Abstract
Behçet’s disease (BD)-like syndrome is an extremely rare situation occurred after Mycobacterium tuberculosis infection and virus infection. We reported a 45-year-old woman who visited our hospital complaining of swollen left ankle, painful genital ulcer, redness in the left eye and skin rash on lower limbs. The patient had a history of pleural tuberculosis and had received anti-tuberculous therapy for one year. Her left cervical lymph node sample demonstrated tubercle bacilli DNA fragmentation. The diagnosis of tuberculous lymphadenitis and Behçet’s disease (BD)-like syndrome were made. This patient’s symptoms remitted following treatment with anti-tuberculous therapy. This case indicates that some microbial infection can trigger the onset of BD-like syndrome in genetically susceptible subjects. However, treatment strategy of BD-like syndrome secondary to infection is totally different from primary BD. The aim of this case report is to present our experience of the different clinical signs and treatment of BD-like syndrome to expedite its early diagnosis in future. Combination of clinical, radiological, immunophenotypic, pathological, and genetic data contribute to improving the rate of diagnosis.
Keywords: Behçet’s disease, Behçet’s disease-like syndrome, Mycobacterium tuberculosis, virus
Introduction
Recurrent oral ulceration, genital ulceration and cutaneous lesions, such as erythema nodosum (EN)-like lesion, are the characterized symptoms of Behçet’s disease (BD) [1]. It is noted that these symptoms could be observed in some microbial infection, particularly in Mycobacterium tuberculosis infection and virus infection. These conditions are called BD-like syndrome secondary to infection. It is resolved by anti-microbial infection treatment instead of corticosteroid and immunosuppressor therapy. Here, we report a case of BD-like syndrome secondary to Mycobacterium tuberculosis infection. Furthermore, we review the literature regarding the association between BD-like syndrome and microbial infection.
Case report
A 45-year-old woman visited our hospital complaining of swollen left ankle, painful genital ulcer and skin rash on lower limbs for twenty days. The patient had a history of pleural tuberculosis three years ago, and had received anti-tuberculous therapy for one year. However, in this consultation, she had no symptoms of fever, chest pain and cough, but did have the symptoms of weight loss and night sweating. On physical examination, vital signs were normal. Redness was observed in her left eye. No oral ulceration was detected. Several enlarged lymph nodes were noted in her left ear posterior, left cervical and right inguinal regions. EN-like skin lesions were found on her both lower legs, distributed on extensor and flexion surface. Arthritis in the left ankle joint was observed (Figure 1). A painful genital ulcer (10 mm×10 mm) with white secretions was detected on the right labia minora. Pathergy testing was positive.
Figure 1.
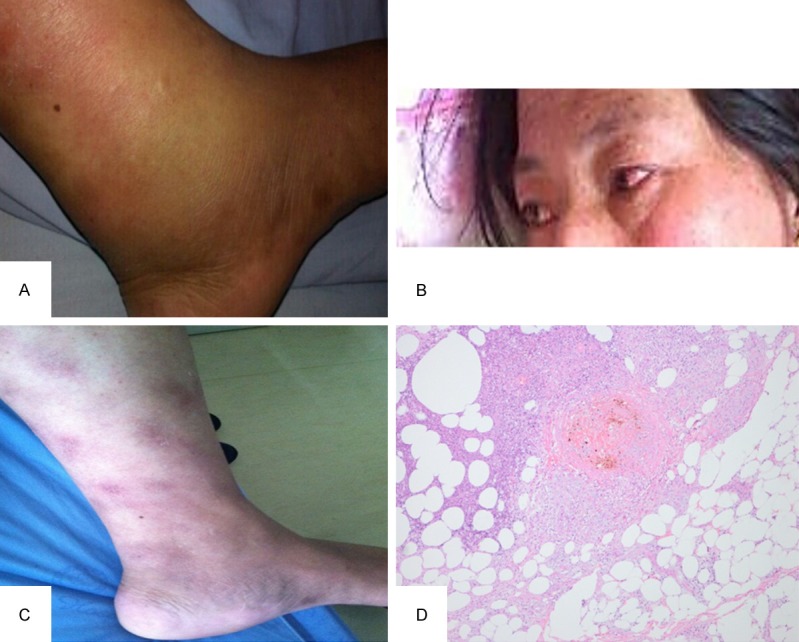
A. Arthritis in the left ankle joint was observed; B. Uveitis was observed in her left eye; C. Erythema nodosum-like skin lesions were found on the lower legs; D. Skin lesion biopsy shows vasculitis in the dermis, subcutaneous thromboangiitis obliterans, fat necrosis and granuloma formation. High-power (original magnification, ×400) magnification.
