Abstract
High-risk human papillomavirus (HPV) is a possible cause of esophageal cancer. However, the molecular pathogenesis of HPV-infected esophageal cancer remains unclear. The expression levels of some microRNAs including miR-125b have been negatively correlated with HPV infection, and miR-125b downregulation is associated with tumorigenesis. In addition, Wnt/β-catenin signaling pathway has been suggested to play an important role in esophageal cancer (EC). We examined miR-125b and Wnt/β-catenin signaling pathway in HPV-16 E6 promoted tumor progression in EC. HPV-16 E6 transfection decreased markedly the expression levels of miR-125b and promoted the colony formation in the Eca 109 and Kyse 150 cell lines, and restoration of miR-125b expression level antagonized the increased colony formation in HPV-16 E6 transfected cell lines. We also demonstrated that overexpression of E6 upregulated the Wnt/β-catenin signaling activity via modulating the multiple regulators including TLE1, GSK3β, and sFRP4. Overexpression of miR-125b restored the expression levels of these proteins. Expression of miR-125b was lower in HPV-16 E6 positive esophageal cancer tissues, and was negatively correlated with E6 mRNA levels. Our results indicate that HPV-16 E6 promotes tumorigenesis in EC via down-regulation of miR-125b, and this underlying mechanism may be involved in the activation of the Wnt/β-catenin signaling pathway.
Keywords: HPV-16 E6, esophageal cancer, miR-125b, Wnt/β-catenin
Introduction
Esophageal cancer (EC) is one of the most common upper gastrointestinal tract malignancies worldwide [1]. There are about 3 times more esophageal cancers in males than females. According to different pathological and etiological characteristics, esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC) are the two main subtypes. ESCC is common frequently found in the developing countries, while EAC is common the western countries [2,3]. Due to the absence of early symptoms, invasiveness of the disease, and its late diagnosis, it is generally associated with a poor prognosis and the 5-year survival is only about 10% [2]. However, the etiology of esophageal cancer is still unclear. Human papilloma virus (HPV) has been suggested as a possible cause of EC. HPV has more than 140 genotypes, which are classified into high-risk and low risk types; and HPV-16 infection is more prevalent than any other high-risk HPV type in most regions of the world [4]. Several lines of clinical studies in different regions of the world have suggested that HPV-16 infection may be an important risk factor for EC [5-7]. Thus, it is important to investigate the mechanism of HPV-16 infection in the development of EC.
MicroRNAs have been described as key regulators in cancer, and play an important role in tumor development either by acting as oncogenes or tumor suppressor genes [8]. Recent studies suggested that some microRNAs are essential in the occurrence and development of the HPV-associated cancers, including EC [6,9,10]. MiR-218 and miR-125b have been found to be negatively correlated with HPV-infected tissues, and studies indeed have shown that HPV mediated miR-218 downregulation is associated with EC [9]. Downregulation of miR-125b has been linked to breast, ovarian, hepatocellular and thyroid carcinomas [11-13], therefore, miR-125b may have a tumor suppressor role, and miR-125b has also been found to be down-regulated in oral squamous cell carcinoma [14]. The role of miR-125b in EC is largely unknown.
The WNT/β-catenin signaling pathway plays essential roles in cell proliferation and differentiation, and deregulated WNT/β-catenin pathway leads to many types of human cancers [15-17]. In esophageal cancer cells, WNT1 induces β-catenin/TCF-dependent transcription, and modulated α-catenin and β-catenin expression was found in esophageal cancer tissues [18-20].
In this study, we first transfect Eca 109 and Kyse 150 cell lines with HPV-16 E6 to investigate whether overexpression of E6 has an effect on the miR-125b expression level and colony formation. We also examined if the altered expression of miR-125b is associated with the activation of Wnt/β-catenin signaling pathway, which has been suggested playing an important role in esophageal cancer [18-20]. Finally, we determined the expression level of miR-125b in HPV-16 E6 negative or positive esophageal cancer tissues.
Materials and methods
Cell lines and culture
The Eca 109 and Kyse 150 cell lines were obtained from Shandong Academy of Medical Sciences (Shandong, China). The cells were cultured with DMEM culture mediums at 37°C in a humidified atmosphere of 5% CO2. When the cell confluence was about 75-80%, the cells were washed by sterile phosphate-buffered saline (PBS). After neutralizing the trypsin (Invitrogen, CA USA) by full medium and washing by PBS, the cells were collected by centrifugation at 1200 g for 3 min. Then the cell pellet was resuspended with fresh full medium correspondingly. Finally, the cells were passaged to four new 100 mm plastic tissue culture dishes and incubated at 37°C in a humidified atmosphere of 5% CO2 to expand the cells for experiments.
