Abstract
Objective: This study aimed to understand the relationship between tissue factor (TF) and laryngeal carcinoma. Methods: Differences in TF expression between pericarcinomatous and carcinomatous tissues were studied in patients with laryngeal carcinoma; the potential clinical significance of the observed differences is discussed. Immunohistochemical, western blot, and RT-PCR analyses were performed to assess the expression of TF at the protein and mRNA levels, and differences between pericarcinomatous and carcinomatous tissues in patients (n = 20) with laryngeal carcinoma were analyzed. Results: Expression of TF was significantly higher in pericarcinomatous tissues than in carcinomatous tissues (P < 0.01); furthermore, the intensity of TF mRNA expression was also significantly stronger in pericarcinomatous than in carcinomatous tissue (P < 0.001). Robust expression of TF was observed in pericarcinomatous tissues but not in carcinomatous tissues. Conclusion: TF may contribute to the carcinogenesis and development of laryngeal carcinoma and may provide a marker for assessment of the degree of malignancy and the progression of laryngeal carcinoma. TF may also provide a new target for therapeutics for human head and neck cancer.
Keywords: Tissue factor, laryngeal carcinoma, pericarcinomatous tissues, carcinomatous tissues
Introduction
Head and neck cancers are malignancies that mostly arise from the mucosal epithelia of the oral cavity, nasal cavity, pharynx, and larynx [1]. The most common histologic type is head and neck squamous cell carcinoma (HNSCC), which accounts for more than 90% of all cases of head and neck cancer [2,3]. Like other types of cancers, HNSCC is believed to arise via a multistep process involving the activation of oncogenes, as well as the inactivation of tumor suppressor genes [4,5].
Tissue factor (TF) is a trans-membrane glycoprotein, which mainly participates in blood clotting. Generally, TF is found to activate factor VII, following with factor X activation, which ultimately activates thrombin and fibrin, which form blood clots [6]. Moreover, it has been demonstrated that there is a close relationship between cancer and TF; TF is known to be involved in cancer angiogenesis, growth, and metastasis [7]. TF has been shown to be an important marker for urological cancer [8,9]. Because of its interactions with downstream signal factors such as factor VII, TF has been confirmed to be the principal coagulation initiator released by cancer cells; it leads to thrombotic effects, which are a major lethal complication of cancer. Mounting evidence has shown that increased TF expression occurs in higher grade gliomas and pancreatic carcinomas, and TF expression is thought to be related to cancer invasion and metastasis. In colorectal cancer, aberrant and increased expression of TF leads to formation of the TF-factor VIIa complex, which induces production of the protease activated receptor (PAR); several kinds of proteases produced by tumor cells are known to induce degradation of the extracellular matrix (EM). TF may be directly linked to mutation of the KRAS oncogene and suppression of the P53 tumor suppressor gene. Depletion of TF was further revealed to promote tumor autophagy and apoptosis by increasing the unfolded protein response (UPR), because accumulation of unfolded or misfolded proteins induces increased endoplasmic reticulum (ER) stress. TF is also involved in several classical apoptosis and immune-activation pathways [10]. In this study, tumorigenesis was considered to be similar to wound healing, which are both relevant to immunoreactivity against new blood vessels, EM proteins, and several types of stromal cells. TGF-β is released by tumor cells and its abundance in tumor stroma plays a key role; it can activate TF expression in stromal cells and endothelial cells of new blood vessels, ultimately leading to cancer invasion and metastasis [7].
Although numerous studies have investigated the relationship between head/neck cancer and TF, the evidence remains inconclusive. Although TF is a prognostic marker for cancer, it is produced throughout the process of tumor progression. Thus, we compared levels of TF expression between pericarcinomatous tissues (PT) and carcinoma tissues (CT) in patients (n = 20) with laryngeal carcinoma to investigate the effect of TF in laryngeal carcinoma.