Laboratory examinations revealed the normal blood test, but the elevated level of C-reactive protein 8.22 mg/L (normal range: <5 mg/L) and erythrocyte sedimentation rate 42.0 mm/h (normal range: <26 mm/h). The antinuclear antibody (ANA) titer was not beyond the critical value, at 1:100. Slightly elevated level was found in rheumatoid factor (RF) of 111.00 IU/ml (normal range <20 IU/ml)). Other assays for anti-extractable nuclear antigen (ENA) antibodies, anti-cyclic citrullinated peptide (CCP) antibodies and anti-keratin (AKA) antibody were negative. The human leukocyte antigen (HLA) type was B27. There were no abnormalities in other serological tests. The Chest computed tomography demonstrated that there was former tuberculous pleurisy with pleural thickening and enlarged calcification of mediastinal pretracheal lymph node. In addition, both PPD skin test and interferon-γ releasing assay by means of QuantiFERONTB gold showed positive results. The biopsy of one EN-like lesion on her right lower limb was performed. The specimen showed the predominant epidermal hyperkeratosis and proliferation of spinous layer without liquefactive degeneration of the basal layer. Vasculitis was noted in the dermis and subcutaneous layer with thrombosis angiitis, necrotic adipocytes and lipogranuloma formation. Also, the histopathology of her left cervical lymph node showed tuberculoid granuloma with caseation, in which Ziehl-Neelsen staining was positive for acid-fast bacilli. Quantitative polymerase chain reaction (qPCR) assay for her lymph node sample demonstrated tubercle bacilli DNA fragmentation (Figure 2).
Figure 2.
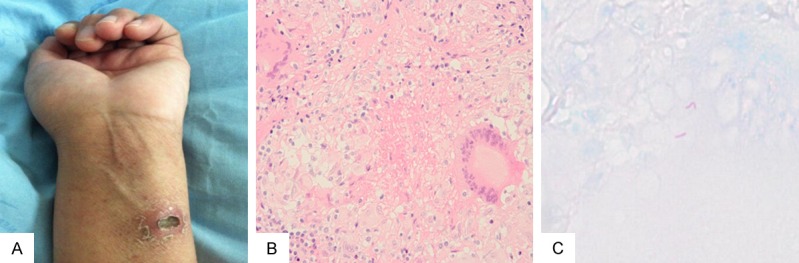
A. PPD skin testing shows positive results. B. Lymph node biopsy from the left cervical lymph node shows chronic granulomatous inflammation with necrosis (HE ×200). C. Ziehl-Neelsen staining revealing a few acid-fast bacilli.
The diagnosis of tuberculous lymphadenitis and BD-like syndrome were made. The patient was treated with quadruple anti-tuberculous chemotherapy, comprising of rifapentine, isoniazid, ethambutol, pyrazinamide. During one year follow-up, her symptoms have completely resolved and have no recurrence under antituberculous treatment.
