Abstract
Objective
To describe the disease characteristics of patients with juvenile idiopathic arthritis (JIA) included in an inception cohort, to analyse how many patients from each JIA category reach an inactive disease state within the first year of specialised care and to determine predictors for attaining inactive disease.
Methods
Patients with JIA were enrolled in this study at 11 large German paediatric rheumatology units within the first 12 months after diagnosis. Laboratory and clinical parameters such as JIA core criteria and data on the medication used were collected every 3 months. Non-parametric statistical testing was performed for the comparison of the JIA core criteria at follow-up. Generalised linear models were used to analyse differences in the rates at which inactive disease was reached and to determine potential predictors.
Results
Of the 695 patients with JIA included in this analysis, approximately 75% experienced a period of inactive disease under treatment with disease-modifying antirheumatic drugs and systemic steroids in most cases with systemic-onset JIA or polyarthritis at least once during the first 12 months in ICON. Significant improvements were observed in all JIA core criteria, in disease activity and in functional status from baseline to the 12-month follow-up. Younger age at onset, a shorter duration between symptom onset and diagnosis and a positive antinuclear antibody status increased the probability of attaining an inactive disease state.
Conclusions
The 12-month outcome of JIA was good under real-life conditions, with half of the patients having attained inactive disease with contemporary treatments. Since a short duration between symptom onset and diagnosis was correlated to a period of inactive disease, children suspected of having JIA should be transferred to specialised care as soon as possible.
Keywords: Juvenile Idiopathic Arthritis, Outcomes research, Epidemiology
Key messages.
What is already known about this subject?
Achievement of inactive disease can be reached on contemporary treatments in juvenile idiopathic arthritis.
What does this study add?
Three of four patients with juvenile idiopathic arthritis (JIA) attain an inactive disease state within the first year under routine paediatric rheumatology care, but marked differences exist among the various JIA categories. Younger age at JIA onset and a shorter time between symptom onset and diagnosis increase the probability of reaching a persistent state of inactive disease.
How might this impact on clinical practice?
The data underline the need for the earliest possible rheumatology care and for optimising the treatment of specific juvenile idiopathic arthritis categories. With these data, families can be informed about their chances of reaching inactive disease in the first year of specialised care.
Introduction
Juvenile idiopathic arthritis (JIA) is the most common chronic inflammatory rheumatic disease and one of the most common causes of disability in childhood. According to the International League of Associations for Rheumatology (ILAR) criteria, the diagnosis of JIA comprises seven categories, each with different characteristics and outcomes that range from a mild oligoarticular course to life-threatening disease. Prognostic indicators would be most helpful to evaluate the risk of an unfavourable disease course and to decide the appropriate treatment, especially in respect of new biological drugs.
JIA outcomes have been studied in several cross-sectional and retrospective studies,1 2 but prospective, longitudinal data would be most relevant to expanding our understanding of the disease course and the prognosis of the different JIA categories. Recently, patients newly diagnosed with JIA were included and followed prospectively in the Nordic JIA cohort study (Scandinavian countries, start 1997,3 4), the Childhood Arthritis Prospective Study (CAPS, UK, start 2001,5) and the Research on Arthritis in Canadian Children Emphasising Outcomes study (ReACCh Out, Canada, start 2005,6 7) to gain insights into the disease course and outcomes under current treatment options and to identify prognostic factors for defined outcomes such as disease activity, function and response to therapy. In 2010, the Inception Cohort of Newly diagnosed patients with juvenile idiopathic arthritis (ICON) was launched with the same goals in Germany, in which 11 paediatric rheumatology centres enrolled patients recently diagnosed with JIA, covering >75% of the incident cases of JIA expected in the population. ICON was established to longitudinally gather data about the disease course and several outcome parameters following routine care in Germany. This study offers a unique opportunity to analyse disease characteristics and treatment responses as well as identify prognostic factors or predictors for outcome measures. Clinical and laboratory parameters, as well as psychosocial and socioeconomic aspects, were recorded and evaluated in terms of their predictive value regarding the disease course, the development of damage and the impact on health-related quality of life.
The aim of the present analysis of ICON data was to describe the cohort in terms of demographic and clinical parameters and to evaluate when, for how long and how many patients with JIA in each category achieve inactive disease (ID) during the first year of paediatric rheumatology care.
Patients and methods
Inception cohort of newly diagnosed patients with JIA (ICON)
ICON was launched as a multicentre-controlled observational cohort study to observe patients with a recent onset of JIA for at least 10 years. Inclusion criterion was a diagnosis of JIA according to the ILAR criteria (arthritis of at least 6 weeks duration without a known cause in individuals <16 years of age8) made within 12 months before enrolment. All consecutively observed patients who met the inclusion criteria were asked to participate at 11 of the largest paediatric rheumatology units in Germany. More than 85% of the patients and their families agreed to participate. Time and personal reasons, lack of interest and language problems were the most frequent causes for non-participation.
Up to 30 June 2014, 954 patients were included in ICON, 695 of whom had already completed the 12-month follow-up (FU) and provided the base for the current study.
Demographic and clinical data, as well as medication and family history, were recorded using standardised questionnaires completed by physicians and parents or patients (8 years of age and older), respectively. Clinical and medication FU data were collected every 3 months during the first year and then every 6 months. At each visit the following JIA core criteria were collected: i) the active joint count, ii) the number of joints with limited range of motion (ROM), iii) the physician's global assessment of disease activity, iv) the patient or parent global assessment of well-being on a numeric rating scale (NRS) (21-point NRS 0–10), v) the Childhood Health Assessment Questionnaire (CHAQ), and vi) the erythrocyte sedimentation rate (ESR) or C reactive protein. Furthermore, morning stiffness (in minutes), active enthesitis count, pain (NRS) and the Paediatric Quality of Life Inventory (PedsQL) generic and rheumatology modules (with a score from 0 being the worst to 100 being the best possible score) were also documented. Additionally, on each visit, the physicians evaluated whether the disease was inactive according to the 2004 criteria defined by Wallace et al10 and, if so, since when (month/year). States of remission on medication (ID on drugs for at least 6 months) and off medication (ID off drugs for at least 12 months) were also defined according to Wallace et al.10 We defined a state of continuously ID for 3 months as an additional outcome parameter; this parameter was added on the basis of the assumption that it may be more suitable than remission on medication in the whole cohort, given the relatively short observation period, or the ID state at just the 12-month FU.
