Abstract
Asthma is a complex inflammatory disease involving the critical actions of several important cytokines. Epidemiological data show that obesity could increase the risk of asthma, and insulin resistance, or metabolic syndrome are an important risk factor for obesity asthma. Some studies identified that upstream of the transcription start site within the TNF-α gene promoter region-308 polymorphism was associated insulin resistance or metabolic disorders, while this site was closely related to asthma. But no research was performed to evaluate the influence of TNF-α-308G/A polymorphism on metabolic syndrome in asthmatic patients. Here, we recruited 248 asthmatic patients, who were separated into asthma with Mets/asthma without Mets groups and 226 matched healthy controls from Hebei Province to evaluate the influence of TNF-α-308G/A polymorphism on metabolic syndrome in asthmatic patients. Single nucleotide polymorphism of TNF-α-308 locus was genotyped using PCR-RFLP. Some biochemical variables were also determined. Our result showed that the genotypic and allelic frequency of rs1800629 did not show significant difference between asthmatic patients and normal controls. However, the frequency of A allele was significantly higher in asthma group with Mets (22.36%) than in controls (15.71%) (P = 0.02; OR = 0.647; 95% CI = 0.447-0.936). After analyzing the relationship between biochemical features of patients and genotype of TNF-α-308G/A, we found levels of LDL cholesterol, TNF-α and insulin, and HOMA-IR were significantly higher in the asthmatic patients carrying the GA and AA genotypes than in the carriers of GG genotype of rs1800629 (P = 0.029, P = 0.022, P = 0.043, respectively). Thus, our data suggested that TNF-α-308G/A variation was related to metabolic phenotype in asthma patients. Furthermore, we first identified TNF-α-308 A allele was the risk factor for asthmatic patients with Mets in Hebei population, China.
Keywords: Asthma, metabolic syndrome, insulin resistance, TNF-α polymorphism
Introduction
Asthma is a chronic allergic disorder of the airways that is characterized by inflammatory infiltrates in the bronchial walls, airway hyperresponsiveness (AHR) and reversible airway obstruction [1]. Currently, the global prevalence of asthma is 1%-18%, with the number of asthma patients totaling around 300 million, and the incidence of this disease is on the rise. The overall prevalence of asthma in China is 1%, but in children is up to 3%, with both increasing. It is believed to be a multifactorial disease whereby genetic factors contribute to its etiology and/or clinical severity [2,3]. However, the genes involved in asthma are still unknown and are likely to be numerous. To date, numerous risk genes have been reported to be associated with asthma susceptibility in various populations. One of the candidate susceptible genes that has been intensely investigated is tumor factor-alpha (TNF-α) [4,5].
TNF-α is a pro-inflammatory cytokine released during allergic responses by both macrophages and mast cells. Several polymorphisms in the promoter region of TNF-α have been associated with different TNF-α expression levels. Of these, the TNF-α-308G/A (also referred to as rs1800629) is the best studied. It involves the substitution of a guanine (G) by an adenine (A) and is associated with an increase in TNF-α expression levels [6,7]. Up to now, a lot of studies of genetic epidemiology have assessed the association of TNF-α gene polymorphisms and risk of asthma in different populations, but conflicting results were obtained due to the heterogeneity of the genetic background among populations. Furthermore, this support the need for replication studies among all ethnic groups.
Metabolic syndrome (Mets) which was firstly described by Reaven et al. as syndrome X, is a major public challenge worldwide [8,9]. The main components of the syndrome are obesity, insulin resistance (IR), hypertension, and dyslipidemia. There is agreement that Mets causes pro-inflammatory and thrombogenic state, leading to late-onset diabetes mellitus and cardiovascular diseases with increased mortality and morbidity. Recently, a great deal of epidemiological data shows that obesity and insulin resistance can increase the risk of asthma [10-13]. Its mechanism is unclear. Several studies have confirmed that TNF-α-308G/A polymorphisms are associated with insulin resistance or metabolic disorders.
Taken together the association of TNF-α gene polymorphisms and asthma, the aim of the present study was to investigate whether SNP in TNF-α-308G/A, rs1800629 is associated with metabolic syndrome in asthmatic patients and further explore the pathogenesis of asthma complicated by metabolic syndrome.
Patients and methods
Ethics statement
The Medical Ethics Committee of Hebei Provincial Chest Hospital approved this study. Written informed consents conforming to the tenets of the Declaration of Helsinki were obtained from each participant prior to the study.