Tissue samples
This study was approved by the Ethics Review Committees of the First Affiliated Hospital of Soochow University, and informed consent was obtained from all patients. A total of 26 patients with esophageal cancer had routine surgery at the Second Hospital of Longyan. Samples tissues taken from these patients were snap-frozen in liquid nitrogen for further real-time polymerase chain reaction (PCR) analysis.
Construct of pcDNA3.1-E6 vector
The E6 DNA fragment of HPV-16 was obtained by PCR from SiHa genomic DNA. The 50 µl volume of the PCR reaction solution will contain 2 µl DNA, 0.25 µl of TAKARA Ex TaqTM (5 units/µl), 5 µl of 10× Ex Taq Buffer, 1 µl of dNTP Mixture (10 mM), 30.25 µl nuclease-free water, 2.5 μl each of forward and reverse primers with EcoRI and HindIII restriction endonuclease target sequences, respectively. The PCR amplification conditions were: 94°C for 10 minutes, 30 cycles of 94°C for 30 seconds, 55°C for 1 minute and 72°C for 3 minutes, then followed by 72°C for 10 minutes. Subsequently, the PCR product will be purified by QIAquick PCR Purification Kit (Qiagen, Valencia, CA). The purified PCR product and pcDNA3.1 vector will be digested by EcoRI and HindIII, respectively. After purification by QIAquick PCR Purification Kit (Qiagen, Valencia, CA) and quantification by using NanoDrop ND-1000 UV-VIS spectrophotometer (Model: 200048602), the digested and purified PCR product and pcDNA3.1 vector will be ligated by T4 DNA ligase to obtain the vector with fragment of HPV-16 E6.
HPV-16 E6 transfection
Eca 109 and Kyse 150 cells were seeded about 30% confluence in the 24-well plates. 500 ng pcDNA3.1-E6 vectors and 1.5 μl of Lipofectamine 2000 reagent were diluted in 25 μl of Opti-MEM separately, incubated at room temperature for 5 min and then mixed gently. The mixture was allowed to stand for 20 min at room temperature before adding to the well. After 24-hour incubation at 37°C for transfection, the cells were collected for further experiments. The cells transfected with empty vector were used for the controls.
HPV-16 E6 siRNA transfection assays
The RNA interference target sequences for HPV-16 E6 siRNA have been previously verified [21,22], the procedures and methods were described previously [23].
MiR-125b mimic transfection assays
MiR-125b mimic were used to increase the expression levels of miR-125b, non-specific sequences were designed as negative control (NC) (Ribobio, Guangzhou, China). Eca 109 and Kyse 150 cells were seeded about 30% confluence in the 24-well plates; and miR-125b mimic or miR-125b NC and pcDNA3.1-E6 vectors were transfected with Lipofecatmine 2000 reagent.
Quantitative real time PCR (qRT-PCR) analysis of E6 mRNA
Total RNA was extracted by homogenization in 1 µl Trizol reagent, followed by chloroform extraction and isopropanol precipitation. A 3 μg sample of total RNA from ECa 109 or Kyse 510 cells was reverse transcribed using SuperScript II Reverse Transcriptase (Invitrogen) and Oligod(T)15 primer. For E6 mRNA amplification, the primers 5’-CGGAATTCATGCACCAAAAGAGAACTGCA-3’ and 5’-CCCAAGCTTACAGCTGGGTTTCTCTACG-3’ were used; and GAPDH were used as an internal control.
qRT-PCR for miR-125b analysis
DNase I-treated total RNA (10 ng) was subjected to microRNA RT-PCR analysis with the TaqMan® miRNA reverse Transcription Kit (Applied Biosystems), miRNA Assays (Applied Biosystems), and a Real-Time Thermocycler 7500 (Applied Biosystems. RNU6B was used as the small RNA reference housekeeping gene. The primers for miR-125b were: 5’-GCCCTCCCTGAGACCCTAAC-3’ and 5’-GTGCAGGGTCCGAGGT-3’.