Discussion
The present case is characterized by genital ulcers, uveitis, arthritis, and ED-like skin lesions. These features as well as a positive result on pathergy testing closely resemble BD. However, this case is not primary BD. The diagnosis of tuberculous lymphadenitis and BD-like syndrome was established for her based on the following points: First, she had the history of pleural tuberculosis which was convinced by her current chest CT; Secondly, her PPD skin test and interferon-γ releasing assay by means of QuantiFERONTB gold showed strongly positive results. The histopathology of her cervical swollen lymph nodes showed chronic granulomatous inflammation with necrosis, with Ziehl-Neelsen staining positive for acid-fast bacilli. Third, all her symptoms completely resolved under antituberculous treatment. In this case, BD-like syndrome is secondary to Mycobacterium tuberculosis infection.
Few case reports mentioned that some microbial infection, in particular Mycobacterium tuberculosis and virus, triggered the onset of pseudo-BD or BD-like syndrome. We reviewed the literature and summarized the clinical manifestations and the possible mechanisms of BD-like syndrome secondary to infection.
The characteristics of documented cases about BD-like syndrome secondary to Mycobacterium tuberculosis infection are listed in Table 1. This condition seems predominant in female, and the average age of disease onset is about 38.6 years. All cases experienced genital ulcer (100%). Most of them suffered from oral ulcer (86%) and arthritis (71%). Ocular lesions were observed in 43% of these patients. However, important organs involvement, for example neurological system, cardiovascular system, and gastrointestinal tract, are not documented in any case. Besides lymph node enlargement is a common symptom, while other symptoms associated with TB infection such as weight loss, night sweating, chest pain and cough are rare. It is worth mentioning that the histopathological feature of EN-like lesion can be classified into two types: One is that panniculitis combined with vasculitis, which is characterized by neutrophilic vascular reaction in the dermis and subcutaneous [2]. The other is tuberculoid granuloma on histopathologic analysis [2-5]. However, in only one case, the specimen from the EN-like lesion had a positive result on polymerase chain reaction (PCR) detection for Mycobacterium tuberculosis [4].
Table 1.
Characteristics of seven cases of Behçet’s disease-like syndrome secondary to Mycobacterium tuberculosis infection
| N (%) | |
|---|---|
| Sex | |
| Male | 2 (29) |
| Female | 5 (71) |
| Nationality | |
| Chinese | 2 (29) |
| Japanese | 3 (42) |
| Indian | 2 (29) |
| Manifestations of BD-like syndrome | |
| Oral ulcer | 6 (86) |
| Genital ulcer | 7 (100) |
| Arthritis | 5 (71) |
| Ocular lesions | 3 (43) |
| Uveitis | 3 |
| Skin lesions | 7 (100) |
| Erythema nodosum | 6 |
| Histopathology | |
| Vasculitis | 2 |
| Tuberculoid granuloma | 3 |
| Acneiform nodules | 1 |
| Vascular involvement | 0 |
| Gastrointestinal involvement | 0 |
| Neurological involvement | 0 |
| Cardiovascular involvement | 0 |
| Pulmonary involvement | 0 |
| Positive Result on pathergy testing | |
| + | 2 |
| ± | 1 |
| NM | 3 |
| Other clinical features | |
| Lymphadenopathy | 5 |
| Fever | 1 |
| Weight loss | 1 |
| Night sweating | 1 |
| MTB infection site | |
| Tuberculous lymphadenitis | 5 (71) |
| Pulmonary tuberculosis | 2 (29) |
NM not mentioned.
In case of time order, the manifestations of BD-like syndrome took place as soon as Mycobacterium tuberculosis infection or in several years after the diagnosis of Mycobacterium tuberculosis infection [3,5,6]. Even, there are two cases reported by Hamill and et al. with BD-like syndrome predating the onset of tuberculosis (TB) [4]. As for the outcome, NSAIDs or traditional immunosuppressive therapy were inefficient in alleviating the symptoms of BD-like syndrome. However, in most cases, anti-TB treatment could lead to the remission of BD-like syndrome.