Laboratory parameters such as antinuclear antibodies (ANA), rheumatoid factor (RF) and human leucocyte antigen (HLA) B27 were also recorded at enrolment. Furthermore, all patients included in ICON received a slit-lamp examination by an ophthalmologist according to the screening guidelines11 by the German study group ‘Uveitis in childhood’. Once uveitis had been diagnosed, it was documented according to the Standardization of Uveitis Nomenclature (SUN12), and patients were observed further by an ophthalmologist who documented disease activity, treatment and possible complications.
Parents and patients 8 years of age and older gave their informed consent for participation.
Participants
Patients selected for this report were enrolled in ICON and had completed at least the 12-month assessment (n=695) with adequate data to ascertain ID state by the criteria published by Wallace et al.10 Among these, 53 (7.6%) did not provide adequate data to ascertain an ID state at the 3-month assessment, 88 (12.7%) at the 6-month assessment and 112 (16.1%) at the 9-month assessment. How missing information on the ID state was handled at each assessment is discussed in the section where the outcome definition is described.
JIA categories—review process/redefinition of ILAR classification
The ILAR diagnoses of all patients were subjected to a review process for JIA category validation by a syntax using the SAS program: exclusion criteria for the respective category were applied to all data sets of this category, and cases with conflicting results were reviewed by two paediatric rheumatologists (KM and CS). Queries were sent to the study centres to clarify category assignment. Finally, according to this review process, 15% patients were reclassified regarding their JIA category diagnosis.
Outcome
As the primary outcome of our study, we analysed how many patients ever reached an ID state according to the ILAR categories; for the patients who reached an ID state, we analysed when and for how long during the first 12 months after enrolment the ID state occurred.
The cumulative time with ID was determined by summing up the sequential time periods of ID during the first 12 months after enrolment. If a visit was missed and the disease state changed from ‘inactive’ at the last visit to ‘active’ at the next available visit, the end of the period of ID was set in the middle of the time period between these two visits. Since this study started before 2011, the 2004 ID criteria were used. Since morning stiffness was also recorded, the 2011 ID criteria of Wallace et al13 were also retrospectively applied for comparisons.
Secondary outcomes were defined as the change (12-month FU compared to baseline) of the JIA core criteria,9 the Juvenile Arthritis Disease Activity Score3-10 (JADAS3-10,14–16), the physician's global assessment of disease activity, patient or parent global assessment and active joint count (up to 10 joints without ESR). This criterion was recently named clinical JADAS-10 (cJADAS-10), and a cJADAS-10 of ≤1 was set to determine ID.16 The CHAQ score, pain, the PedsQL score and the percentage of patients with a history of uveitis at the 12-month FU were also secondary outcomes. Furthermore, we looked for predictors for the achievement of a more stable period of ID by comparing patients who had continuously ID during the last quarter of the first year in ICON with patients who did not reach such a continuum of disease inactivity. We analysed invariant parameters such as gender, age, ANA status, HLA-B27, RF and the time between symptom onset and diagnosis.
Statistical analysis
Non-parametric statistical testing was performed for the comparison of the JIA core criteria, other clinical parameters and scores both at baseline and at 12-month FU. The Wilcoxon rank-sum test for the comparison of medians and the McNemar test for the comparison of categorical variables was used for the analyses of change of JIA core criteria, scores and clinical parameters between baseline and 12-month FU. Generalised linear models were used for (1) the analyses of differences in the rates of reaching an ID, (2) the comparison of the percentage of time with ID between JIA categories within the first 12 months of observation and (3) determining potential predictor variables for reaching a continuously ID from months 9 to 12. The mean of each JIA category was compared with the mean of the total sample (grand mean centring). Data analyses were performed using SAS software (V.9.3; SAS Institute Inc, Cary, North Carolina, USA).
Results
Patients
Six hundred ninety-five patients were included in this analysis. The median duration in months from diagnosis to study enrolment was 1.6 for the cohort, 0.9 for RF− PA, 2.5 for RF+ PA, 1.8 for oligoarthritis (OA) and 2.9 for patients with systemic-onset JIA (soJIA). Demographic parameters and disease characteristics at study enrolment are summarised in Table 1.
Table 1.
Demographic data of patients at enrolment in ICON
| n | 695 |
|---|---|
| Female/male, n (%) | 457 (66)/238 (34) |
| Median; IQR | |
| Age at diagnosis, years | 6.5; 3.0–11.3 |
| Age at study enrolment, years | 6.9; 3.2–11.6 |
| Time from symptom onset to diagnosis, months | 3.0; 1.0–6.0 |
| Time from diagnosis to enrolment, months | 1.6; 0.4–4.6 |
| JIA categories | n/% |
| OA | 313/45.0 |
| Polyarthritis RF negative (RF– PA) | 195/28.1 |
| Polyarthritis RF positive (RF+ PA) | 10/1.4 |
| PsA | 28/4.0 |
| ERA | 74/10.7 |
| soJIA | 25/3.6 |
| UA | 50/7.2 |
| n/% tested | |
| ANA positive | 393/59.1 |
| RF positive | 21/3.8 |
| HLA-B27 positive | 106/20.1 |
ANA, antinuclear antibodies; ERA, enthesitis-related arthritis; HLA, human leucocyte antigen; JIA, juvenile idiopathic arthritis; OA, oligoarthritis; PsA, Psoriatic arthritis; RF, rheumatoid factor; soJIA, systemic-onset JIA; UA, undifferentiated arthritis.
Treatment
Up to enrolment, 95% of all patients with JIA had received non-steroidal anti-inflammatory drugs, and 42% received a conventional synthetic disease-modifying antirheumatic drug (csDMARD). Almost 4% were already being treated with a biologic DMARD (bDMARD), whereas 58% were completely DMARD naïve. Half of the patients had received intra-articular glucocorticoid injections and 33% had received systemic glucocorticoids (20% low dose (<0.2 mg/kg body weight/day), 7% high dose (≥0.2 mg/kg body weight/day) and 20% pulse therapy). The median time from symptom onset and from diagnosis to the initiation of a DMARD therapy was 3.9 months (IQR 2–7.7) and 0.3 months (IQR 0.1–1.3), respectively. Patients who had already received a DMARD before enrolment were treated for 1.7 months (median, IQR 0.7–4.2). The medication the patients received during the first year of specialised care is summarised in table 4.