Western blotting
Western blot analysis was performed using anti-glycogen synthase kinase 3 beta (GSK3beta) (1:3000, Abcam Cambridge, MA, USA); anti-TLE1 (1:2500, Abcam Cambridge, MA, USA); anti-secreted Frizzled-related proteins 4 (sFRP4) (1:2500, Abcam Cambridge, MA, USA); anti-beta-catenin (1:3000, Abcam Cambridge, MA, USA), and GAPDH was used a loading control.
Colony formation assay
For the colony formation assay, 500 transfected cells were plated in a 6-well plate for 9 days. Colonies were fixed with methanol/acetone (1:1) and stained with crystal violet.
Statistical analysis
All statistical analysis was carried out using GraphPad Prism version 5 (GraphPad Prism version 5.0, Inc. California, USA). The differences of E6 mRNA levels, miR-125b levels, and protein levels were analyzed by one-way ANOVA followed by Bonferroni’s multiple comparison tests. All data are expressed as mean ± s.e.m. Differences were considered significant when P < 0.05.
Results
HPV-16 E6 mRNA expression levels in Eca 109 and Kyse 510 cells with different treatments
The E6 mRNA expression levels in Eca 109 and Kyse 150 cells were determined by qRT-PCR analysis. The E6 mRNA was hardly detected in the Eca 109 and Kyse 510 cell lines transfected with empty vector (the mRNA levels relative to GAPDH were 0.214 ± 0.007 and 0.202 ± 0.004, respectively); and Eca 109 and Kyse 150 cell lines transfected with pcDNA3.1-E6 vector had a significant increase in the E6 mRNA levels; silencing of HPV-16 E6 significantly decreased E6 mRNA levels in both Eca 109 and Kyse 150 cell lines transfected with pcDNA3.1-E6 vector (P < 0.05; n = 3, Figure 1).
Figure 1.
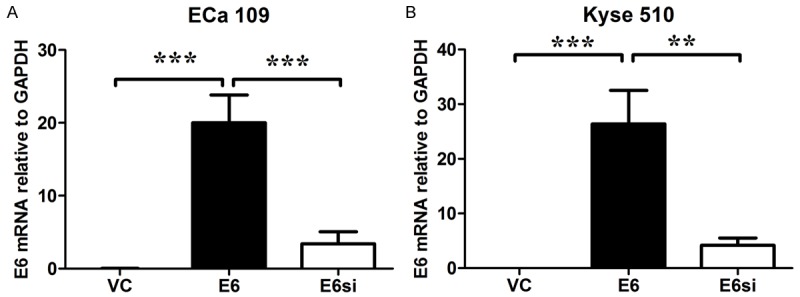
HPV-16 E6 mRNA expression levels in ECa 109 and Kyse 150 cells received different treatments. The E6 mRNA expression levels relative to GAPDH in ECa 109 and Kyse 150 cells were shown. Data represents the mean ± s.e.m. of 3 determinations. VC = vector control (cells transfected with empty vector); E6 = cells transfected with HPV E6, E6si = cells transfected with HPV E6 and silenced by E6 siRNA. Significant differences between groups were indicted as **P < 0.01, ***P < 0.001 (One-way ANOVA followed by Bonferroni’s multiple comparison tests).
HPV-16 E6 mediated miR-125b expression in Eca 109 and Kyse 150 cells
The miR-125b expression levels in Eca 109 and Kyse 150 cells were measured by real-time PCR; and data showed that the miR-125b level was significantly lower in HPV-16 E6 transfected Eca 109 and Kyse 150 cells than their relative controls, respectively (P < 0.05; n = 3, Figure 2). Transfection of miR-125brestored the miR-125b levels in cells transfected with HPV-16 E6 (P < 0.05; n = 3, Figure 2).
Figure 2.