Participants
A total of 248 patients with asthma were defined according to the criteria of the Global Initiative for Asthma (GINA), and 226 matched healthy controls were enrolled in this study from Hebei Provincial Chest Hospital Pulmonology outpatient clinic. All subjects are Han Chinese. Respiratory symptoms and medications were assessed in detail and pulmonary function tests were performed in a standard fashion using electronic spirometer (MIR), for every subject. The inclusion criteria for controls were as follows: no symptoms or history of asthma or other pulmonary diseases; no symptoms or history of atopy; negative skin prick test results with a battery of common aeroallergens; and absence of first-degree relatives with a history of asthma or atopy; without autoimmune or inflammatory disease.
All the subjects’ height and weight were measured by the same person using the same equipment. Body mass index was calculated by dividing body weight to height square (kg/m2). Biochemical features, including total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL), and high-density lipoprotein cholesterol (HDL) were collected for further study.
Diagnosis of metabolic syndrome
For the diagnosis of metabolic syndrome, modified Chinese Diabetes Society(CDS) diagnostic criteria were used. The CDS definition for Mets required impaired glucose tolerance plus two of the following three disorders: obesity (waist-to-hip ratio ≥ 0.9 cm in men or ≥ 0.85 cm in women), dyslipidemia (triglyceride level ≥ 1.7 mmol/L and/or HDL cholesterol level ≤ 1.03 mmol/L in men or ≤ 1.29 mmol/L in women), and high blood pressure (systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg). Glucose concentration was determined using the glucose oxidase method, and insulin concentration was determined using a radioimmunoassay (Diagnostic Products Corp, Los Angeles, CA). To assess insulin resistance, homeostasis model assessment of insulin resistance (HOMA-IR) index was calculated as follows: [fasting insulin (μU/mL)] × [fasting glucose (mmol/L)]/22.5. The HOMA-IR index is a mathematical model designed by Mathews et al. [14].
Genotyping of SNP in TNF-α-308G/A, rs1800629
Genome DNA from whole blood cells of each sample was extracted by using Blood Genomic DNA Miniprep Kit (Axygen, USA) according to the manufacturer’s instructions. Genotyping for the TNF-α-308G/A polymorphisms in genomic DNA was performed using the PCR and restriction fragment length polymorphism (RFLP). The genomic region encompassing the-308G/A polymorphism was amplified using the following primers: forward 5’-AGGCAATAGGTTTTGAGGGCCAT-3’ and reverse 5’-TCCCTGCTCCGATTCCG-3’. Polymerase chain reaction products were generated in a 10 μL reaction volume containing 50 ng of genomic DNA, 1 × PCR buffer, 2 mmol/L MgCl2, 0.2 mmol/L of each dNTP, 1 μmol/L of each primer, and 0.25 U of Taq DNA polymerase (Invitrogen Corporation, Carlsbad, CA). Cycling conditions consisted of an initial denaturation step at 94°C for 5 minutes, followed by 35 cycles of denaturation at 94°C for 30 seconds, annealing at 60°C for 30 seconds, and extension at 72°C for 30 seconds and a final elongation step at 72°C for 1 minute. Polymerase chain reaction products were digested with 2 U of NcoI restriction enzyme at 37°C, according to the manufacturer’s instructions (New England BioLabs, Ipswich, MA). The-308G allele contains an NcoI restriction site not present in the-308A allele; thus, in the presence of the-308G allele, the PCR product (107 bp) is cut into 2 fragments of 80 and 27 bp in length [15].
Assay of serum TNF-α levels
The serum level of TNF-α was determined by ELISA Quantikine Human TNF-α immunoassay kit (Biosource, USA). The lower limit of detection ranged from 5 to 5.5 pg/mL. Assay was carried out according to the manufacturers’ instructions.
Statistic analysis
Data were statistically described in terms of mean ± standard deviation (SD), or frequencies (number of cases) and percentages as required depending on their distribution. The Hardy-Weinberg equilibrium (HWE) was assessed for each variation to identify the deviation. The differences of the genotypes and alleles of TNF-α-308G/A between patients and normal controls were evaluated by using Pearson Chi-square test. Exact test was used instead when the expected frequency is less than 5. The odds ratio (OR) and 95% confidence intervals (95% CI) were calculated. Unpaired Student’s t test or Mann-Whitney tests were used for two-group comparisons. Because of skewed distributions, log-transformed values for HDL cholesterol, LDL cholesterol, insulin, HOMA-IR, and TNF-α were used in analyses and back-transformed for data presentation. Statistical analysis of data was performed using the SPSS software package 16.0 (SPSS Inc. USA). P-value less than 0.05 was considered statistically significant.