Table 4.
Percentage of patients treated with a certain substance at enrolment in ICON, at the 6-month and 12-month FU
| ILAR category | NSAIDs |
Systemic steroids |
MTX |
Biologics |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FU in months | 0 | 6 | 12 | 0 | 6 | 12 | 0 | 6 | 12 | 0 | 6 | 12 |
| All patients with JIA | 95.2 | 72.0 | 51.3 | 32.7 | 26.7 | 14.2 | 42.1 | 65.4 | 64.7 | 3.5 | 15.2 | 20.6 |
| Persistent oligoarthritis | 95.1 | 65.9 | 51.0 | 9.9 | 8.8 | 6.1 | 21.3 | 34.5 | 39.2 | 0.4 | 2.7 | 4.6 |
| Extended oligoarthritis | 98.5 | 75.4 | 46.3 | 38.8 | 35.1 | 17.9 | 50.8 | 84.2 | 85.1 | 1.5 | 7.0 | 20.9 |
| Polyarthritis RF negative | 95.1 | 81.4 | 53.2 | 51.7 | 40.7 | 19.2 | 66.5 | 95.5 | 88.2 | 3.5 | 20.3 | 27.1 |
| Polyarthritis RF positive | 100.0 | 80.0 | 50.0 | 80.0 | 60.0 | 60.0 | 70.0 | 80.0 | 80.0 | 0.0 | 40.0 | 40.0 |
| Psoriatic arthritis | 94.1 | 72.4 | 55.9 | 41.2 | 27.6 | 20.6 | 47.1 | 79.3 | 82.4 | 2.9 | 20.7 | 32.4 |
| Enthesitis-related arthritis | 94.8 | 72.2 | 64.9 | 26.0 | 26.4 | 14.3 | 36.4 | 68.1 | 67.5 | 5.2 | 19.4 | 24.7 |
| Systemic arthritis | 88.9 | 36.0 | 29.6 | 96.3 | 68.0 | 33.3 | 55.6 | 64.0 | 63.0 | 29.6 | 64.0 | 66.7 |
| Undifferentiated arthritis | 95.5 | 81.1 | 38.6 | 27.3 | 18.9 | 6.8 | 31.8 | 62.2 | 56.8 | 4.6 | 27.0 | 36.4 |
FU, follow-up; JIA, juvenile idiopathic arthritis; ILAR, International League of Associations for Rheumatology; MTX, methotrexate; NSAIDs, non-steroidal anti-inflammatory drugs; RF, rheumatoid factor.
Table 3.
Comparison between patients with inactive versus active disease during the last quarter of the first year in ICON
| Continuously ID from months 9 to 12 | Not continuously ID disease from months 9 to 12 | Multivariate |
|||
|---|---|---|---|---|---|
| OR | 95% CI | p Value | |||
| Female/male, n (%) | 183 (66.3)/93 (33.7) | 274 (65.4)/145 (34.6) | 1.27 | 0.88 to 1.84 | 0.198 |
| Age at symptom onset Median in years (IQR) |
4.5 (2.5–9.2) | 6.6 (2.9–11.1) | 0.96 | 0.92 to 1.00 | 0.040 |
| Time from symptom onset to diagnosis Median in months (IQR) |
2.0 (1.0–5.0) | 3.0 (1.0–7.0) | 0.97 | 0.95 to 0.99 | 0.014 |
| JIA category, n (%) | |||||
| Oligoarthritis, persistent | 100 (36.2) | 148 (35.4) | 1.00 | – | |
| Oligoarthritis, extended | 25 (9.1) | 40 (9.5) | 1.04 | 0.58 to 1.83 | 0.898 |
| Polyarthritis RF negative | 80 (29.0) | 115 (27.5) | 1.36 | 0.90 to 2.06 | 0.143 |
| Polyarthritis RF positive | 5 (1.8) | 5 (1.2) | 4.46 | 0.74 to 32.24 | 0.113 |
| Psoriatic arthritis | 8 (2.9) | 20 (4.8) | 0.84 | 0.34 to 1.91 | 0.682 |
| Enthesitis-related arthritis | 21 (7.6) | 53 (12.7) | 0.87 | 0.41 to 1.81 | 0.723 |
| Systemic arthritis | 16 (5.8) | 9 (2.2) | 3.35 | 1.37 to 8.67 | 0.001 |
| Undifferentiated arthritis | 21 (7.6) | 29 (6.9) | 1.49 | 0.73 to 3.00 | 0.266 |
| ANA positive, n (% tested) | 176 (65.4) | 217 (54.8) | 1.51 | 1.04 to 2.20 | 0.029 |
| RF positive, n (% tested) | 7 (3.2) | 14 (4.1) | 0.55 | 0.12 to 1.98 | 0.392 |
| HLA-B27 positive, n (% tested) | 36 (18.4) | 70 (21.2) | 1.21 | 0.69 to 2.11 | 0.511 |
Significant p values (<0.05) are shown in bold.
ANA, antinuclear antibodies, HLA B27, human leucocyte antigen B27; ID, inactive disease; JIA, juvenile idiopathic arthritis; RF, rheumatoid factor.
At the 12-month FU, 14% of all patients were still receiving systemic glucocorticoids. Only 1.7% of patients were on a high-dose oral corticosteroid treatment, 9% on low-dose corticosteroid treatment and 6% had received methylprednisolone pulse therapy within the past 3 months. During the first year of observation, 60% of patients received intra-articular corticosteroid injections; the highest percentage was in patients with persistent OA (78%), followed by patients with extended OA (73%) and RF− PA (56%). Sixty-seven per cent were treated with csDMARDs and 22% were treated with bDMARDs. The following bDMARDs were used during the observation period: etanercept in 13.1% of patients, adalimumab in 4.9%, golimumab in 2%, anakinra in 1.2%, tocilizumab in 1.9% and canakinumab in 0.9%. BDMARD use differed among the various JIA categories. Patients with enthesitis-related arthritis (ERA) and polyarticular JIA (extended OA, RF− polyarthritis, RF+ polyarthritis) were almost exclusively treated with tumour necrosis factor (TNF) antagonists in 30% and in 28%, respectively. In contrast, patients with soJIA were mostly treated with interleukin (IL) 1 (44%) and IL-6 blockers (40%).