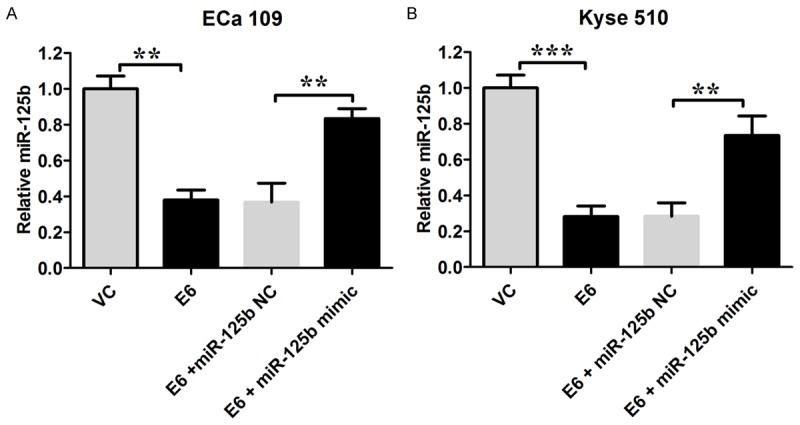
HPV-16 E6 down-regulated miR-125b expression in ECa 109 and Kyse 150 cells. The miR-125b expression levels relative to RNU6B in Eca 109 and Kyse 150 cells were shown. VC = vector control (cells transfected with empty vector); E6 = cells transfected with HPV E6. Data represents the mean ± s.e.m. of 3 determinations. The relative miR-125b level in the VC group was arbitrarily assigned as 1. Significant differences between groups were indicted as **P < 0.01, ***P < 0.001 (One-way ANOVA followed by Bonferroni’s multiple comparison tests).
HPV-16 E6 modulates Wnt/β-catenin signaling activity via miR-125b
In the HPV-16 E6 transfected cells, the protein levels of β-catenin were significantly higher than their relative controls, while the protein expression levels of sFRP4, GSK3β, and TLE1 were down-regulated (n = 3, Figure 3). Overexpression of miR-125b in Eca 109 and Kyse 150 cells transfected by HPV-16 E6 restored the protein levels of β-catenin, sFRP4, GSK3β, and TLE1, respectively (n = 3, Figure 3).
Figure 3.
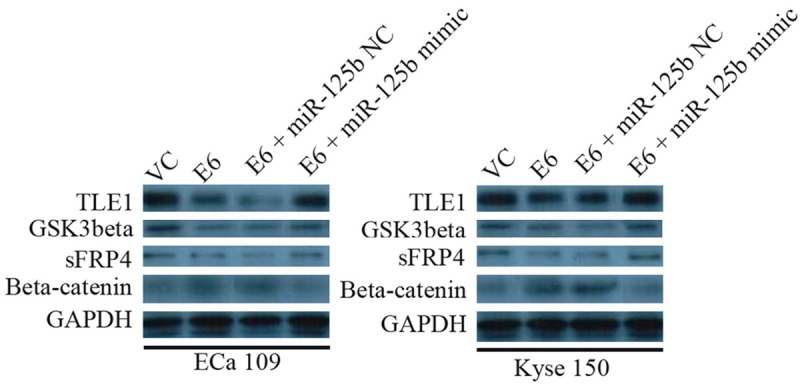
Western blot analysis of TLE1, GSK3beta, sFRP4, and beta-catenin in Eca 109 and Kyse 150 cells. VC = vector control (cells transfected with empty vector); E6 = cells transfected with HPV-16 E6.
HPV-16 E6 promote cell proliferation via down-regulation of miR-125b
The number of colonies in HPV-16 E6 transfected Eca 109 and Kyse 150 cells were significantly increased compared to their relative controls, respectively (Eca 109: 123.8 ± 6.6 vs. 249.0 ± 8.4; Kyse 150: 126.6 ± 14.5 vs. 244.0 ± 24.8; P < 0.05; n = 4-5, Figure 4). Overexpression of miR-125b in the cells transfected by HPV-16 E6 significantly decreased the number of colonies (P < 0.05; n = 4-5, Figure 4).
Figure 4.