Results
In this study, 248 asthmatic (108 males and 140 females) and 226 controls (112 males and 114 females) were screened for rs1800629 polymorphisms using PCR-RFLP methods. The mean age of asthmatic patients was 46.48 years, and mean age of matched controls was 46.58 years. There were no significant differences between two groups with regarded to gender and age distribution. Table 1 showed the general characteristics of the studied subjects. The incidence of Mets in asthma group was about triple that of control (61.29% vs. 22.81%). Ninety-six (38.71%) patients in asthma group and 37 (16.23%) patients in control group had Hypertension. Body mass index, glucose and insulin blood levels, HOMA-IR and serum TNF-α levels were significantly higher in asthma patients (P < 0.001 for both parameter). There were no differences between lipid panels including total cholesterol, triglycerides, LDL cholesterol, and HDL cholesterol.
Table 1.
Anthropometric and biochemical data of patients and healthy controls
| Characteristic | Asthma | Control | P-value |
|---|---|---|---|
| Male [n (%)] | 108 (43.54) | 112 (49.56) | 0.342 |
| Age (years) | 46.48 ± 10.25 | 46.58 ± 12.25 | 0.765 |
| Mets [n (%)] | 152 (61.29) | 52 (22.81) | < 0.001 |
| Hypertension [n (%)] | 96 (38.71) | 37 (16.23) | < 0.001 |
| BMI (kg/m2) | 24.25 ± 1.98 | 22.34 ± 1.01 | < 0.001 |
| Fasting insulin (μU/mL) | 14.8 ± 8.2 | 8.1 ± 7.1 | < 0.001 |
| Fasting glycemia (mmol/L) | 6.14 ± 1.24 | 4.21 ± 0.74 | < 0.001 |
| HOMA-IR | 3.9 ± 2.3 | 1.6 ± 1.7 | < 0.001 |
| Total cholesterol (mmol/L) | 5.34 ± 0.87 | 5.52 ± 1.08 | 0.574 |
| Triglycerides (mmol/L) | 1.81 ± 0.76 | 1.79 ± 0.36 | 0.637 |
| HDL cholesterol (mmol/L) | 1.03 ± 0.48 | 1.29 ± 0.45 | 0.067 |
| LDL cholesterol (mmol/L) | 3.02 ± 1.41 | 2.65 ± 1.26 | 0.059 |
| TNF-α (pg/ml) | 53.21 ± 3.84 | 27.93 ± 6.50 | < 0.001 |
Mets, metabolic syndrome; BMI indicates body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Firstly, the frequency of genotypes and alleles of TNF-α gene SNP rs1800629 was detected in asthmatic patients and controls. HWE of rs1800629 in patients and controls were listed in Table 2, and the results showed allelic distribution of rs1800629 was not deviated from HWE in both case and control populations. The genotypic and allelic frequency of rs1800629 did not show significant difference between asthmatic patients and normal controls. Then, Genotype and allele frequency of rs1800629 were detected in metabolic syndrome patients and normal control (Table 3). The genotypic and allelic frequency of rs1800629 between asthmatic patients and normal controls did not show significant difference.
Table 2.
Genotype and allele frequency of rs1800629 and Pearson’s chi-square test in asthmatic patients and normal control
| Genotype/Allele | Patients (n = 248) | Controls (n = 226) | P-value | OR (95% CI) |
|---|---|---|---|---|
|
|
|
|||
| HWE* P = 0.25 | HWE P = 0.21 | |||
| GG | 165 | 187 | 0.610 | 0.905 (0.616-1.330) |
| GA | 76 | 37 | 0.075 | 0.822 (0.680-1.487) |
| AA | 7 | 2 | 0.122 | 4.560 (0.556-37.422) |
| G | 406 | 411 | 0.318 | 0.841 (0.598-1.182) |
| A | 90 | 41 |
Chi-square test for deviation from the Hardy-Weinberg equilibrium (a value of P < 0.001 was regarded as a deviation from the HWE).
Table 3.
Genotype and allele frequency of rs1800629 and Pearson’s chi-square test in metabolic syndrome patients and normal control
| Genotype/Allele | Patients (n = 204) | Controls (n = 270) | P-value | OR (95% CI) |
|---|---|---|---|---|
|
|
|
|||
| HWE* P = 0.82 | HWE P = 0.42 | |||
| GG | 132 | 219 | 0.559 | 0.869 (0.542-1.392) |
| GA | 64 | 49 | 0.921 | 1.258 (0.787-2.010) |
| AA | 8 | 2 | 0.350 | 3.358 (0.347-32.543) |
| G | 366 | 493 | 0.356 | 0.813 (0.524-1.267) |
| A | 42 | 46 |
Chi-square test for deviation from the Hardy-Weinberg equilibrium (a value of P < 0.001 was regarded as a deviation from the HWE).