Materials and methods
Patients
A total of 20 patients with laryngeal carcinoma (aged 44-79 years, with a median age of 59 years) who underwent surgery between July 2013 and December 2013 were recruited for this study from the Renmin Hospital of Wuhan University (Wuhan, China). Patients were classified by histological outcome. Clinical data, including age, gender, lymph metastasis, and stage and grade of cancer were collected. No patients received any preoperative chemotherapy or radiotherapy. The clinical characteristics of the patients are shown in Table 1. The disease stage was determined according to the 2002 TNM classification of the International Union Against Cancer (UICC, Geneva, Switzerland). The histological grades of the tumors were determined according to the degree of differentiation (Broders’ classification). Ethical approval was granted by the Renmin Hospital of Wuhan University. Informed written consent was granted by patients who donated tissue.
Table 1.
20 cases patients’ characteristics of human laryngeal carcinoma
| Characteristics | Cases (%) |
|---|---|
| Age, years (%) | |
| < 60 | 11 (55%) |
| ≥ 60 | 9 (45%) |
| Gender (%) | |
| Male | 15 (75%) |
| Female | 5 (25%) |
| Location (%) | |
| Supraglottic | 6 (30%) |
| Glottic | 14 (70%) |
| Fuhrman/Histological grade (%) | |
| I | 7 (35%) |
| II | 9 (45%) |
| III | 4 (20%) |
| TNM stage (%) | |
| I | 5 (25%) |
| II | 9 (45%) |
| III | 3 (15%) |
| IV | 3 (15%) |
| Lymph node status (%) | |
| Negative | 8 (40%) |
| Positive | 12 (60%) |
Immunohistochemistry
Fresh tissue specimens, including carcinomatous tissues and pericarcinomatous tissues (taken from the surgery safety margin, from normal laryngeal mucosal tissues adjacent to the tumor edge, to 0.2-1.0 cm beyond the tumor, and confirmed by pathological examination) (as controls) were obtained directly after surgery. Part of the tissue was frozen in liquid nitrogen and frozen at -80°C until RNA and protein extraction. Immunohistochemical analysis was conducted to identify the expression of TF in these sections. CT and PT for each patient were sectioned at 8-µm thickness and stored at -80°C. Sections were fixed in acetone for 10 min and then incubated with anti-TF antibody (1:100 dilution; Abcam, USA); nuclei were stained with DAPI (DAPI, Sigma-Aldrich, St Louis, MO, USA). Images were acquired with a Zeiss fluorescent microscope (Carl Zeiss, Toronto, ON, Canada).
Western blot analyses
To determine TF expression levels in CT and PT, protein was extracted from frozen tissue samples. Briefly, tissues were lysed in a buffer containing 20 mM Tris (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 25 mM sodium pyrophosphate, 1 mM NaF, 1 mM glycerophosphate, 0.1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride, 2 μg/ml leupeptin, and 10 μg/ml aprotinin. In all, 50 μg of total cell lysate was separated on SDS-PAGE gels and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore; Billerica, MA, USA). Membranes were blocked with 5% nonfat dry milk in Tris-buffered saline and Tween 20 (TBS-T) buffer and then incubated with the following primary antibodies: TF (1:1000 dilution; Abcam, USA) and GAPDH (1:1000 dilution; Santa Cruz, CA, USA) at 4°C overnight. Samples were then incubated for 1 h at room temperature with horseradish peroxidase (HRP)-conjugated anti-rabbit secondary antibody (1:1000; Santa Cruz, CA, USA) as a secondary antibody. Signals were detected using an ECL Western Blotting Kit (Amersham Pharmacia Biotech, Piscataway, NJ, USA). For quantification, the density of bands was measured using the Quantity One software (Bio-Rad, Hercules, CA, USA).