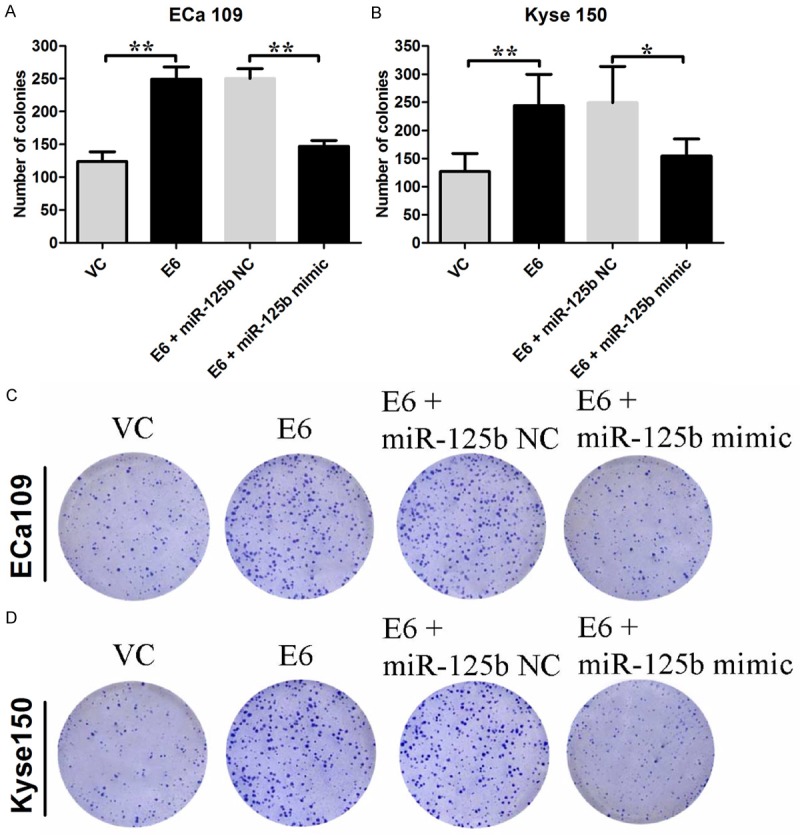
Effects of HPV-16 E6 on the colony formation in Eca 109 and Kyse 150 cells. The number of colonies for (A) Eca 109 cells, and (B) Kyse 150 cells received different treatments were shown. Representative colony formations for (C) Eca 109 cells and (D) Kyse 150 cells received different treatments were shown. VC = vector control (cells transfected with empty vector); E6 = cells transfected with HPV E6. Data represents the mean ± s.e.m. of 3 determinations. Significant differences between groups were indicted as *P<0.05, **P<0.01 (One-way ANOVA followed by Bonferroni’s multiple comparison tests).
MiR-125b is down-regulated in HPV-16 E6 positive esophageal cancer tissues
The expression of miR-125b in 13 HPV-16 E6 positive esophageal cancer tissues and 13 HPV-16 E6 negative esophageal cancer tissues were detected by qRT-PCR. The expression level of miR-125b in HPV-16 E6 positive esophageal cancer tissues was significantly higher than in HPV-16 E6 negative cancer tissues (P < 0.05; n = 13, Figure 5A); and the expression level of E6 mRNA was negatively correlated with miR-125b level (n = 13; Figure 5B).
Figure 5.
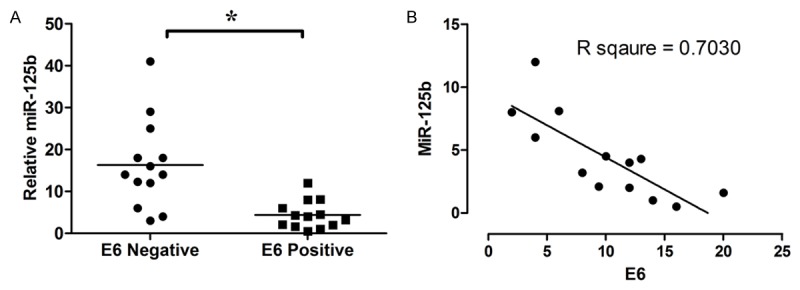
MiR-125b is negatively correlated with expression level of E6 mRNA in HPV-16 E6 positive esophageal cancer tissues. A: MiR-125b is down-regulated in HPV-16 E6 positive esophageal cancer tissues; B: Correlation of miR-125b levels to HPV-16 E6 mRNA levels in HPV-16 E6 positive esophageal cancer tissues. Data represents the mean ± s.e.m. of 13 determinations. Significant differences between groups were indicted as *P < 0.05 (unpaired t-test).
Discussion
HPV-16 E6 promotes tumorigenesis in EC via down-regulation of miR-125b, and this underlying mechanism may be involved in the activation of the Wnt/β-catenin signaling pathway.