On the basis of Mets criteria, the asthmatic patients were further subgrouped as asthma with Mets and asthma without Mets ones. As shown in Table 4, among the asthmatic patients with Mets, the GG genotype of the rs1800629 was found in 59.21% (90/152) of the cases, whereas the GA and AA were present in 36.84% (56/152) and 3.95% (6/152) of the asthmatic patients with Mets, respectively. In the asthmatic patients without Mets, the GG, GA and AA genotypes were found in 78.13% (75/96), 20.83% (20/96) and 1.04% (1/96) of the cases, respectively. The frequency of a allele was significantly higher in asthma group with Mets (22.36%) than in controls (15.71%) (P = 0.02; OR = 0.647; 95% CI = 0.447-0.936). While asthmatic patients without Mets failed to show any such difference (P = 0.16; OR = 1.440; 95% CI = 0.864-2.461).
Table 4.
The comparison of genotype and allele frequency of TNF-α site in asthmatic subgroups and the control group
| Groups (N) | Genotype (%) | Allele (%) | |||
|---|---|---|---|---|---|
|
|
|
||||
| GG | GA | AA | G | A | |
| As with Mets (152) | 90 (59.21) | 56 (36.84) | 6 (3.95) | 236 (77.63) | 68 (22.36) |
| As without Mets (96) | 75 (78.13) | 20 (20.83) | 1 (1.04) | 170 (88.54) | 22 (11.46) |
| Controls (226) | 187 (69.03) | 37 (30.53) | 2 (0.44) | 381(84.29) | 71 (15.71) |
| Control with Mets (52) | 42 (80.77) | 8 (15.38) | 2 (3.84) | 92 (88.47) | 12 (11.54) |
| Control without Mets (174) | 145 (83.33) | 29 (16.67) | 0 | 290 (83.33) | 29 (16.67) |
χ2 (df = 1), (As with Mets vs. Controls) = 5.37; P = 0.02; OR = 0.647; 95% CI = 0.447-0.936; χ2 (df = 1), (As without Mets vs. Controls) = 5.22; P = 0.16; OR = 1.440; 95% CI = 0.864-2.461, χ2 (df = 1), (As with Mets vs. As without Mets) = 9.431; P = 0.002; OR = 0.449; 95% CI = 0.267-0.755. χ2 (df = 1), (As with Mets vs. Mets) = 4.28; P = 0.25; OR= 0.847; 95% CI = 0.745-1.106.
In the patients with asthma, anthropometric and biochemical parameters were analyzed according to the genotypes of rs1800629. For statistical purposes, the carriers of the A allele in the heterozygous and homozygous states were treated together (Table 5). We observed that the levels of LDL cholesterol and insulin, and HOMA-IR were significantly higher in the asthmatic patients carrying the GA and AA genotypes than in the carriers of GG genotype of rs1800629 (P = 0.029, P = 0.022, P = 0.043, respectively). Furthermore, results revealed at among asthmatic patients (Figure 1), the rs1800629 GA and GG genotypes exhibited significantly higher TNF-α serum levels than that of GG genotype (56.21 ± 1.63 vs. 51.43 ± 1.52, P < 0.001).
Table 5.
Anthropometric and biochemical parameters in patients with asthma according to TNF-α genotypes for -308G/A polymorphisms
| Characteristic | Genotype | P-value | |
|---|---|---|---|
|
| |||
| GG (n = 165) | GA + AA (n = 83) | ||
| Age (years) | 46.91 ± 13.1 | 46.02 ± 14.1 | 0.757 |
| BMI (kg/m2) | 24.12 ± 2.98 | 24.37 ± 2.47 | 0.521 |
| Fasting insulin (μU/mL) | 11.13 ± 3.64 | 15.72 ± 8.31 | 0.022 |
| Fasting glycemia (mmol/L) | 6.11 ± 1.85 | 6.30 ± 2.14 | 0.528 |
| HOMA-IR | 2.64 ± 0.69 | 4.20 ± 2.37 | 0.043 |
| Total cholesterol (mmol/L) | 5.34 ± 0.97 | 4.72 ± 1.08 | 0.074 |
| Triglycerides (mmol/L) | 1.98 ± 0.92 | 1.72 ± 0.86 | 0.473 |
| HDL cholesterol (mmol/L) | 1.01 ± 0.24 | 1.05 ± 0.17 | 0.185 |
| LDL cholesterol (mmol/L) | 2.55 ± 1.26 | 3.13 ± 1.04 | 0.029 |
Figure 1.