Real-time PCR (RT-PCR) was performed using the Platinum Quantitative RT-PCR Thermo Script One-step Master Mix Reagents Kit (Invitrogen). The reaction was optimized for a volume of 25 µl. Total RNA from samples was homogenized and extracted using TRIzol (Invitrogen) according to the manufacturer’s instructions. First-strand cDNA was synthesized from 1 mg of total RNA in a 20-ml total volume using a recombinant reverse transcriptase (Roche) with random primers. Real-time reactions were carried out using a CFX96 real-time PCR detection system (Bio-Rad) with SYBR Green. Relative RNA levels indicate the quantity of TF RNA normalized to GAPDH RNA levels. Relative TF RNA levels indicate the quantity of RNA of the specific gene normalized to RNA levels of GAPDH. The following primer pairs were used: TF, 5’-CAAACCCGTCAATCAAGTCTAC and 3’-CTTCACATCCTTCACAATCTCG; GAPDH (internal control), 5’-CCCCATGGTGTCTGAGCG and 3’-CGACAGTCAGCCGCATCTT.
Statistical analysis
Data were analyzed using the GraphPad PRISM software (GraphPad Software, Inc., USA). Results are expressed as mean ± SEM. Data were analyzed using a t-test and one-way analysis of variance (ANOVA). P < 0.05 was considered to indicate statistical significance.
Results
The location of TF expression
To investigate the significance and location of TF expression in laryngocarcinoma tissue, samples from all patients were analyzed. Immunostaining showed that TF was strongly expressed in PT from all patients (n = 20), but was not expressed in CT. Surprisingly, strongest TF expression was observed in connective tissue, which is constituted of macrophages, fibroblasts, and stroma, regardless of the surrounding structure of the samples (Figure 1).
Figure 1.
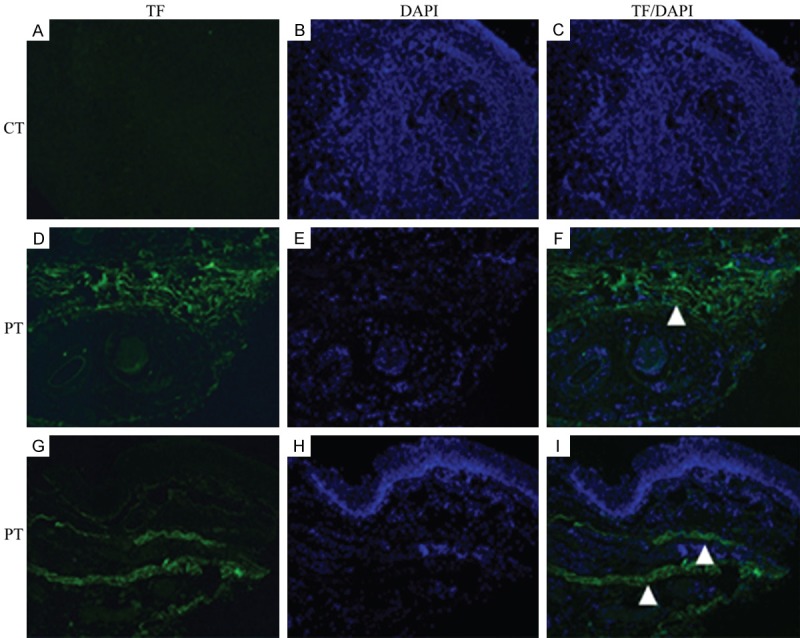
TF expressed much more in peri-carcinomatous tissue (PT) than in carcinomatous tissue (CT). A-I. The sections from carcinomatous tissues were stained with TF (green) and DAPI (blue). A-C. For CT, even all of the sections just showed nuclei staining with DAPI (blue), none displayed TF staining (green). D-F. The sections of PT around vessel, as observed, the interstitial parts showed the TF expression (white triangle, green), co-staining with DAPI for nuclei (blue). G-I. The sections of PT around epithelium, like two triangle point, under the epithelium, robust TF signal appeared in connective tissue (green) with the nuclei staining (blue) (Magnification, ×630). All the patients were performed immunohistochemistry analyses. All of the images represent more than 10 random fields from one section.