Many studies have investigated the association between HPV and esophageal cancer [5,7,24], but few reports have explored the molecular mechanism of HPV infection in esophageal cancer. In this study, we report for the first time that E6-mediated miR-125b is responsible for the tumor progression in HPV-16-infected Eca 109 and Kyse 150 cell lines. HPV infection has been shown to be involved in the development of human cancers including cervical and oropharyngeal carcinomas via altering the levels of several microRNAs [6,9,10]. There is also evidence showing that miR-125b is negatively correlated with HPV-infected tissues [25]. MiR-125b is highly conserved miRNA common among various species; downregulation of miR-125b has been linked to breast, ovarian, hepatocellular and thyroid carcinomas [11-13], therefore, miR-125b may have a tumor suppressor role, and miR-125b has been found to be down-regulated in oral squamous cell carcinoma [14]. The present study showed that miR-125b is downregulated in E6 infected Eca 109 and Kyse 150 cell lines, and the downregulation is related to the increased colony formation in these cell lines. By comparing HPV-16 E6 positive and HPV-16 E6 negative esophageal cancer tissues, we further confirmed that miR-125b is down-regulated in HPV-16 E6 positive esophageal cancer tissues and is inversely correlated with the expression levels of E6 mRNA. These results suggest that miR-125b plays an important role in tumor progression in HPV-16 E6-infected esophageal cancer.
The Wnt/β-catenin signaling pathway is a well-characterized pathway involved in oncogenesis in many systems [26,27]. Wnt/β-catenin signaling pathway plays an important role in the regulation of cell proliferation, differentiation, migration and cell death [28,29]. Dysregulation of Wnt/β-catenin signaling pathway has been linked to many cancers [30]. Understanding the underlying mechanisms may reveal novel targets for cancer therapy. In the present study, we found that HPV E6 activates Wnt/β-catenin pathway in different layers trough direct downregulation of sFRP4, GSK3beta, and TLE1, and this might be due to the down-regulation of miR-125b. sFRP4 is a putative Wnt-binding receptor, and several studies suggested that sFRP4 may be a tumor suppressor [31]. Studies have shown that sFRP4 is decreased in endometrial cancer cells and can inhibit cell growth [32]; it can also increase the sensitivity of glioma stem-like cells to chemotherapy by inducing apoptosis through the inhibition of Wnt/β-catenin signaling [33]. The molecular mechanism underlying sFRP4 protein downregulation in Eca 109 and Kyse 150 cell lines remained largely unclear, and it is possible that miR-125b targets the sFRP4-3’UTR to inhibit sFRP4 expression [34]. Further studies may be needed to determine the direct targets of miR-125b on these regulators including TLE1, sFRP4, GSK3beta and beta-catenin. It may also be worthy to examine if HPV-16 E6 could modulate downstream targets such as c-myc and cyclin D1 in Wnt/β-catenin signaling pathway.
In summary, we demonstrate that E6 is responsible for the colony formation via down-regulation of miR-125b in HPV-infected Eca 109 and Kyse 150 cell lines, which is associated with Wnt/β-catenin signaling pathway. Therefore, we suggest that miR-125b can be targeted to suppress the progression and metastasis of esophageal cancer, especially in HPV-infected esophageal cancer.
Acknowledgements
This work was supported by the grant (KL11C221611025) from Science and Technology Project of Suzhou city.
Disclosure of conflict of interest
None.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Hongo M, Nagasaki Y, Shoji T. Epidemiology of esophageal cancer: Orient to Occident. Effects of chronology, geography and ethnicity. J Gastroenterol Hepatol. 2009;24:729–735. doi: 10.1111/j.1440-1746.2009.05824.x. [DOI] [PubMed] [Google Scholar]