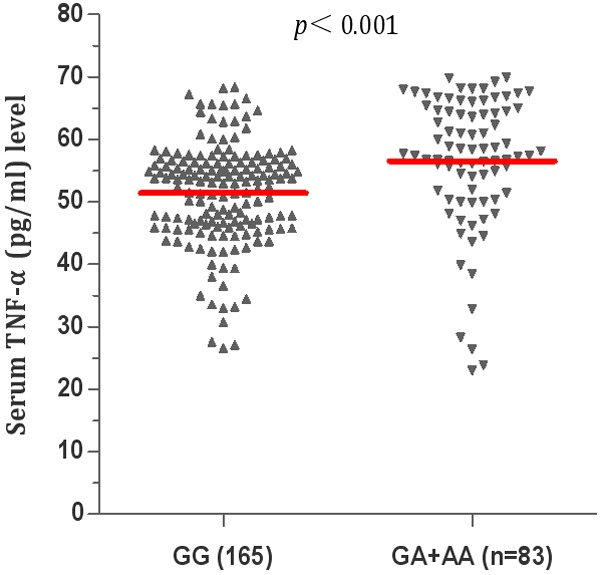
Comparison of serum levels of TNF-α in asthma group under the genotypes of rs1800629 polymorphisms. GG genotype of rs1800629 in patients with asthma (n = 165), GT and TT genotypes of rs1800629 in patients with asthma (n = 83).
Discussion
Asthma is a complex inflammatory disease involving the critical actions of several important cytokines. The proinflammatory cytokines, such as TNF-α, are found in airways and are known to induce inflammatory responses and regulate immunity [16]. TNF-α has important biological effects on airway inflammation and remodeling. Several studies have shown that high serum TNF-α level is linked to hyperresponsiveness in asthma. Furthermore, the concentration of TNF-α was found to be elevated in asthmatic airways and sputum. TNF-α has many effects relevant to the pathogenesis of asthma, including neutrophil release, epithelial cell barrier permeability, macrophage activation, recruitment of inflammatory infiltrates, effectiveness of the local and systemic inflammatory response, and amplification of the effects of other proinflammatory cytokines. Because secretion of cytokine is genetically regulated at the level of transcription, and the linkage of TNF-α polymorphisms with the genotype of asthma has been demonstrated in accumulating studies [17-19].
TNF-α gene is located in the class III region of the human major histocompatibility complex (MHC) on chromosome 6p21 [20,21]. Among the several single nucleotide polymorphisms (SNPs) identified in TNF-α, TNF-α rs1800629 is the most extensively studied. The A allele of this polymorphism can lead to high binding affinity of nuclear factors to the TNF promoter, resulting in a high level of transcription activity and secretion levels of TNF-α. So, it was suggested to have a significant functional effect [22]. A number of studies have tried to determine whether the polymorphism of TNF-α rs1800629 influences TNF-α expression, susceptibility to asthma, but no accordant result was obtained due to the heterogeneity of the genetic background among populations [23-25]. Whether genetic variations of the TNF-α rs1800629 conferred susceptibility to asthmatic patients in Chinese was puzzled.
In this study, we analyzed TNF-α gene SNP rs1800629 in 248 asthmatic patients and 226 matched controls from Hebei, China. In order to exclude the gender bias, the percentage of males was extremely similar in patients (43.54%, 108/248) and controls (49.56%, 112/226). No difference was found between genotypic and allelic frequency of rs1800629 in asthmatic patients and normal controls. This result was in contrast with other previous studies that the TNF-α rs1800629 polymorphism was strongly associated with the risk of asthma. According to stratified analysis by ethnicity, Zhang et al. showed significant associations were showed in Asians, but not Caucasians. However, Yang et al. meta-analysis suggested the positive association was shown in West Asians and South Asians, but not in East Asians. It is possible that different genetic backgrounds and environmental exposure may account for these differences.
Additionally, our results revealed asthmatic subjects with genotypes carrying at least one A alles (AA and AG genotypes) exhibited significantly higher TNF-α serum levels than that of GG genotype (Figure 1). It has been shown previously that individuals carrying the GA genotype have higher amounts of TNF-α mRNA, and serum protein levels, than individuals with the G/G genotype. Similarly, Louis et al. reported that cells stimulated with lipopolysacharide, from individuals with the rs1800629 A allele, expressed more TNF-α than did the cells from individuals that were homozygous for the G allele.
TNF-α rs1800629 polymorphism was suggested to have a significantly functional effect, with the A allele being associated with higher constitutive and inducible levels of transcription for TNF-α than the G allele [26]. The A allele of this polymorphism has been reported to be correlated with an increase in transcription activity and secretion levels of TNF-α [6,27]. The TNF-α rs1800629 A allele leads to high binding affinity of nuclear factors to the TNF promoter and gives a high level of gene transcription. Several studies have shown that high serum TNF-α level is linked to hyper responsiveness in asthma. In addition, TNF-α was found in increased concentration in asthmatic airways, in lavage fluid from asthmatic lungs and induced sputum from subjects with severe asthma [23,28,29].