Outcomes
Comparing the JIA core criteria at baseline with the data of the 12-month FU, we observed a significant improvement in all parameters in the composite score JADAS3-10 and in the patients’ physical functioning and health-related quality of life (table 5). At the 12-month FU, the patients were specifically treated for their JIA for approximately 14 months (median). Twenty-five patients in ICON already had a diagnosis of extended OA at inclusion in ICON; another 40 (14%) of the 288 remaining patients with OA at inclusion developed an extended disease course during the first year of observation in ICON. Notably, approximately 28% of patients with OA (persistent as well as extended) and RF− PA were in remission on medication at 12-month FU, as were an even greater number of patients with soJIA (33%), but only about one-fifth of all patients with PsA and ERA were in remission on medication.
Table 5.
Comparison of JIA core criteria,† scores and clinical parameters at baseline and at 12-month FU
| Enrolment | 12-month FU | |
|---|---|---|
| JIA core criteria† | ||
| Active joint count, median, IQR | 2, 1–4 | 0, 0–1* |
| Active joint count=0, n (%) | 118 (17.0) | 456 (65.7)* |
| Limited ROM joint count, median, IQR | 2, 1–4 | 0, 0–2* |
| Limited ROM joint count=0, n (%) | 128 (18.7) | 366 (53.9)* |
| Physician's global assessment of disease activity, NRS, median, IQR | 3.5, 1.0–5.0 | 0.5, 0.0–1.5* |
| Physician's global assessment of disease activity=0 | 52 (7.5) | 290 (41.9)* |
| Parent's global assessment of well-being, median, IQR | 2.5, 1.0–4.0 | 1.0, 0.5–2.0* |
| Parent's global assessment of well-being=0 | 74 (10.7) | 190 (28.7)* |
| CHAQ, median, IQR | 0.4, 0.0–1.0 | 0.0, 0.0–0.4* |
| CHAQ=0, n (%) | 211 (30.5) | 382 (57.6)* |
| ESR, mm/h, median, IQR | 15.0, 8.0–31.0 | 9.0, 5.0–15.0* |
| ESR≤20 mm/h, n (% of tested) | 363 (62) | 376 (83.6) |
| CRP, mg/L, median, IQR | 2.5, 0.4–8.5 | 0.6, 0.1–2.8* |
| CRP≤5 mg/L, n (% of tested) | 417 (65.1) | 413 (86.0) |
| Active enthesitis, n (%) | 55 (8.0) | 17 (2.5) |
| History of uveitis, n (%) | 59 (8.5) | 82 (11.8) |
| JADAS3-10, median, IQR | 9.0, 5.0–14.0 | 2.0, 0.5–5.0* |
| JADAS3-10≤1, n (%) | 42 (6.1) | 264 (40.0)* |
| PedsQL, median, IQR parent | 71.7, 56.5–84.8 | 89.1, 77.4–95.7* |
| PedsQL, median, IQR patient | 78.3, 66.3–89.1 | 91.3, 80.4–97.8* |
| Pain, NRS, median, IQR | 2.0, 0.5–5.0 | 0.5, 0.0–2.0* |
| Pain=0, n (%) | 143 (20.8) | 308 (46.5)* |
| Inactive disease according to 2004 criteria by Wallace et al10 (%) | 40 (6.8) | 375 (54.0)* |
| Continuously inactive for 3 months according to 2004 criteria by Wallace et al10 (%) | – | 276 (39.7) |
| Remission on medication according to 2004 criteria by Wallace et al10 (%) | – | 183 (26.3) |
| Inactive disease according to 2011 criteria by Wallace et al13 (%) | 37 (6.1) | 363 (52.2) |
| Remission on medication according to 2011 criteria by Wallace et al13 (%) | – | 181 (26.0) |
*p<0.001.
†JIA core criteria: Defined according to Giannini et al.9
CHAQ, Child Health Assessment Questionnaire, 0–3; CRP: C reactive protein, cut-off for normal values: ≤5 mg/L; FU, follow-up; ESR: erythrocyte sedimentation rate; cut-off for normal values: ≤20 mm/h; JADAS3-10, Juvenile Arthritis Disease Activity Score 3–10, 0–30; JIA, JIA, juvenile idiopathic arthritis; NRS, numeric rating scale; PedsQL: Paediatric Quality of Life Inventory, 0–100; ROM, range of motion.
Inactive disease
Approximately three-fourth of all patients had experienced a period of ID at least once during the first 12 months in the ICON cohort, with marked differences regarding the categories: 88% of patients with soJIA and almost 80% of patients with persistent OA, but only half of all patients with ERA (55%) reached an ID state in the first year after enrolment.
The shortest time from diagnosis to ID was observed in patients with soJIA (median 5.1 months), and the longest time in patients with ERA and extended OA (median 9.0 months).
Patients with soJIA had the longest duration of the first period of ID (median 7.0 months). This duration was shortest in patients with RF+ PA (median 2.8 months).
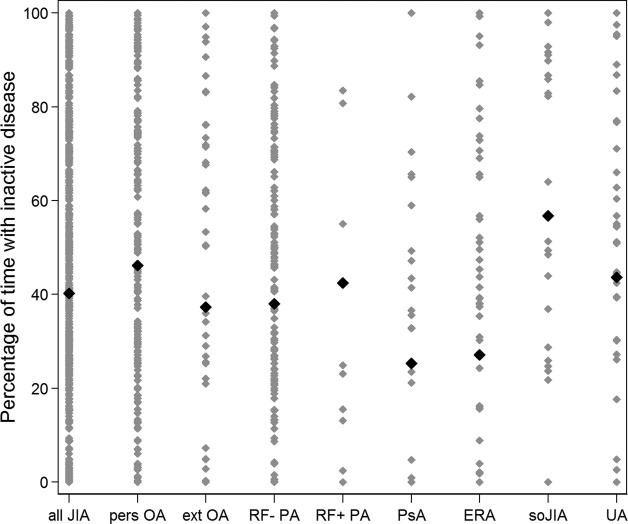
The percentage of time spent with ID during the first 12 months after enrolment in ICON differed significantly among the different subgroups, with 57% in patients with soJIA—which equals 6.8 months—and only 27% (3.2 months) in patients with ERA. However, there was also a large variation within each subgroup (table 6 and figure 1).