- 3.Zeng H, Zheng R, Guo Y, Zhang S, Zou X, Wang N, Zhang L, Tang J, Chen J, Wei K, Huang S, Wang J, Yu L, Zhao D, Song G, Chen J, Shen Y, Yang X, Gu X, Jin F, Li Q, Li Y, Ge H, Zhu F, Dong J, Guo G, Wu M, Du L, Sun X, He Y, Coleman MP, Baade P, Chen W, Yu XQ. Cancer survival in China, 2003-2005: a population-based study. Int J Cancer. 2015;136:1921–1930. doi: 10.1002/ijc.29227. [DOI] [PubMed] [Google Scholar]
- 4.Trottier H, Franco EL. The epidemiology of genital human papillomavirus infection. Vaccine. 2006;24(Suppl 1):S1–15. doi: 10.1016/j.vaccine.2005.09.054. [DOI] [PubMed] [Google Scholar]
- 5.Hardefeldt HA, Cox MR, Eslick GD. Association between human papillomavirus (HPV) and oesophageal squamous cell carcinoma: a meta-analysis. Epidemiol Infect. 2014;142:1119–1137. doi: 10.1017/S0950268814000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li M, He XY, Zhang ZM, Li S, Ren LH, Cao RS, Feng YD, Ji YL, Zhao Y, Shi RH. MicroRNA-1290 promotes esophageal squamous cell carcinoma cell proliferation and metastasis. World J Gastroenterol. 2015;21:3245–3255. doi: 10.3748/wjg.v21.i11.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yong F, Xudong N, Lijie T. Human papillomavirus types 16 and 18 in esophagus squamous cell carcinoma: a meta-analysis. Ann Epidemiol. 2013;23:726–734. doi: 10.1016/j.annepidem.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Esquela-Kerscher A, Slack FJ. Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 9.Jiang Z, Song Q, Yang S, Zeng R, Li X, Jiang C, Ding W, Zhang J, Zheng Y. Serum microRNA-218 is a potential biomarker for esophageal cancer. Cancer Biomark. 2015;15:381–9. doi: 10.3233/CBM-150480. [DOI] [PubMed] [Google Scholar]
- 10.Qi Y, Li X, Zhao S. miR-29b inhibits the progression of esophageal squamous cell carcinoma by targeting MMP-2. Neoplasma. 2015;62:384–390. doi: 10.4149/neo_2015_046. [DOI] [PubMed] [Google Scholar]
- 11.Visone R, Pallante P, Vecchione A, Cirombella R, Ferracin M, Ferraro A, Volinia S, Coluzzi S, Leone V, Borbone E, Liu CG, Petrocca F, Troncone G, Calin GA, Scarpa A, Colato C, Tallini G, Santoro M, Croce CM, Fusco A. Specific microRNAs are downregulated in human thyroid anaplastic carcinomas. Oncogene. 2007;26:7590–7595. doi: 10.1038/sj.onc.1210564. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Yan LX, Wu QN, Du ZM, Chen J, Liao DZ, Huang MY, Hou JH, Wu QL, Zeng MS, Huang WL, Zeng YX, Shao JY. miR-125b is methylated and functions as a tumor suppressor by regulating the ETS1 proto-oncogene in human invasive breast cancer. Cancer Res. 2011;71:3552–3562. doi: 10.1158/0008-5472.CAN-10-2435. [DOI] [PubMed] [Google Scholar]
- 13.Zhao A, Zeng Q, Xie X, Zhou J, Yue W, Li Y, Pei X. MicroRNA-125b induces cancer cell apoptosis through suppression of Bcl-2 expression. J Genet Genomics. 2012;39:29–35. doi: 10.1016/j.jgg.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Peng SC, Liao CT, Peng CH, Cheng AJ, Chen SJ, Huang CG, Hsieh WP, Yen TC. MicroRNAs MiR-218, MiR-125b, and Let-7g predict prognosis in patients with oral cavity squamous cell carcinoma. PLoS One. 2014;9:e102403. doi: 10.1371/journal.pone.0102403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barker N, Clevers H. Catenins, Wnt signaling and cancer. Bioessays. 2000;22:961–965. doi: 10.1002/1521-1878(200011)22:11<961::AID-BIES1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 16.Clements WM, Wang J, Sarnaik A, Kim OJ, MacDonald J, Fenoglio-Preiser C, Groden J, Lowy AM. beta-Catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res. 2002;62:3503–3506. [PubMed] [Google Scholar]
- 17.Polakis P. Wnt signaling in cancer. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kadowaki T, Shiozaki H, Inoue M, Tamura S, Oka H, Doki Y, Iihara K, Matsui S, Iwazawa T, Nagafuchi A, et al. E-cadherin and alpha-catenin expression in human esophageal cancer. Cancer Res. 1994;54:291–296. [PubMed] [Google Scholar]
- 19.Mizushima T, Nakagawa H, Kamberov YG, Wilder EL, Klein PS, Rustgi AK. Wnt-1 but not epidermal growth factor induces beta-catenin/T-cell factor-dependent transcription in esophageal cancer cells. Cancer Res. 2002;62:277–282. [PubMed] [Google Scholar]