Moreover, it has been reported that BD-like syndrome occurred secondary to virus infection (Table 2). All of the three documented cases are female patients. The appearance of BD-like syndrome usually took place shortly after virus infection, with the shorter time interval compared to that in Mycobacterium tuberculosis infection. All of the cases experienced orogenital ulceration. Ocular inflammation, but not major organ involvement, was indicated. As for skin lesions, multiple erythematous papulovesicular eruptions were described besides EN-like lesions [7]. Constitutional symptoms appeared in the three cases including fever, fatigue, anorexia, weight loss and swollen lymph nodes. Thalidomide or topical corticosteroid showed little effective in alleviating the symptoms of BD-like syndrome. Anti-virus therapy greatly improved the symptoms of BD-like syndrome in three cases [7-9].
Table 2.
Characteristics of three cases of Behçet’s disease-like syndrome secondary to virus infection
| Age/sex | General manifestations | oral ulcers and genital ulcers | Eye lesions | Skin lesion | Arthritis | Major organ involvement | Virus infection | Resolution |
|---|---|---|---|---|---|---|---|---|
| 65/F [7] | Fever Fatigue Wasting Enlargement of lymphnodes | + | - | Erythematous papules and vesicles | - | - | Chronic active EBV infection | Famciclovirdapsone |
| 13/F [8] | Fever | + | Uveitis | Vesicles | + | - | HSV-1 reactivation | Valacyclovir |
| 27/F [9] | Fatigue, Fevers, Night sweats, AnorexiasPhotophobia | + | Conjunctivitis | EN | + | - | HIV infection | Prednisone thalidomide, without improvement; Colchicine improvement without recur |
EN erythema nodosum.
The aetiopathogenesis of BD-like syndrome has largely unknown. BD-like syndrome might occur in the predisposed individuals. HLA-B51 is a well-known genetic factor in patients with primary BD. However, among the seven cases listed in Table 1, only two cases had HLA-B51 type. The genetic background conferring such susceptibility for BD-like syndrome may differ from that encountered in primary BD. In addition, a molecular-mimicry based pathogenic mechanism may be involved in the development of BD-like syndrome. Human protein 60-kD heat shock protein (HSP60) is investigated as a candidate autoantigen in primary BD [10]. HSP60 is observed to be over expressed locally in oral ulcers, epidermal regions of active skin lesions such as erythema nodosum and in intestinal BD lesions [11-13]. Recent study recognized HSP-60 as a ligand for TLR-2 and TLR-4, which may suggest that the function of HSP-60 as an endogenous “danger” signal to the immune system followed by rapid inflammatory cytokine release and the enhancement of adaptive Th1-type responses [14]. HSP65 antigen obtained from Mycobacterium tuberculosis has a high homology with human HSP60 [15]. Mycobacterium tuberculosis HSP65 displays molecular mimicry to human HSP60, resulting in an immunologic cross-reaction and the subsequent development of BD-like syndrome.
Conclusion
In conclusion, some microbial infection, such as Mycobacterium tuberculosis and virus, can trigger the onset of BD-like syndrome in genetically susceptible subjects. However, treatment strategy of BD-like syndrome secondary to infection is totally different from primary BD. It is important to differentiate BD-like syndrome from primary BD. Clinical features, such as lymph node enlargement, fever, and weight loss, should be paid more attention to screen suspected BD patients for microbial infection.
Disclosure of conflict of interest
None.
