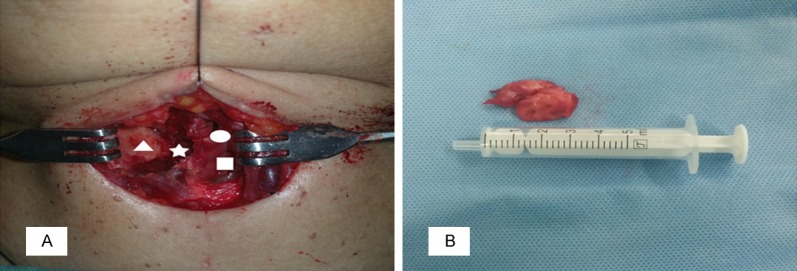
Intraoperative findings. A. View of surgical cavity, it showed that the mass was excised completely from the right side of the paraglottic space (asterisk), the paries medialis of the thyroid cartilage (triangle), the right ventricular band (circular) and the right vocal cord (square) were exposed. B. A part of the resected mass was whitish-grey in color, measured 3.5 cm × 3.0 cm × 3.0 cm in total.
On microscopic examination, numerous plasma cells and histiocytes were present and focally admixed with sparse eosinophils and neutrophils. The normal lymph node contained histiocytes, lymphocytes, and plasma cells (Figure 4A). Intermixed with the inflammatory cells and fibrotic collagen fibers were scattered large histiocytes. Postoperative pathologic examination with immunochemical staining revealed that the large histiocytes with emperipolesis were immunoreactive for the S-100 protein (Figure 4B), CD68 (Figure 4C) and lysozyme (Figure 4D) staining were positive, and CD1a staining was negative (Figure 4E). Based on the immunohistochemical findings and the morphologic features, a final diagnosis of RDD in the right paraglottic space was established, and no additional treatment was administered. Subsequent to post-operative anti-inflammatory and symptomatic treatment, an electronic laryngoscopic image showed that the right vocal cord was exposed, the right vocal cord mucosal became hyperemia and swelling, bilateral ventriculus larynges size were basically identical and the movement of the right vocal cord was good (Figure 1B).
Figure 4.
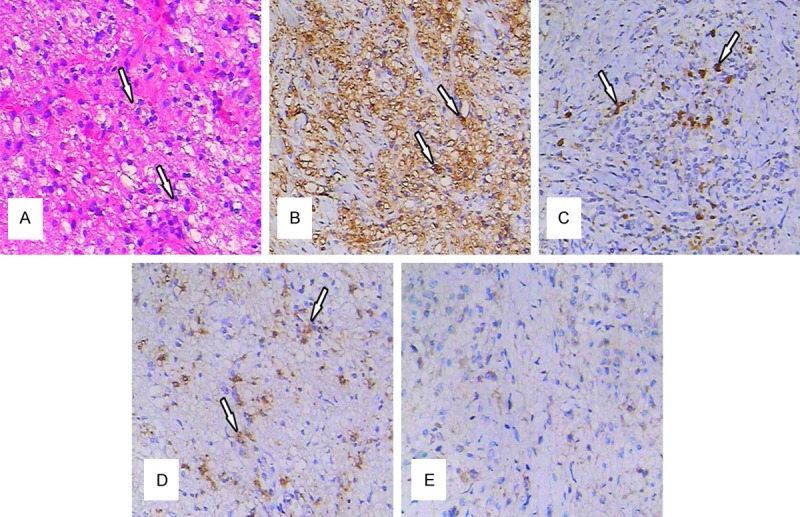
(A) Histopathological findings (H&E staining): The normal lymph node architecture was altered by a massive sinusoidal dilation of histiocytes in the inflammatory background (original magnification ×200). (B) Immunohistological findings: Some of the histiocytes exhibited S-100 (+) (original magnification ×200). (C) Immunohistological findings: Some of the histiocytes exhibited CD68 (+) (original magnification ×200). (D) Histiocytes exhibited immunoreactivity for lysozyme (+) (original magnification ×200) and (E) CD1a (-) (original magnification ×200).