Table 6.
Patients reaching an ID during the first 12 months of observation in ICON
| Ever ID n (%) |
p Value | Percentage of time with ID during the first 12 months, mean % of subgroup (SD) | p Value | Duration from symptom onset to ID in months, median, IQR | Duration from diagnosis to ID in months, median, IQR | |
|---|---|---|---|---|---|---|
| All patients with JIA | 510 (73.4) | 40 (35) | 11.0, 7.0–15.1 | 7.9, 3.9–10.9 | ||
| Persistent oligoarthritis | 200 (79.7) | 0.062 | 46 (36) | 0.021 | 10.0, 6.0–13.0 | 6.0, 3.0–10.0 |
| Extended oligoarthritis | 42 (67.7) | 0.323 | 37 (38) | 0.592 | 14.0, 10.9–18.5 | 9.0, 6.0–13.0 |
| Polyarthritis RF negative | 144 (73.9) | 0.852 | 38 (34) | 0.583 | 11.0, 7.8–14.2 | 8.0, 4.0–11.0 |
| Polyarthritis RF positive | 9 (90.0) | 0.168 | 43 (42) | 0.769 | 17.0, 9.9–18.0 | 7.0, 6.0–12.0 |
| Psoriatic arthritis | 19 (55.9) | 0.014 | 25 (29) | 0.011 | 13.0, 5.9–17.0 | 7.0, 5.0–11.0 |
| Enthesitis-related arthritis | 41 (55.4) | 0.001 | 27 (32) | 0.002 | 15.9, 11.8–20.0 | 9.0, 3.7–12.0 |
| Systemic arthritis | 22 (88.0) | 0.078 | 57 (35) | 0.008 | 7.0, 4.0–10.0 | 5.1, 3.0–9.0 |
| Undifferentiated arthritis | 33 (75.0) | 0.767 | 44 (35) | 0.426 | 13.0, 7.0–20.0 | 7.2, 3.9–12.0 |
Significant p values (<0.05) are shown in bold, comparing the means of all patients with JIA within a category using regression analysis.
ID, inactive disease; JIA, juvenile idiopathic arthritis; RF, rheumatoid factor.
Figure 1.
Bold diamonds are the mean percentage of time with inactive disease during the first 12 months within the subgroup. Each diamond represents one patient. JIA, juvenile idiopathic arthritis; pers OA, persistent oligoarthritis; ext OA, extended oligoarthritis; RF− PA, rheumatoid factor negative polyarthritis; RF+ PA, rheumatoid factor positive polyarthritis; PsA, psoriatic arthritis; ERA, enthesitis-related arthritis; soJIA, systemic-onset juvenile idiopathic arthritis.
At the 12-month FU, 40% of the patients had achieved a continuously ID for at least 3 months. The treatment of patients with versus without continuously ID at the 12-month FU is given in table 2. Patients with ID at the 12-month FU had been treated less often with corticosteroids (5.8% vs 13.8%) and DMARDs (60.9% vs 68.5%) during the 3 months prior to the 12-month FU than those not being in an ID state. In general, patients who were treated with DMARDs and/or corticosteroids had a lower probability of attaining a state of ID than those who were not treated with such substances. However, patients starting DMARDs and corticosteroids had a higher disease activity than those not treated with such substances.
Table 2.
Last 3 months treatment in patients with versus without continuously ID at the 12-month follow-up
| Patients on systemic glucocorticoids |
Patients on |
|||||||
|---|---|---|---|---|---|---|---|---|
| Low dose |
High dose |
csDMARDs |
bDMARDs |
|||||
| Not continuously ID for at least 3 months n (%) |
Continuously ID for at least 3 months n (%) |
Not continuously ID for at least 3 months n (%) |
Continuously ID for at least 3 months n (%) |
Not continuously ID for at least 3 months n (%) |
Continuously ID for at least 3 months n (%) |
Not continuously ID for at least 3 months n (%) |
Continuously ID for at least 3 months n (%) |
|
| All patients with JIA | 43 (10.3) | 14 (5.1) | 31 (7.4) | 3 (1.9) | 282 (67.3) | 166 (60.1) | 106 (25.3) | 34 (12.3) |
| Persistent oligoarthritis | 6 (4.0) | 0 (0.0) | 4 (2.7) | 1 (1.0) | 67 (44.4) | 31 (31.0) | 10 (6.6) | 1 (1.0) |
| Extended oligoarthritis | 5 (13.5) | 2 (8.0) | 4 (10.8) | 0 (0.0) | 31 (83.8) | 21 (84.0) | 10 (27.0) | 2 (8.0) |
| Polyarthritis RF negative | 16 (13.9) | 8 (10.0) | 13 (11.3) | 1 (1.3) | 100 (87.0) | 72 (90.0) | 42 (36.5) | 12 (15.0) |
| Polyarthritis RF positive | 3 (60.0) | 1 (20.0) | 1 (20.0) | 0 (0.0) | 4 (80.0) | 4 (80.0) | 3 (60.0) | 1 (20.0) |
| Psoriatic arthritis | 3 (12.0) | 0 (0.0) | 3 (12.0) | 0 (0.0) | 20 (80.0) | 8 (88.9) | 11 (44.0) | 0 (0.0) |
| Enthesitis-related arthritis | 5 (9.4) | 0 (0.0) | 3 (5.7) | 0 (0.0) | 36 (67.9) | 14 (66.7) | 13 (24.5) | 4 (19.1) |
| Systemic arthritis | 4 (44.4) | 3 (18.8) | 1 (11.1) | 1 (6.3) | 7 (77.8) | 8 (50.0) | 7 (77.8) | 10 (62.5) |
| Undifferentiated arthritis | 1 (4.2) | 0 (0.0) | 2 (8.3) | 0 (0.0) | 17 (70.8) | 8 (40.0) | 10 (41.7) | 4 (20.0) |
bDMARD, biologic disease-modifying antirheumatic drug; csDMARDs, conventional synthetic DMARD; ID, inactive disease; JIA, juvenile idiopathic arthritis; RF, rheumatoid factor.