These increases in serum TNF-α level may be attributed to its being a proinflammatory cytokine. TNF-α has many effects relevant to the pathogenesis of asthma, including neutrophil release, epithelial cell barrier permeability, macrophage activation, recruitment of inflammatory infiltrates, effectiveness of the local and systemic inflammatory response, and amplification of the effects of other proinflammatory cytokines [30].
In the patients with asthma, anthropometric and biochemical parameters were analyzed under the genotypes of rs1800629. We observed the levels of LDL cholesterol and insulin, and HOMA-IR content of AA+GA carriers were higher than that of the AA carriers. This finding is in contrast with a study conducted by Chang et al., who reported an association between the asthma patients and an insulin resistance state in relation with the AA + GA genotypes of the rs1800629 polymorphism. TNF-α suppresses insulin induced tyrosine phosphorylation of insulin receptor and its substrates which may affect insulin sensitivity [31,32]. LDL cholesterol is sometimes referred to as bad cholesterol because they can transport their content of fat molecules into artery walls, attract macrophages, and thus drive atherosclerosis. LDL cholesterol pose a risk for cardiovascular disease when they invade the endothelium and become oxidized, since the oxidized forms are more easily retained by the proteoglycans [33]. A complex set of biochemical reactions regulates the oxidation of LDL particles, chiefly stimulated by presence of necrotic cell debries and free radicals in the endothelium [34]. To our knowledge, this is the first time to identify that SNP rs1800629 in the TNF-α conferred to IR and TBIL value in China asthmatic patients. All these results indicated that though the rs1800629 was not genetic susceptible factor for asthmatic patients, it associated with clinical features such as serum LDL cholesterol and insulin.
When the asthmatic patients were further subgrouped as asthma with Mets and asthma without Mets according to diagnosis of Mets criteria, significant association was observed between this polymorphism and asthmatic patients with Mets susceptibility. While asthmatic patients without Mets failed to show any such difference. This might suggest that the TNF-α rs1800629 polymorphism may play a role in the etiology of Mets patients with asthma. Due to the limited sample size in this study, we suggested more Mets patients with asthma should be collected for further verification. To our knowledge, It is the first time to verify SNP rs1800629 polymorphism could impact on asthmatic patients with Mets in Chinese, and this result was partially consistent with high expression of TNF-α mRNA and TNF-α -stimulated genes in Mets patients [35].
Therefore, Carrier of TNF-α-308A locus, high level of LDL-C and decrement in lung function lead to insulin resistance. Thus, it results in an increased incidence of metabolic syndrome in asthmatic patients. So, people with asthma carrying rs1800629 A allele should be early intervention, such as reducing inflammation, weight control, improve insulin resistance, and improve lung function. These measurements can effectively reduce the metabolic syndrome and atherosclerosis occurs, thereby reducing asthma, heart and cerebrovascular disease incidence and mortality.
Conclusion
In summary, though no any relationship between genotypes and alleles in rs1800629 and asthma susceptibility was revealed, rs1800629 A allele increases insulin resistance and LDL cholesterol in asthmatic patients. Furthermore, we identified rs1800629 was the risk factor for asthmatic patients with Mets in Hebei population.
Acknowledgements
We thank all the participants in this study. This study was supported by Training Program Foundation for Talent of Hebei Provincial Chest Hospital (HPCH201326148) and the Natural Science Foundation of Hebei Province (H2014206208).
Disclosure of conflict of interest
None.
References
- 1.Cohn L, Elias JA, Chupp GL. Asthma: mechanisms of disease persistence and progression. Annu Rev Immunol. 2004;22:789–815. doi: 10.1146/annurev.immunol.22.012703.104716. [DOI] [PubMed] [Google Scholar]
- 2.Braman SS. The global burden of asthma. Chest. 2006;130:4S–12S. doi: 10.1378/chest.130.1_suppl.4S. [DOI] [PubMed] [Google Scholar]