Discussion
RDD is also known as sinus histiocytosis with massive lymphadenopathy and is generally a benign idiopathic histiocytic disorder with a distinctive clinical appearance and unique pathological features [3]. The disease can affect any age group, but the majority of reported cases have occurred in the first two decades of life [4]. Clinically, males are more likely to be affected with a male-to-female ratio of 5:1 [5]. Bilateral, massive and painless cervical lymphadenopathy is the most frequent initial symptom of the disease and is present in 87% of the cases [6]. Extranodal involvement was previously thought to be uncommon, but some more recent reports have suggested that extranodal involvement is present in approximately 25%-43% of cases [7-12]. Extranodal involvement most commonly includes the skin, soft tissues, upper respiratory tract, the ophthalmic, bone tissues, the central nervous system, the genitourinary tract, the liver, spleen, gastrointestinal tract and cardiac tissues. Lymph node involvement alone is found in 57% of patients [33]. To date, in the English literature, fewer than 30 cases of RDD in the larynx have been reported, and none of these cases occurred in the paraglottic space. This case might be the first reported case of RDD at the level of the paraglottic space.
Patients with masses of the upper respiratory tract may exhibit a variety of symptoms, such as foreign body sensation, dysphagia, dyspnea, voice changes, cough, and stridor. In this case, the patient had a lesion on the right side of the paraglottic space. Her primary symptoms were progressive voice hoarseness, dyspnea, and foreign body sensation, which were unobservable and atypical compared with the symptoms of other diseases. The current case was discovered incidentally. The majority of the masses in the larynx is strongly concerning in terms of larynx cancer and includes hemangiomas, lipomas, chondromas, Langerhans cell histiocytosis, and metastatic carcinomas. It is difficult to differentiate RDD from these conditions. Moreover, RDD results in slow-growing masses that are often present for long periods before becoming symptomatic. So RDD in the paraglottic space is easy to misdiagnose and missed diagnosis.
The pathogenesis of RDD remains still unknown, but several theories have been proposed. A disturbance of cell-mediated immunity, a primary viral infection, and autoimmune mechanisms have all been proposed as possible etiologies of this disorder [13-15]. Moreover, some infectious agents, such as Epstein-Barr virus and herpesvirus 6 have been identified in visceral and cutaneous lesions and related to the pathogenesis and clinical manifestations of RDD, although a viral genesis has not yet been confirmed [16,17]. At present, the general consensus favors the role of idiopathic histiocytosis in the development of RDD. The histiocytes in RDD are thought to represent an unusual type of activated macrophage. It has been suggested that a cytokine-mediated migration of monocytes may be involved in the accumulation and activation of histiocytes [4].
Regarding clinical behavior, RDD is generally considered to be benign process that is typically self-limiting, asymptomatic, naturally healing and eventually spontaneously resolving. Systemic therapy is not necessary. There is evidence that exclusive nodal involvement may not require therapy because this manifestation often spontaneously remits [18-20]. In the setting of RDD, 20% of cases exhibit spontaneous regression without therapy [21]. Nevertheless, some severe cases of RDD require abundant attention. The optimal therapy for RDD has yet to be determined because of studies of systematic treatments have been undertaken. The treatment strategy for RDD is based on the characteristics of the individual situation, such as the clinical manifestations and the extranodal involvement of the vital organs. A previous study revealed that the therapeutic modalities for RDD include corticosteroids (prednisolone), cryotherapy, radiotherapy, surgery (typically used for severe RDD-induced complication, such as massive adenopathy, airway obstruction or disfigurement), high-dose thalidomide and alkylating agents (e.g., cyclophosphamide) [22,23].
For patients with resectable lesions, surgical excision may relieve the compression of vital structures, alleviate local damage and preserve function. Surgery is the most effective method of treatment, and it is also essential for diagnosis [24,25]. In the present case, complete lump excision treatment might have been the best choice because the patient’s mass was located in the paraglottic space, which is a vital organ, and could have caused life-threatening complications, for example mass enlargement could have induced symptomatic complications such as upper airway obstruction. Although some complications exist, previous reports have demonstrated that complete surgical resection is the mainstay of therapy because it elicits symptom reversal, has a low rate of recurrence, and results in excellent long-term prognoses [26-28]. Aggressive behavior and mortality are rarely observed after the resection of these lesions.