TF expression is higher in pericarcinomatous than in carcinomatous tissue
To investigate differences in TF expression between PT and CT, TF protein was extracted from all tissue samples. A protein gel blot assay showed that TF was more strongly expressed in PT than in CT (Figure 2A), although the difference in expression between the two tissue types was small in some patients. Semi-quantitative analysis of TF expression was used to determine statistical significance; TF expression was significantly stronger in PT than in CT (P < 0.01) (Figure 2B).
Figure 2.
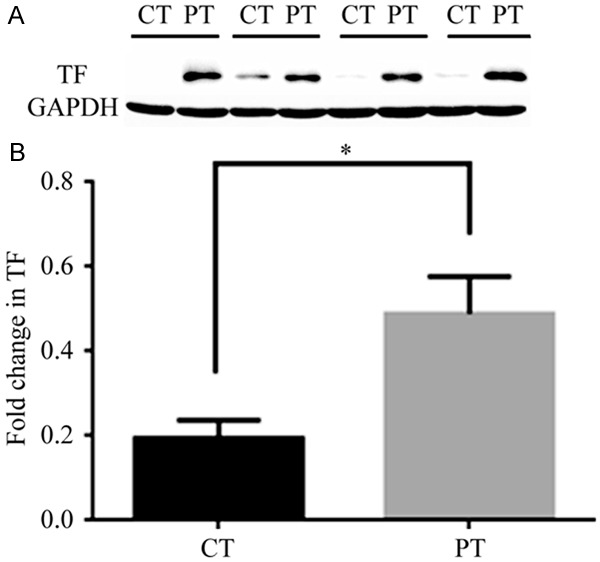
Protein gel blot analysis (Western blotting analysis) was used to show the difference of TF expression between carcinomatous tissue (CT) and peri-carcinomatous tissue (PT) for all the patients (n = 20). TF protein expression was significantly higher in PT compared with that from CT. A. Take just 4 patients’ TF western blotting for the example. B. The relative intensities of TF and GAPDH were quantified by densitometric scanning of the film with Quantity One software. The value of PT (GAPDH and TF) was used as a control (set at 1). The fold change in TF expression was calculated by the ratio of TF/GAPDH. The graph was generated by Graph pad PRISM software (n = 20) (*P < 0.01 vs. other).
PT showed higher levels of TF mRNA than did CT in laryngeal carcinoma
TF expression was further examined using RT-PCR. Quantification of TF mRNA showed that TF expression was significantly higher in PT than in CT. mRNA levels were calculated using the ratio between TF and GAPDH. Concordant with our other results, TF mRNA expression was significantly lower in CT than in PT (P < 0.001) (Figure 3).
Figure 3.
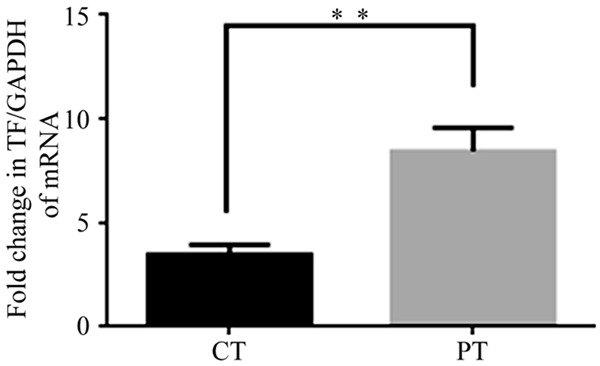
TF mRNA levels were further assessed by qRT-PCR. Compare to the level of TF mRNA transcription in carcinomatous tissue (CT), it was much higher in peri-carcinomatous tissue (PT), the GAPDH and TF mRNA levels were quantified by absolute qRT-PCR, and the plasmids comprising full-length of each gene were used for quantification of the template. GAPDH was employed as an internal reference. The fold change in TF expression was calculated by the ratio of TF mRNA copy number/GAPDH mRNA copy number. The graph was generated by Graph pad PRISM software (n = 20) (**P < 0.001 vs. other).