- 20.Nakanishi Y, Ochiai A, Akimoto S, Kato H, Watanabe H, Tachimori Y, Yamamoto S, Hirohashi S. Expression of E-cadherin, alpha-catenin, beta-catenin and plakoglobin in esophageal carcinomas and its prognostic significance: immunohistochemical analysis of 96 lesions. Oncology. 1997;54:158–165. doi: 10.1159/000227681. [DOI] [PubMed] [Google Scholar]
- 21.Jiang M, Milner J. Selective silencing of viral gene expression in HPV-positive human cervical carcinoma cells treated with siRNA, a primer of RNA interference. Oncogene. 2002;21:6041–6048. doi: 10.1038/sj.onc.1205878. [DOI] [PubMed] [Google Scholar]
- 22.Yoshinouchi M, Yamada T, Kizaki M, Fen J, Koseki T, Ikeda Y, Nishihara T, Yamato K. In vitro and in vivo growth suppression of human papillomavirus 16-positive cervical cancer cells by E6 siRNA. Mol Ther. 2003;8:762–768. doi: 10.1016/j.ymthe.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Cheng YW, Wu MF, Wang J, Yeh KT, Goan YG, Chiou HL, Chen CY, Lee H. Human papillomavirus 16/18 E6 oncoprotein is expressed in lung cancer and related with p53 inactivation. Cancer Res. 2007;67:10686–10693. doi: 10.1158/0008-5472.CAN-07-1461. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Gao C, Yang Y, Zhou F, Li M, Jin Q, Gao L. Systematic review with meta-analysis: the association between human papillomavirus infection and oesophageal cancer. Aliment Pharmacol Ther. 2014;39:270–281. doi: 10.1111/apt.12574. [DOI] [PubMed] [Google Scholar]
- 25.Nuovo GJ, Wu X, Volinia S, Yan F, di Leva G, Chin N, Nicol AF, Jiang J, Otterson G, Schmittgen TD, Croce C. Strong inverse correlation between microRNA-125b and human papillomavirus DNA in productive infection. Diagn Mol Pathol. 2010;19:135–143. doi: 10.1097/PDM.0b013e3181c4daaa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katoh M, Katoh M. WNT signaling pathway and stem cell signaling network. Clin Cancer Res. 2007;13:4042–4045. doi: 10.1158/1078-0432.CCR-06-2316. [DOI] [PubMed] [Google Scholar]
- 27.van Amerongen R, Bowman AN, Nusse R. Developmental stage and time dictate the fate of Wnt/beta-catenin-responsive stem cells in the mammary gland. Cell Stem Cell. 2012;11:387–400. doi: 10.1016/j.stem.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 28.Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 29.Robinson JA, Chatterjee-Kishore M, Yaworsky PJ, Cullen DM, Zhao W, Li C, Kharode Y, Sauter L, Babij P, Brown EL, Hill AA, Akhter MP, Johnson ML, Recker RR, Komm BS, Bex FJ. Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem. 2006;281:31720–31728. doi: 10.1074/jbc.M602308200. [DOI] [PubMed] [Google Scholar]
- 30.Marson A, Foreman R, Chevalier B, Bilodeau S, Kahn M, Young RA, Jaenisch R. Wnt signaling promotes reprogramming of somatic cells to pluripotency. Cell Stem Cell. 2008;3:132–135. doi: 10.1016/j.stem.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Chen WD, Pretlow TP, Yang B, Akiyama Y, Van Engeland M, Toyota M, Tokino T, Hinoda Y, Imai K, Herman JG, Baylin SB. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36:417–422. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- 32.Carmon KS, Loose DS. Secreted frizzled-related protein 4 regulates two Wnt7a signaling pathways and inhibits proliferation in endometrial cancer cells. Mol Cancer Res. 2008;6:1017–1028. doi: 10.1158/1541-7786.MCR-08-0039. [DOI] [PubMed] [Google Scholar]
- 33.Warrier S, Balu SK, Kumar AP, Millward M, Dharmarajan A. Wnt antagonist, secreted frizzled-related protein 4 (sFRP4), increases chemotherapeutic response of glioma stem-like cells. Oncol Res. 2013;21:93–102. doi: 10.3727/096504013X13786659070154. [DOI] [PubMed] [Google Scholar]
- 34.Ge C, Wu S, Wang W, Liu Z, Zhang J, Wang Z, Li R, Zhang Z, Li Z, Dong S, Wang Y, Xue Y, Yang J, Tan Q, Wang Z, Song X. miR-942 promotes cancer stem cell-like traits in esophageal squamous cell carcinoma through activation of Wnt/beta-catenin signalling pathway. Oncotarget. 2015;6:10964–77. doi: 10.18632/oncotarget.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]