It is difficult to distinguish many of laryngeal masses from each other based on clinical appearance and imaging. The diagnosis is more complex when the right paraglottic space is the only organ affected and there is no lymph node involvement. On imaging, the lesions are similar to laryngeal carcinomas and appear as homogeneous, lobulated, and isointense lesions in the right paraglottic space that exhibit strong homogeneous enhancement on T2-weighted images. However, histology and immunohistochemistry can help distinguish RDD from other malignant disorders. RDD is characterized by a variety of chronic inflammatory cells that are dominated by lymphocytes and plasma cells [29-31]. There are also scattered and abundant foamy macrophages and histiocytes engulf lymphocytes, plasma cells and polymorphonuclear leucocytes, and emperipolesis may occur [32]. Emperipolesis signifies the phagocytosis of autologous lymphocytes and is characteristic of RDD but is typically difficult to appreciate in extranodal lesions because there is usually an increased level of fibrosis that may obscure the histiocytes, which are present in only 70% of cases [33]. Typically, the histiocytes stain positive for the S-100 protein and CD68, and negative staining for CD1a can confirm the diagnosis of RDD.
In summary, we described a rare case of extra-nodal RDD that arose primarily in the paraglottic space. Although it is extremely uncommon, all otolaryngology surgeons should be aware of RDD in the larynx. RDD has an atypical clinical presentation and thus may easily be misdiagnosed. The present cases could have progressed to cause life-threatening conditions. It is important to recognize and distinguish RDD from other tumors that occur in this anatomic region. Therefore, a high degree of suspicion and a thorough pathological review are necessary to diagnose this rare clinical entity. Accurate diagnosis of this lesion may prevent unnecessary extensive work-ups for other diseases and prevent over treatment.
Disclosure of conflict of interest
None.
References
- 1.Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy. A newly recognized benign clinicopathological entity. Arch Pathol. 1969;87:63–70. [PubMed] [Google Scholar]
- 2.Becker M, Gaiser T, Middel P, Rompel R. Clinicopathologic challenge. Destombes-Rosai-Dorfman disease (DRDD) (sinus histiocytosis with massive lymphadenopathy) Int J Dermatol. 2008;47:125–127. doi: 10.1111/j.1365-4632.2008.03376.x. [DOI] [PubMed] [Google Scholar]
- 3.Cohen-Barak E, Rozenman D, Schafer J, Krausz J, Dodiuk-Gad R, Gabriel H, Shani-Adir A. An unusual co-occurrence of Langerhans cell histiocytosis and Rosai-Dorfman disease: report of a case and review of the literature. Int J Dermatol. 2014;53:558–563. doi: 10.1111/ijd.12051. [DOI] [PubMed] [Google Scholar]
- 4.Foucar E, Rosai J, Dorfman RF. Sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): review of the entity. Semin Diagn Pathol. 1990;7:19–73. [PubMed] [Google Scholar]
- 5.Landim FM, Rios Hde O, Costa CO, Feitosa RG, Rocha Filho FD, Costa AA. Cutaneous Rosai-Dorfman disease. An Bras Dermatol. 2009;84:275–278. doi: 10.1590/s0365-05962009000300010. [DOI] [PubMed] [Google Scholar]
- 6.Chang LY, Kou T, Chan HL. Extranodal Rosai-Dorfman disease with cutaneous. ophthalmic and laryngeal involvement: report of a case treated with isotretinoin. Int J Dermatol. 2002;41:888–891. doi: 10.1046/j.1365-4362.2002.01675.x. [DOI] [PubMed] [Google Scholar]