Predictors for achieving a continuously ID from months 9 to 12
We also found significant differences among the JIA categories when comparing the patients who were continuously inactive during the last quarter of the first year in ICON with the patients who did not reach this period of continuously ID. Furthermore, we analysed general parameters such as gender, age at onset and the duration between symptom onset and diagnosis and such descriptors as ANA status, the presence of RF and HLA-B27 as possible predictors for achieving an ID state. In univariate and multivariate analyses, younger age at onset, a shorter duration between symptom onset and diagnosis and a positive ANA status increased the probability of attaining a period of continuously ID at the end of the first year of observation (table 3).
Discussion
ID is the ultimate therapeutic goal in JIA. Furthermore, it has been shown that a period of ID early in the disease course is of prognostic value to the later clinical course.17–19 The inception cohort ICON was initiated to evaluate the clinical courses and long-term outcomes of patients with JIA under real-life conditions. In this paper, we describe the demographic and disease characteristics of the patients enrolled in ICON, and we analyse how many patients with JIA in each subgroup on contemporary treatments reached a state of ID during the first 12 months after enrolment and when and for how long they did so.
The demographic and clinical parameters documented in ICON are rather comparable to data from other inception cohort studies: Two-thirds are female with a median age at diagnosis of 6.5 years, which is somewhat younger than in CAPS and ReACCH Out,5 20 and approximately half of the patients suffered from oligoarticular disease. The median active joint count at baseline was 2, and the physician's global assessment of disease activity was 3.5, which are remarkably similar to the values of the JIA core criteria described for patients enrolled in the aforementioned inception cohorts.
In ICON, all core set parameters, CHAQ, PedsQL and the composite score JADAS3–10, documented at enrolment had improved significantly after 12 months of paediatric rheumatology care. This is in line with data from the CAPS study that also included patients diagnosed with JIA ≤12 months previously and that showed significant improvements in the same ranges in all core set parameters at the 1-year FU.19
Approximately three-fourth of all patients in ICON attained a state of ID during the first 12 months after enrolment. This is in agreement with results from Anink et al,21 who stated that, in a retrospective analysis of all patients with JIA followed between the years 2003 and 2007 for at least 1 year at the Emma Children's Hospital in Amsterdam, 77% reached ID. This cohort was skewed towards the more severe end point, meaning that almost half of all patients had a polyarticular JIA, and the median disease duration of all patients with JIA was 32 months. The median time to reach ID was 10 months for the whole JIA cohort, with a median length of 4 months. Similarly, Ringold et al22 reported that 78.3% of all polyarticular patients with JIA who were treated at Seattle Children's Hospital in the years 2000–2006 and followed for an average of 30 months reached an ID state within the first year. They did so after 8 months (median), and the median duration of this ID period was 6 months. Our results are very similar to these published data: the median time to attain a period of ID from symptom onset was 11.0 months; from diagnosis, it was 7.9 months with a median duration of 4.9 months.
Reaching ID is of course no achievement per se; it depends on the treatment that is being administered. The patients followed in ICON were treated relatively intensively with intra-articular steroids in 60%, csDMARDs in 67% and bDMARDs in 22% of cases during the first 14 months of disease. This treatment approach, including the type of substances used, corresponds to current recommendations of treatment in JIA.23–25 The therapy of patients with soJIA in ICON reflects this well. BDMARDs that inhibit IL-1 or IL-6 are recommended as the first glucocorticoid sparing therapies for children with active soJIA.25 These substances, approved for soJIA in Germany in 2011 and 2013, already received more than 80% of the soJIA cases within the first year in ICON. Patients with soJIA were those who achieved most frequently and most rapidly a state of ID during the first months of rheumatology care. However, most of the patients with soJIA were on bDMARD therapy, which was shown to enable such patients to reach ID in most cases.26 In contrast, patients with enthesitis-related arthritis (ERA) reached a state of ID less frequently (55%) and after a mean period of 15.9 months after symptom onset. Only a small proportion of children with ERA received biologics, although it is known that patients with this subtype respond poorly to conventional DMARDs such as methotrexate. This may be at least partially attributable to the licensing situation, with approval of etanercept and adalimumab for use in ERA as recently as 2013 and 2014, respectively. However, a recently published retrospective study found that patients with ERA had attained an ID state least often (24%) in comparison with patients in other JIA categories 1 year after the initiation of the TNF inhibitor.27 In addition, it has to be kept in mind that the Wallace criteria for ID in JIA do not include a measure for enthesitis and, therefore, may not display all aspects of disease (in)activity in this JIA category. Compared to enrolment in ICON, when 56% of patients with ERA suffered from enthesitis, 16% still had clinically active enthesitis at the 12-month FU. Recently, Weiss et al28 used data from the Childhood Arthritis and Rheumatology Research Alliance (CARRA) registry to demonstrate that ERA is associated with higher pain intensity and poorer health status in comparison with other categories of JIA.
The first investigation to focus on sequential time periods of active and ID was published by Wallace et al10 in 2005. Also, for the first time in this study, the preliminary criteria for ID were used to analyse the data of 437 patients with JIA who were followed for at least 4 years. As in our study, the cumulative time spent in a state of ID during the FU period was 40% among all patients and differed significantly between the JIA categories.
Magnani et al17 showed in a retrospective study that patients with JIA who attained an ID state at least once in the first 5 years of their disease less frequently experienced long-term joint damage, and they also showed a trend towards less functional impairment and radiographic changes. Interestingly, the clinical indicators of JIA severity at baseline did not significantly differ among patients who had an ID state at only one visit, at two or more visits and those who did not attain an ID state at all. This indicates that baseline features may not be appropriate to predict a certain disease course.