Discussion
TF plays a critical role in the blood coagulation cascade; it is located on the surface of several types of cells such as epithelial cells and muscle cells surrounding blood vessels [11,12]. Recently, the relationship between TF expression and tumors has received increasing attention, and TF has been shown to discriminate and regulate cell signal transduction, inflammation, angiogenesis, as well as tumor growth and metastasis [13,14]. However, the mechanism and details remain unclear, and few studies have focused on the influence of TF in neck and head cancers. PT, which is located around tumors, is sometimes treated as a type of healthy tissue; however, it is located between completely healthy tissue and tumor tissue.
The results of this study showed that TF expression was present in PT samples from all patients with laryngeal carcinoma, but was not present in CT or normal tissue (tissue sampled from very far away from tumor tissue samples). Furthermore, immunostaining showed that the TF signal was very strong in fibrous connective tissues, some of which was taken from locations surrounding vessels and lymph glands, and some from subcutaneous tissue. Macrophages, fibroblasts, and other cell types are distributed in connective tissue with intercellular substance. These types of cells are thought to express TF during tumorigenesis, which has been shown in several studies [15-17]. Tumorigenesis has been shown to be similar to normal cutaneous wound healing, which includes an inflammatory reaction, as well as increase and accumulation of TF-expressing macrophages [18,19]. Furthermore, connective tissue is usually considered to function as part of the structural skeleton and to function as a connection between organs; the connective tissue showed strong TF expression, suggesting that TF can transmit some cancer cell signals that participate in the tumor formation process.
Quantification of TF mRNA and protein revealed that TF expression was much higher in PT than in CT, and it confirmed previous results from an immunofluorescence analysis of TF expression. In conclusion, for the first time, our findings demonstrate a relationship between TF protein expression and head and neck cancer. Surprisingly, immunohistochemical, western blot, and RT-PCR analyses showed that TF expression was higher in PT, especially in fibroconnective tissue, than in CT. These data strongly suggest that TF is likely to be involved in delivery of cancer cell signaling in laryngeal carcinoma; in addition, TF expression by mesenchymal cells (macrophages and fibroblasts) is related to the tumor formation process, which has been shown to be similar to the inflammatory reaction involved in wound healing or thrombus formation [7,18,20]. Thus, TF represents an important and novel target for head and neck cancer research; however, the underlying mechanism requires further investigation.
Acknowledgements
The authors thank Professor Weiqi Yao, Wuhan University School of Basic Medical Sciences, School of Chemistry and Life Sciences, Hubei University of Education for their technical assistance and manuscript advising. We also thank the patients who gave consent to use their tissue for research. And the third group of “hanyang ‘talents plan’--Bin Wang”.
Disclosure of conflict of interest
None.
References
- 1.Russell NS, Bartelink H, Snow GB. Head and neck cancer. N Engl J Med. 1993;328:1783–1784. [PubMed] [Google Scholar]
- 2.Argiris A, Karamouzis MV, Raben D, Ferris RL. Head and neck cancer. Lancet. 2008;371:1695–1709. doi: 10.1016/S0140-6736(08)60728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillison ML, D’Souza G, Westra W, Sugar E, Xiao W, Begum S, Viscidi R. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 4.Bose P, Brockton NT, Dort JC. Head and neck cancer: from anatomy to biology. Int J Cancer. 2013;133:2013–2023. doi: 10.1002/ijc.28112. [DOI] [PubMed] [Google Scholar]