- 7.Eiras Jda C, Schettini AP, Lima LL, Tubilla LH, Oliveira RM. Cutaneous Rosai-Dorfman disease: a case report. An Bras Dermatol. 2010;85:687–690. doi: 10.1590/s0365-05962010000500014. [DOI] [PubMed] [Google Scholar]
- 8.Wang KH, Chen WY, Liu HN, Huang CC, Lee WR, Hu CH. Cutaneous Rosai-Dorfman disease: clinicopathological profiles, spectrum and evolution of 21 lesions in six patients. Br J Dermatol. 2006;154:277–286. doi: 10.1111/j.1365-2133.2005.06917.x. [DOI] [PubMed] [Google Scholar]
- 9.Shi XY, Ma DL, Fang K. Cutaneous Rosai-Dorfman disease presenting as a granulomatous rosacea-like rashes. Chin Med J (Engl) 2011;124:793–794. [PubMed] [Google Scholar]
- 10.Gaitonde S. Multifocal, extranodal sinus histiocytosis with massive lymphadenopathy: an overview. Arch Pathol Lab Med. 2007;131:1117–1121. doi: 10.5858/2007-131-1117-MESHWM. [DOI] [PubMed] [Google Scholar]
- 11.Lauwers GY, Perez-Atayde A, Dorfman RF, Rosai J. The digestive system manifestations of Rosai-Dorfman disease (sinus histiocytosis with massive lymphadenopathy): review of 11 cases. Hum Pathol. 2000;31:380–385. doi: 10.1016/s0046-8177(00)80254-3. [DOI] [PubMed] [Google Scholar]
- 12.Nathwani RA, Kenyon L, Kowalski T. Rosai-Dorfman disease of the colon. Gastrointest Endosc. 2008;68:194–196. doi: 10.1016/j.gie.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Levine PH, Jahan N, Murari M, Manak M, Jaffe ES. Detection of human herpesvirus 6 in tissues involved by sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease) J Infect Dis. 1992;166:291–295. doi: 10.1093/infdis/166.2.291. [DOI] [PubMed] [Google Scholar]
- 14.Tsang WY, Yip TT, Chan JK. The Rosai-Dorfman disease histiocytes are not infected by Epstein-Barr virus. Histopathology. 1994;25:88–90. doi: 10.1111/j.1365-2559.1994.tb00604.x. [DOI] [PubMed] [Google Scholar]
- 15.Mehraein Y, Wagner M, Remberger K, Füzesi L, Middel P, Kaptur S, Schmitt K, Meese E. Parvovirus B19 detected in Rosai-Dorfman disease in nodal and extranodal manifestations. J Clin Pathol. 2006;59:1320–1326. doi: 10.1136/jcp.2005.029850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Setareh M, Zahra M, Vahid M, Farah S. Generalized lymphadenopathy in infancy: A case report. Iran J Pediatr. 2013;23:105–108. [PMC free article] [PubMed] [Google Scholar]
- 17.Pinto DC, Vidigal Tde A, Castro BD, Santos BH, Ousa NJ. Rosai-Dorfman disease in the differential diagnosis of cervical lymphnode. Braz J Otorhinolaryngol. 2008;74:632–635. doi: 10.1016/S1808-8694(15)30616-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long E, Lassalle S, Cheikh-Rouhou R, Hofman V, Lacour JP, Hofman P. Intestinal occlusion caused by Rosai-Dorfman disease mimicking colonic diverticulitis. Pathol Res Pract. 2007;203:233–237. doi: 10.1016/j.prp.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 19.Lauwers GY, Perez-Atayde A, Dorfman RF, Rosai J. The digestive system manifestations of Rosai-Dorfman disease (sinus histiocytosis with massive lymphadenopathy): review of 11 cases. Hum Pathol. 2000;31:380–385. doi: 10.1016/s0046-8177(00)80254-3. [DOI] [PubMed] [Google Scholar]
- 20.Shukla D, Veillon DM, Abreo F, Cotelingam JD. Pathologic quiz case: a 55-year-old woman with a history of treated Hodgkin disease and a persistent abdominal mass: extranodal gastrointestinal Rosai-Dorfman disease. Arch Pathol Lab Med. 2003;127:1527–1528. doi: 10.5858/2003-127-1527-PQCAYW. [DOI] [PubMed] [Google Scholar]