References
- 1.Criteria for diagnosis of Behcet’s disease. International Study Group for Behcet’s Disease. Lancet. 1990;335:1078–1080. [PubMed] [Google Scholar]
- 2.Fukui S, Takizawa Y, Kubota N, Okamoto T, Hishima T. Tuberculous lymphadenitis and the appearance of Behcet’s disease-like symptoms. Intern Med. 2014;53:805–808. doi: 10.2169/internalmedicine.53.1663. [DOI] [PubMed] [Google Scholar]
- 3.Shinoda K, Hayashi R, Taki H, Hounoki H, Makino T, Nomoto K, Shimizu T, Tobe K. Pseudo-Behcet’s disease associated with tuberculosis: A case report and review of the literature. Rheumatol Int. 2014;34:1471–1474. doi: 10.1007/s00296-014-2998-y. [DOI] [PubMed] [Google Scholar]
- 4.Hamill M, Remedios D, Kapembwa M. Orogenital ulceration with overlapping tuberculosis: Epiphenomenon or expanding spectrum of Behcet disease. J Low Genit Tract Dis. 2006;10:219–222. doi: 10.1097/01.lgt.0000225897.29466.e4. [DOI] [PubMed] [Google Scholar]
- 5.Duan H, Zhao X, Xiao W. Tuberculous allergic Behcet’s disease syndrome: a case report. Cli J Postgrad Med. 2011;34:76–77. (in Chinese) [Google Scholar]
- 6.Sharma A, Dogra S, Pinto B, Sharma K, Singh R, Dhir V, Sharma SK, Kakkar N, Radotra B, Singh S. Poncet’s disease presenting as Pseudo-Behcet’s disease. Int J Rheum Dis. 2013;16:483–485. doi: 10.1111/1756-185X.12121. [DOI] [PubMed] [Google Scholar]
- 7.Park BM, Ahn JS, Lee JB, Won YH, Yun SJ. Chronic active Epstein-Barr virus infection-associated hydroa vacciniforme-like eruption and Behcet’s-like orogenital ulcers. Dermatology. 2013;226:212–216. doi: 10.1159/000348709. [DOI] [PubMed] [Google Scholar]
- 8.Sugata K, Enomoto Y, Sugiyama H, Fujita A, Miyake F, Asano Y, Yoshikawa T. Single episode of Behcet’s disease-like symptoms caused by herpes simplex virus reactivation. Pediatr Int. 2009;51:577–578. doi: 10.1111/j.1442-200X.2009.02869.x. [DOI] [PubMed] [Google Scholar]
- 9.Buskila D, Gladman DD, Gilmore J, Salit IE. Behcet’s disease in a patient with immunodeficiency virus infection. Ann Rheum Dis. 1991;50:115–116. doi: 10.1136/ard.50.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saruhan-Direskeneli G, Celet B, Eksioglu-Demiralp E, Direskeneli H. Human HSP 60 peptide responsive T cell lines are similarly present in both Behcet’s disease patients and healthy controls. Immunol Lett. 2001;79:203–208. doi: 10.1016/s0165-2478(01)00280-2. [DOI] [PubMed] [Google Scholar]
- 11.Deniz E, Guc U, Buyukbabani N, Gul A. HSP 60 expression in recurrent oral ulcerations of Behcet’s disease. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:196–200. doi: 10.1016/j.tripleo.2010.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Nara K, Kurokawa MS, Chiba S, Yoshikawa H, Tsukikawa S, Matsuda T, Suzuki N. Involvement of innate immunity in the pathogenesis of intestinal Behcet’s disease. Clin Exp Immunol. 2008;152:245–251. doi: 10.1111/j.1365-2249.2008.03626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ergun T, Ince U, Eksioglu-Demiralp E, Direskeneli H, Gurbuz O, Gurses L, Aker F, Akoglu T. HSP 60 expression in mucocutaneous lesions of Behcet’s disease. J Am Acad Dermatol. 2001;45:904–909. doi: 10.1067/mjd.2001.117728. [DOI] [PubMed] [Google Scholar]
- 14.Tursen U. Pathophysiology of the Behcet’s Disease. Patholog Res Int. 2012;2012:493015. doi: 10.1155/2012/493015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Direskeneli H. Innate and Adaptive Responses to Heat Shock Proteins in Behcet’s Disease. Genet Res Int. 2013;2013:249157. doi: 10.1155/2013/249157. [DOI] [PMC free article] [PubMed] [Google Scholar]