- 3.Koppelman GH, Nawijn MC. Recent advances in the epigenetics and genomics of asthma. Curr Opin Allergy Clin Immunol. 2011;11:414–419. doi: 10.1097/ACI.0b013e32834a9573. [DOI] [PubMed] [Google Scholar]
- 4.Ober C, Yao TC. The genetics of asthma and allergic disease: a 21st century perspective. Immunol Rev. 2011;242:10–30. doi: 10.1111/j.1600-065X.2011.01029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang G, Chen J, Xu F, Bao Z, Yao Y, Zhou J. Association between tumor necrosis factor-alpha rs1800629 polymorphism and risk of asthma: a meta-analysis. PLoS One. 2014;9:e99962. doi: 10.1371/journal.pone.0099962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louis E, Franchimont D, Piron A, Gevaert Y, Schaaf-Lafontaine N, Roland S, Mahieu P, Malaise M, De Groote D, Louis R, Belaiche J. Tumour necrosis factor (TNF) gene polymorphism influences TNF-αlpha production in lipopolysaccharide (LPS)-stimulated whole blood cell culture in healthy humans. Clin Exp Immunol. 1998;113:401–406. doi: 10.1046/j.1365-2249.1998.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma S, Sharma A, Kumar S, Sharma SK, Ghosh B. Association of TNF haplotypes with asthma, serum IgE levels, and correlation with serum TNF-αlpha levels. Am J Respir Cell Mol Biol. 2006;35:488–495. doi: 10.1165/rcmb.2006-0084OC. [DOI] [PubMed] [Google Scholar]
- 8.Kassi E, Pervanidou P, Kaltsas G, Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48. doi: 10.1186/1741-7015-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–1454. doi: 10.1161/01.CIR.0000158483.13093.9D. [DOI] [PubMed] [Google Scholar]
- 10.Forno E, Han YY, Muzumdar RH, Celedón JC. Insulin resistance, metabolic syndrome, and lung function in US adolescents with and without asthma. J Allergy Clin Immunol. 2015;136:304–11. e8. doi: 10.1016/j.jaci.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garmendia JV, Moreno D, Garcia AH, De Sanctis JB. Metabolic syndrome and asthma. Recent Pat Endocr Metab Immune Drug Discov. 2014;8:60–66. doi: 10.2174/1872214807666140107151023. [DOI] [PubMed] [Google Scholar]
- 12.Serafino-Agrusa L, Spatafora M, Scichilone N. Asthma and metabolic syndrome: Current knowledge and future perspectives. World J Clin Cases. 2015;3:285–292. doi: 10.12998/wjcc.v3.i3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Assad N, Qualls C, Smith LJ, Arynchyn A, Thyagarajan B, Schuyler M, Jacobs DR Jr, Sood A. Reply: body mass index is a stronger predictor than the metabolic syndrome for future asthma in women. Am J Respir Crit Care Med. 2014;189:232–233. doi: 10.1164/rccm.201308-1488LE. [DOI] [PubMed] [Google Scholar]
- 14.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 15.Guzman-Flores JM, Escalante M, Sanchez-Corona J, Garcia-Zapien AG, Cruz-Quevedo EG, Munoz-Valle JF, Moran-Moguel MC, Saldana-Cruz AM, Flores-Martinez SE. Association analysis between -308G/A and -238G/A TNF-αlpha gene promoter polymorphisms and insulin resistance in Mexican women with gestational diabetes mellitus. J Investig Med. 2013;61:265–269. doi: 10.2310/JIM.0b013e31827b98c9. [DOI] [PubMed] [Google Scholar]
- 16.Nakae S, Suto H, Berry GJ, Galli SJ. Mast cell-derived TNF can promote Th17 cell-dependent neutrophil recruitment in ovalbumin-challenged OTII mice. Blood. 2007;109:3640–3648. doi: 10.1182/blood-2006-09-046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bilolikar H, Nam AR, Rosenthal M, Davies JC, Henderson DC, Balfour-Lynn IM. Tumour necrosis factor gene polymorphisms and childhood wheezing. Eur Respir J. 2005;26:637–646. doi: 10.1183/09031936.05.00071104. [DOI] [PubMed] [Google Scholar]
- 18.Trajkov D, Mirkovska-Stojkovikj J, Arsov T, Petlichkovski A, Strezova A, Efinska-Mladenovska O, Sandevska E, Gogusev J, Spiroski M. Association of cytokine gene polymorphisms with bronchial asthma in Macedonians. Iran J Allergy Asthma Immunol. 2008;7:143–156. [PubMed] [Google Scholar]
- 19.Jiffri EH, Elhawary NA. The impact of common tumor necrosis factor haplotypes on the development of asthma in children: an Egyptian model. Genet Test Mol Biomarkers. 2011;15:293–299. doi: 10.1089/gtmb.2010.0157. [DOI] [PubMed] [Google Scholar]