In another retrospective study, Albers et al found that the time with active disease in the first 2 years was the most significant factor associated with the duration of active disease in the following years. The lowest median percentage of time with active disease was found in patients with persistent OA (47%), followed by RF− PA (58%) and extended OA (70%), which is markedly similar to our results in ICON. However, although the median time with active disease differed significantly between the categories of JIA, individual patients could have a wide range of activity; this result was also observed in ICON. Therefore, the authors concluded that the JIA subtype by itself may not be valid as a prognostic factor for an individual patient.
We did not analyse disease activity, active, painful or swollen joint count or the composite score JADAS at inclusion as possible predictors for achieving a period of ID because we assumed that these parameters may have already changed during the treatment of a significant portion of patients. The same applied to the assessment of treatment effects. In ICON, patients starting DMARDs or corticosteroids had a higher disease activity than those not treated with DMARDs or corticosteroids. Even at the 12-month FU, patients on bDMARDs, csDMARDs and/or corticosteroids were less frequently in a state of continuously ID compared with those not treated with these substances. Without adjusting for differences in patients’ baseline characteristics (before DMARD/corticosteroid start) to take confounding by indication into account, conclusions about the treatment effects are impossible. Such adjustment could not be done for the whole ICON cohort due to treatment naivety in only approximately half of the patients at inclusion.
Therefore, we analysed invariant parameters such as gender, age, ANA status, HLA-B27, RF and the time between symptom onset and diagnosis to determine if they were of predictive value. Female gender, positive ANA status, younger age at onset and a shorter duration between symptom onset and diagnosis were found to be predictive in univariate and multivariate analyses. This is, in part, in line with results from the ReaACCh Out study, where younger age at diagnosis and study enrolment and a shorter time from symptom onset to diagnosis were correlated with ID at 6 months, although no correlation was observed for gender or ANA status.6 In a trial of early aggressive therapy in polyarticular JIA, Wallace et al29 also demonstrated that patients with a shorter disease duration at enrolment were more likely to attain clinically ID at 6 months.
In the context of therapy, the results of the ICON study are especially notable because they are non-interventional, real-life data obtained under current treatment conditions as standard of care.
We have to state the possible limitations of our study: comparing the demographic parameters and subgroup distribution of the patients included in ICON presented here with population-based patients with JIA data collected in the German paediatric rheumatology database (GPRD),30 the proportion of patients with polyarthritis is higher in ICON (almost 30% vs 20%), which suggests the possibility of a selection bias. We may speculate that the specialised centres that participated in this observation study treat a slightly greater number of patients with polyarticular disease than their colleagues who participate in the GPRD representing the whole paediatric rheumatology community. Moreover, we had to address missing visits (fortunately <10% of all visits from baseline to 12-month FU) while calculating the cumulative time that patients spent with ID, and we may have incorrectly estimated this time by setting the end of the period of ID in the middle of the time period between the two available visits. Furthermore, we used the definition of ID defined by Wallace et al10 for our analysis in all JIA categories, even though they were developed for oligoarticular, polyarticular and systemic disease and have not been evaluated in ERA or PsA to date.
In conclusion, through the ICON study, we have added additional information about the course and outcome of JIA that was obtained under real-life conditions with current treatment regimens. The outcome 12 months after enrolment was good, with all clinical and functional scores being significantly improved within the same or even greater range (notably polyarthritis) as in other cohorts. Compared with baseline disease characteristics, the percentage of time with ID during the first years of JIA and the early response to treatment—irrespective of the JIA category, age at onset, gender or laboratory markers—may be valuable for predicting the outcome in the following years regarding disease course and function. Since a short duration between symptom onset and diagnosis was demonstrated to be predictive of achieving a period of ID, children with joint problems should be transferred to specialised care as soon as possible.
Acknowledgments
The authors would like to thank all the physicians who were engaged in the ICON cohort: Tilmann Kallinich, Berlin; Hans-Iko Huppertz, Bremen; Ivan Foeldvari, Hamburg; Angelika Thon, Hannover; Kirsten Mönkemöller, Köln; Anton Hospach, Stuttgart and Jasmin Kümmerle-Deschner, Tübingen. They also thank all patients and their parents for their participation in ICON.
Footnotes
Funding: The ICON study is funded by a research grant of the Federal ministry of education and research (BMBF, FKZ 01ER0812).
Competing interests: None declared.
Ethics approval: The study protocol was approved by the ethics committee of the Charité—Universitätsmedizin Berlin. Parents and patients 8 years of age and older gave their informed consent for participation.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
References
- 1.Flatø B, Lien G, Smerdel A et al. Prognostic factors in juvenile rheumatoid arthritis: a case-control study revealing early predictors and outcome after 14.9 years. J Rheumatol 2003;30:386–93. [PubMed] [Google Scholar]
- 2.Oen K, Malleson PN, Cabral DA et al. Disease course and outcome of juvenile rheumatoid arthritis in a multicenter cohort. J Rheumatol 2002;29:1989–99. [PubMed] [Google Scholar]
- 3.Nordal E, Zak M, Aalto K et al. Ongoing disease activity and changing categories in a long-term nordic cohort study of juvenile idiopathic arthritis. Arthritis Rheum 2011;63:2809–18. 10.1002/art.30426 [DOI] [PubMed] [Google Scholar]
- 4.Berntson L, Nordal E, Aalto K et al. HLA-B27 predicts a more chronic disease course in an 8-year followup cohort of patients with juvenile idiopathic arthritis. J Rheumatol 2013;40:725–31. 10.3899/jrheum.121257 [DOI] [PubMed] [Google Scholar]