- 21.Lima FB, Barcelos PS, Constâncio AP, Nogueira CD, Melo-Filho AA. Rosai-Dorfman disease with spontaneous resolution: case report of a child. Rev Bras Hematol Hemoter. 2011;33:312–314. doi: 10.5581/1516-8484.20110083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becker MR, Gaiser T, Middel P, Rompel R. Clinicopathologic challenge. Destombes-Rosai-Dorfman disease (DRDD) (sinus histiocytosis with massive lymphadenopathy) Int J Dermatol. 2008;47:125–127. doi: 10.1111/j.1365-4632.2008.03376.x. [DOI] [PubMed] [Google Scholar]
- 23.Chan CC, Chu CY. Dapsone as a potential treatment for cutaneous Rosai-Dorfman disease with neutrophilic predominance. Arch Dermatol. 2006;142:428–430. doi: 10.1001/archderm.142.4.428. [DOI] [PubMed] [Google Scholar]
- 24.Adeleye AO, Amir G, Fraifeld S, Shoshan Y, Umansky F, Spektor S. Diagnosis and management of Rosai-Dorfman disease involving the central nervous system. Neurol Res. 2010;32:572–578. doi: 10.1179/016164109X12608733393836. [DOI] [PubMed] [Google Scholar]
- 25.Zhang JT, Tian HJ, Lang SY, Wang XQ. Primary intracerebral Rosai-Dorfman disease. J Clin Neurosci. 2010;17:1286–1288. doi: 10.1016/j.jocn.2010.01.048. [DOI] [PubMed] [Google Scholar]
- 26.Pulsoni A, Anghel G, Falcucci P, Matera R, Pescarmona E, Ribersani M, Villivà N, Mandelli F. Treatment of sinus histiocytosis with massive lymphadenopathy (Rosai-Dorfman disease): report of a case and literature review. Am J Hematol. 2002;69:67–71. doi: 10.1002/ajh.10008. [DOI] [PubMed] [Google Scholar]
- 27.Purav P, Ganapathy K, Mallikarjuna VS, Annapurneswari S, Kalyanaraman S, Reginald J, Natarajan P, Bapu KR, Balamurugan M. Rosai-Dorfman disease of the central nervous system. J Clin Neurosci. 2005;12:656–659. doi: 10.1016/j.jocn.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Forest F, N’Guyen AT, Fesselet J, Metellus P, Bouvier C, de Paula AM, Roche PH, Figarella-Branger D. Meningeal Rosai-Dorfman disease mimicking meningioma. Ann Hematol. 2014;93:937–940. doi: 10.1007/s00277-013-1994-8. [DOI] [PubMed] [Google Scholar]
- 29.Hinduja A, Aguilar LG, Steineke T, Nochlin D, Landolfi JC. Rosai-Dorfman disease manifesting as intracranial and intraorbital lesion. J Neurooncol. 2009;92:117–120. doi: 10.1007/s11060-008-9733-z. [DOI] [PubMed] [Google Scholar]
- 30.Sundaram C, Uppin SG, Prasad BC, Sahu BP, Devi MU, Prasad VS, Purohit AK. Isolated Rosai Dorfman disease of the central nervous system presenting as dural-based and intraparenchymal lesions. Clin Neuropathol. 2005;24:112–117. [PubMed] [Google Scholar]
- 31.Johnson MD, Powell SZ, Boyer PJ, Weil RJ, Moots PL. Dural lesions mimicking meningiomas. Hum Pathol. 2002;33:1211–1226. doi: 10.1053/hupa.2002.129200. [DOI] [PubMed] [Google Scholar]
- 32.Andriko JA, Morrison A, Colegial CH, Davis BJ, Jones RV. Rosai-Dorfman disease isolated to the central nervous system: a report of 11 cases. Mod Pathol. 2001;14:172–178. doi: 10.1038/modpathol.3880278. [DOI] [PubMed] [Google Scholar]
- 33.Favara BE, Feller AC, Pauli M, Jaffe ES, Weiss LM, Arico M, Bucsky P, Egeler RM, Elinder G, Gadner H, Gresik M, Henter JI, Imashuku S, Janka-Schaub G, Jaffe R, Ladisch S, Nezelof C, Pritchard J. Contemporary classification of histiocytic disorders. The WHO Committee On Histiocytic/Reticulum Cell Proliferations. Reclassification Working Group of the Histiocyte Society. Med Pediatr Oncol. 1997;29:157–166. doi: 10.1002/(sici)1096-911x(199709)29:3<157::aid-mpo1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]