- 20.A genome-wide search for asthma susceptibility loci in ethnically diverse populations. The Collaborative Study on the Genetics of Asthma (CSGA) Nat Genet. 1997;15:389–392. doi: 10.1038/ng0497-389. [DOI] [PubMed] [Google Scholar]
- 21.Daniels SE, Bhattacharrya S, James A, Leaves NI, Young A, Hill MR, Faux JA, Ryan GF, le Souef PN, Lathrop GM, Musk AW, Cookson WO. A genome-wide search for quantitative trait loci underlying asthma. Nature. 1996;383:247–250. doi: 10.1038/383247a0. [DOI] [PubMed] [Google Scholar]
- 22.Schluter B, Erren M, Schotte H, Junker R, Rust S, Assmann G. The mutagenically separated polymerase chain reaction is a rapid and reliable method for genotyping of the tumour necrosis factor-alpha promoter polymorphism (-308 G/A) Clin Chim Acta. 2002;320:135–138. doi: 10.1016/s0009-8981(02)00054-2. [DOI] [PubMed] [Google Scholar]
- 23.Shin HD, Park BL, Kim LH, Jung JH, Wang HJ, Kim YJ, Park HS, Hong SJ, Choi BW, Kim DJ, Park CS. Association of tumor necrosis factor polymorphisms with asthma and serum total IgE. Hum Mol Genet. 2004;13:397–403. doi: 10.1093/hmg/ddh036. [DOI] [PubMed] [Google Scholar]
- 24.Wang JY, Liou YH, Wu YJ, Hsiao YH, Wu LS. An association study of 13 SNPs from seven candidate genes with pediatric asthma and a preliminary study for genetic testing by multiple variants in Taiwanese population. J Clin Immunol. 2009;29:205–209. doi: 10.1007/s10875-008-9256-6. [DOI] [PubMed] [Google Scholar]
- 25.Attia J, Thakkinstian A, D’Este C. Meta-analyses of molecular association studies: methodologic lessons for genetic epidemiology. J Clin Epidemiol. 2003;56:297–303. doi: 10.1016/s0895-4356(03)00011-8. [DOI] [PubMed] [Google Scholar]
- 26.Hohjoh H, Nakayama T, Ohashi J, Miyagawa T, Tanaka H, Akaza T, Honda Y, Juji T, Tokunaga K. Significant association of a single nucleotide polymorphism in the tumor necrosis factor-alpha (TNF-αlpha) gene promoter with human narcolepsy. Tissue Antigens. 1999;54:138–145. doi: 10.1034/j.1399-0039.1999.540204.x. [DOI] [PubMed] [Google Scholar]
- 27.Puthothu B, Bierbaum S, Kopp MV, Forster J, Heinze J, Weckmann M, Krueger M, Heinzmann A. Association of TNF-αlpha with severe respiratory syncytial virus infection and bronchial asthma. Pediatr Allergy Immunol. 2009;20:157–163. doi: 10.1111/j.1399-3038.2008.00751.x. [DOI] [PubMed] [Google Scholar]
- 28.Obase Y, Shimoda T, Mitsuta K, Matsuo N, Matsuse H, Kohno S. Correlation between airway hyperresponsiveness and airway inflammation in a young adult population: eosinophil, ECP, and cytokine levels in induced sputum. Ann Allergy Asthma Immunol. 2001;86:304–310. doi: 10.1016/S1081-1206(10)63303-0. [DOI] [PubMed] [Google Scholar]
- 29.Martin RJ, Chu HW, Honour JM, Harbeck RJ. Airway inflammation and bronchial hyperresponsiveness after Mycoplasma pneumoniae infection in a murine model. Am J Respir Cell Mol Biol. 2001;24:577–582. doi: 10.1165/ajrcmb.24.5.4315. [DOI] [PubMed] [Google Scholar]
- 30.Bradley JR. TNF-mediated inflammatory disease. J Pathol. 2008;214:149–160. doi: 10.1002/path.2287. [DOI] [PubMed] [Google Scholar]
- 31.Suriano AR, Sanford AN, Kim N, Oh M, Kennedy S, Henderson MJ, Dietzmann K, Sullivan KE. GCF2/LRRFIP1 represses tumor necrosis factor alpha expression. Mol Cell Biol. 2005;25:9073–9081. doi: 10.1128/MCB.25.20.9073-9081.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalziel B, Gosby AK, Richman RM, Bryson JM, Caterson ID. Association of the TNF-αlpha -308 G/A promoter polymorphism with insulin resistance in obesity. Obes Res. 2002;10:401–407. doi: 10.1038/oby.2002.55. [DOI] [PubMed] [Google Scholar]
- 33.Beisiegel U, Weber W, Ihrke G, Herz J, Stanley KK. The LDL-receptor-related protein, LRP, is an apolipoprotein E-binding protein. Nature. 1989;341:162–164. doi: 10.1038/341162a0. [DOI] [PubMed] [Google Scholar]
- 34.Prado KB, Shugg S, Backstrand JR. Low-density lipoprotein particle number predicts coronary artery calcification in asymptomatic adults at intermediate risk of cardiovascular disease. J Clin Lipidol. 2011;5:408–413. doi: 10.1016/j.jacl.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Padrón-Morales J, Sanz C, Dávila I, Muñoz-Bellido F, Lorente F, Isidoro-García M. Polymorphisms of the IL12B, IL1B, and TNFA genes and susceptibility to asthma. J Investig Allergol Clin Immunol. 2013;23:487–494. [PubMed] [Google Scholar]