- 5.Adib N, Hyrich K, Thornton J et al. Association between duration of symptoms and severity of disease at first presentation to paediatric rheumatology: results from the Childhood Arthritis Prospective Study. Rheumatology 2008;47:991–5. 10.1093/rheumatology/ken085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oen K, Tucker L, Huber AM et al. Predictors of early inactive disease in a juvenile idiopathic arthritis cohort: results of a Canadian multicenter, prospective inception cohort study. Arthritis Rheum 2009;61:1077–86. 10.1002/art.24539 [DOI] [PubMed] [Google Scholar]
- 7.Guzman J, Oen K, Tucker LB et al. The outcomes of juvenile idiopathic arthritis in children managed with contemporary treatments: results from the ReACCh-Out cohort. Ann Rheum Dis 2015;74:1854–60. 10.1136/annrheumdis-2014-205372 [DOI] [PubMed] [Google Scholar]
- 8.Petty RE, Southwood TR, Manners P et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004;31:390–2. [PubMed] [Google Scholar]
- 9.Giannini EH, Ruperto N, Ravelli A et al. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum 1997;40:1202–9. [DOI] [PubMed] [Google Scholar]
- 10.Wallace CA, Ruperto N, Giannini E et al. Preliminary criteria for clinical remission for select categories of juvenile idiopathic arthritis. J Rheumatol 2004;31:2290–4. [PubMed] [Google Scholar]
- 11.Heiligenhaus A, Niewerth M, Ganser G et al. Prevalence and complications of uveitis in juvenile idiopathic arthritis in a population-based nation-wide study in Germany: suggested modification of the current screening guidelines. Rheumatology 2007;46:1015–19. 10.1093/rheumatology/kem053 [DOI] [PubMed] [Google Scholar]
- 12.Jabs DA, Nussenblatt RB, Rosenbaum JT et al. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol 2005;140:509–16. 10.1016/j.ajo.2005.03.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace CA, Giannini EH, Huang B et al. American College of Rheumatology provisional criteria for defining clinical inactive disease in select categories of juvenile idiopathic arthritis. Arthritis Care Res 2011;63:929–36. 10.1002/acr.20497 [DOI] [PubMed] [Google Scholar]
- 14.Consolaro A, Ruperto N, Bazso A et al. Development and validation of a composite disease activity score for juvenile idiopathic arthritis. Arthritis Rheum 2009;61:658–66. 10.1002/art.24516 [DOI] [PubMed] [Google Scholar]
- 15.McErlane F, Beresford MW, Baildam EM et al. Validity of a three-variable Juvenile Arthritis Disease Activity Score in children with new-onset juvenile idiopathic arthritis. Ann Rheum Dis 2013;72:1983–8. 10.1136/annrheumdis-2012-202031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Consolaro A, Negro G, Chiara Gallo M et al. Defining criteria for disease activity states in nonsystemic juvenile idiopathic arthritis based on a three-variable juvenile arthritis disease activity score. Arthritis Care Res 2014;66:1703–9. 10.1002/acr.22393 [DOI] [PubMed] [Google Scholar]
- 17.Magnani A, Pistorio A, Magni-Manzoni S et al. Achievement of a state of inactive disease at least once in the first 5 years predicts better outcome of patients with polyarticular juvenile idiopathic arthritis. J Rheumatol 2009;36:628–34. 10.3899/jrheum.080560 [DOI] [PubMed] [Google Scholar]
- 18.Albers HM, Brinkman DMC, Kamphuis SSM et al. Clinical course and prognostic value of disease activity in the first two years in different subtypes of juvenile idiopathic arthritis. Arthritis Care Res 2010;62:204–12. [DOI] [PubMed] [Google Scholar]
- 19.Hyrich KL, Lal SD, Foster HE et al. Disease activity and disability in children with juvenile idiopathic arthritis one year following presentation to paediatric rheumatology. Results from the Childhood Arthritis Prospective Study. Rheumatology 2010;49:116–22. 10.1093/rheumatology/kep352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oen K, Duffy CM, Tse SML et al. Early outcomes and improvement of patients with juvenile idiopathic arthritis enrolled in a Canadian multicenter inception cohort. Arthritis Care Res 2010;62:527–36. 10.1002/acr.20044 [DOI] [PubMed] [Google Scholar]
- 21.Anink J, Dolman KM, Merlijn van den Berg J et al. Two-year outcome of juvenile idiopathic arthritis in current daily practice: what can we tell our patients? Clin Exp Rheumatol 2012;30:972–8. [PubMed] [Google Scholar]
- 22.Ringold S, Seidel KD, Koepsell TD et al. Inactive disease in polyarticular juvenile idiopathic arthritis: current patterns and associations. Rheumatology 2009;48:972–7. 10.1093/rheumatology/kep144 [DOI] [PubMed] [Google Scholar]
- 23.Beukelman T. Treatment advances in systemic juvenile idiopathic arthritis. F1000prime Rep 2014;6:21 10.12703/P6-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dueckers G, Guellac N, Arbogast M et al. Evidence and consensus based GKJR guidelines for the treatment of juvenile idiopathic arthritis. Clin Immunol 2012;142:176–93. 10.1016/j.clim.2011.10.003 [DOI] [PubMed] [Google Scholar]
- 25.Ringold S, Weiss PF, Beukelman T et al. 2013 update of the 2011 American College of Rheumatology recommendations for the treatment of juvenile idiopathic arthritis: recommendations for the medical therapy of children with systemic juvenile idiopathic arthritis and tuberculosis screening among children receiving biologic medications. Arthritis Rheum 2013;65:2499–512. 10.1002/art.38092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woerner A, Uettwiller F, Melki I et al. Biological treatment in systemic juvenile idiopathic arthritis: achievement of inactive disease or clinical remission on a first, second or third biological agent. RMD Open 2015;1:e000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Donnithorne KJ, Cron RQ, Beukelman T. Attainment of inactive disease status following initiation of TNF-α inhibitor therapy for juvenile idiopathic arthritis: enthesitis-related arthritis predicts persistent active disease. J Rheumatol 2011;38:2675–81. 10.3899/jrheum.110427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weiss PF, Beukelman T, Schanberg LE et al. Enthesitis-related arthritis is associated with higher pain intensity and poorer health status in comparison with other categories of juvenile idiopathic arthritis: the Childhood Arthritis and Rheumatology Research Alliance Registry. J Rheumatol 2012;39:2341–51. 10.3899/jrheum.120642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace CA, Giannini EH, Spalding SJ et al. Trial of early aggressive therapy in polyarticular juvenile idiopathic arthritis. Arthritis Rheum 2012;64:2012–21. 10.1002/art.34343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Minden K, Niewerth M, Listing J et al. Health care provision in pediatric rheumatology in Germany—national rheumatologic database. J Rheumatol 2002;29:622–8. [PubMed] [Google Scholar]