- 5.Gaykalova DA, Mambo E, Choudhary A, Houghton J, Buddavarapu K, Sanford T, Darden W, Adai A, Hadd A, Latham G, Danilova LV, Bishop J, Li RJ, Westra WH, Hennessey P, Koch WM, Ochs MF, Califano JA, Sun W. Novel insight into mutational landscape of head and neck squamous cell carcinoma. PLoS One. 2014;9:e93102. doi: 10.1371/journal.pone.0093102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodsell DS. The molecular perspective: tissue factor. Oncologist. 2006;11:849–850. doi: 10.1634/theoncologist.11-7-849. [DOI] [PubMed] [Google Scholar]
- 7.Lwaleed BA, Lam L, Lasebai M, Cooper AJ. Expression of tissue factor and tissue factor pathway inhibitor in microparticles and subcellular fractions of normal and malignant prostate cell lines. Blood Coagul Fibrinolysis. 2013;24:339–343. doi: 10.1097/MBC.0b013e32835e98a6. [DOI] [PubMed] [Google Scholar]
- 8.Lwaleed BA, Francis JL, Chisholm M. Monocyte tissue factor levels in patients with urological tumours: an association between tumour presence and progression. BJU Int. 1999;83:476–482. doi: 10.1046/j.1464-410x.1999.00944.x. [DOI] [PubMed] [Google Scholar]
- 9.Lwaleed BA, Francis JL, Chisholm M. Urinary tissue factor levels in patients with bladder and prostate cancer. Eur J Surg Oncol. 2000;26:44–49. doi: 10.1053/ejso.1999.0739. [DOI] [PubMed] [Google Scholar]
- 10.Tian M, Wan Y, Tang J, Li H, Yu G, Zhu J, Ji S, Guo H, Zhang N, Li W, Gai J, Wang L, Dai L, Liu D, Lei L, Zhu S. Depletion of tissue factor suppresses hepatic metastasis and tumor growth in colorectal cancer via the downregulation of MMPs and the induction of autophagy and apoptosis. Cancer Biol Ther. 2011;12:896–907. doi: 10.4161/cbt.12.10.17679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol. 2004;24:1015–1022. doi: 10.1161/01.ATV.0000130465.23430.74. [DOI] [PubMed] [Google Scholar]
- 12.Mackman N, Tilley RE, Key NS. Role of the extrinsic pathway of blood coagulation in hemostasis and thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:1687–1693. doi: 10.1161/ATVBAHA.107.141911. [DOI] [PubMed] [Google Scholar]
- 13.Kasthuri RS, Taubman MB, Mackman N. Role of tissue factor in cancer. J. Clin. Oncol. 2009;27:4834–4838. doi: 10.1200/JCO.2009.22.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole M, Bromberg M. Tissue factor as a novel target for treatment of breast cancer. Oncologist. 2013;18:14–18. doi: 10.1634/theoncologist.2012-0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vrana JA, Stang MT, Grande JP, Getz MJ. Expression of tissue factor in tumor stroma correlates with progression to invasive human breast cancer: paracrine regulation by carcinoma cell-derived members of the transforming growth factor beta family. Cancer Res. 1996;56:5063–5070. [PubMed] [Google Scholar]
- 16.Mantovani A, Romero P, Palucka AK, Marincola FM. Tumour immunity: effector response to tumour and role of the microenvironment. Lancet. 2008;371:771–783. doi: 10.1016/S0140-6736(08)60241-X. [DOI] [PubMed] [Google Scholar]
- 17.Breij EC, de Goeij BE, Verploegen S, Schuurhuis DH, Amirkhosravi A, Francis J, Miller VB, Houtkamp M, Bleeker WK, Satijn D, Parren PW. An antibody-drug conjugate that targets tissue factor exhibits potent therapeutic activity against a broad range of solid tumors. Cancer Res. 2014;74:1214–1226. doi: 10.1158/0008-5472.CAN-13-2440. [DOI] [PubMed] [Google Scholar]
- 18.Han X, Guo B, Li Y, Zhu B. Tissue factor in tumor microenvironment: a systematic review. J Hematol Oncol. 2014;7:54. doi: 10.1186/s13045-014-0054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu AJ. Tissue factor, blood coagulation, and beyond: an overview. Int J Inflam. 2011;2011:367284. doi: 10.4061/2011/367284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackman N. The role of tissue factor and factor VIIa in hemostasis. Anesth Analg. 2009;108:1447–1452. doi: 10.1213/ane.0b013e31819bceb1. [DOI] [PMC free article] [PubMed] [Google Scholar]


