Abstract
Bilateral septic arthritis of the shoulder is uncommon in the immunocompetent patient with no previous risk factors for joint infection, and is thus easily missed. Septic arthritis is associated with significant rates of morbidity and mortality. Early diagnosis and management is the key to a favourable outcome; septic arthritis should be considered as a differential diagnosis in the unwell patient presenting with shoulder pain and reduced range of joint movement. We present a case of a 47-year-old previously fit and well man with bilateral shoulder septic arthritis. We will also review the current literature on management and long-term outcomes of patients with septic arthritis of the glenohumeral joint.
Background
Septic arthritis is a common differential for the unwell patient presenting with pain and reduced range of movement in a joint, and has an incidence of approximately 2 per 100 000.1 Polyarticular septic arthritis is uncommon, and accounts for only 12% of all cases of septic arthritis.1 2 Furthermore, the shoulder joint is involved in only 3% of cases, and in just 1% of those with multiple joint involvement.2 Owing to its rarity, diagnosis can often be difficult and delayed.
Mortality secondary to septic arthritis has been reported to be as high as 50% despite adequate fluid resuscitation, timely washout of the affected joint and aggressive antibiotic therapy.3 The need for early recognition is crucial for an increased chance of survival. Furthermore, early intervention is more likely to prevent downstream permanent joint and cartilaginous damage in survivors.3 4 That being said, even those who have received prompt treatment can have a permanent reduction in the range of movement of the shoulder.
The majority of patients with septic arthritis have existing comorbidities that predispose them to infection, such as rheumatoid arthritis, immunodeficiency, malignancy, heart disease and diabetes.5 Therefore, young fit patients with no risk factors are easily missed. This case report aims to highlight the importance of early recognition and management in one such individual. Prior to this there has been only one other such case of polyarticular upper limb septic arthritis in an otherwise well patient.
Case presentation
We present a case of a 47-year-old previously fit and well man, a chef, with a 1-week history of progressively worsening bilateral shoulder pain, with associated reduced range of movement. He had no significant medical history and no risk factors for septic arthritis such as diabetes, immunosuppression, heart defects or malignancy. He weighed 76 kg and, at a height of 1.75 m, had a body mass index of 24.5. For 3 days prior to admission, he experienced recurrent intermittent episodes of fever and rigours. He presented to the accident and emergency department with clinical signs of septicaemia; he had a temperature of 39.5°C, was clammy and tachycardic at 120 bpm, and had a systolic blood pressure of 90 mm Hg. Examination of the cardiovascular, respiratory and abdominal systems revealed no abnormality. Both shoulders appeared mildly erythematous, and hot to the touch. The patient was unable to actively move his shoulder joints. Attempts at passive movements were extremely painful, and the shoulder joints appeared locked in a neutral position.
Investigations
Shoulder X-rays showed no bony abnormality or evidence of effusion (figure 1). Investigations to rule out other infectious foci were performed. Chest X-ray and urinalysis were unremarkable. White cells were elevated at 14.1×109/L (normal range 4–11×109/L), with a neutrophilia of 10.9×109/L. C reactive protein was raised at 387 mg/L (normal range 0–10 mg/L).
Figure 1.
Shoulder X-rays showing no underlying bony abnormality.
Aspiration was performed on both shoulder joints. Over 20 mLs of purulent fluid was obtained from each shoulder. Aspirate from the right shoulder yielded a presence of leucocytes, with 80% polymorphs and 20% lymphocytes. The left shoulder yielded 90% polymorphs and 10% lymphocytes. Both the joint aspirate culture and blood cultures taken at the time of admission grew Staphylcoccus aureus.
Investigations for autoimmune diseases, tuberculosis and HIV were negative. An echocardiogram showed no underlying cardiac abnormality. As previously mentioned, the patient had no risk factors to make him susceptible to infection.
Differential diagnosis
The differential diagnoses for acute onset bilateral shoulder pain include septic arthritis, trauma and inflammatory autoimmune arthritis. Our patient's acutely unwell presentation, raised inflammatory markers and positive blood and joint aspirate cultures on a background of no preceding triggers or comorbidities, pointed to septic arthritis.
Treatment
Given his clinical presentation, the patient was started on aggressive fluid resuscitation and intravenous flucloxacillin (2 g four times daily). He was taken to theatre for an emergency arthroscopic washout of both shoulders (figure 2A, B). Large amounts of purulent fluid were obtained during the procedure. Fortunately, the shoulder joints appeared undamaged, with no erosion of the articular surfaces.
Figure 2.
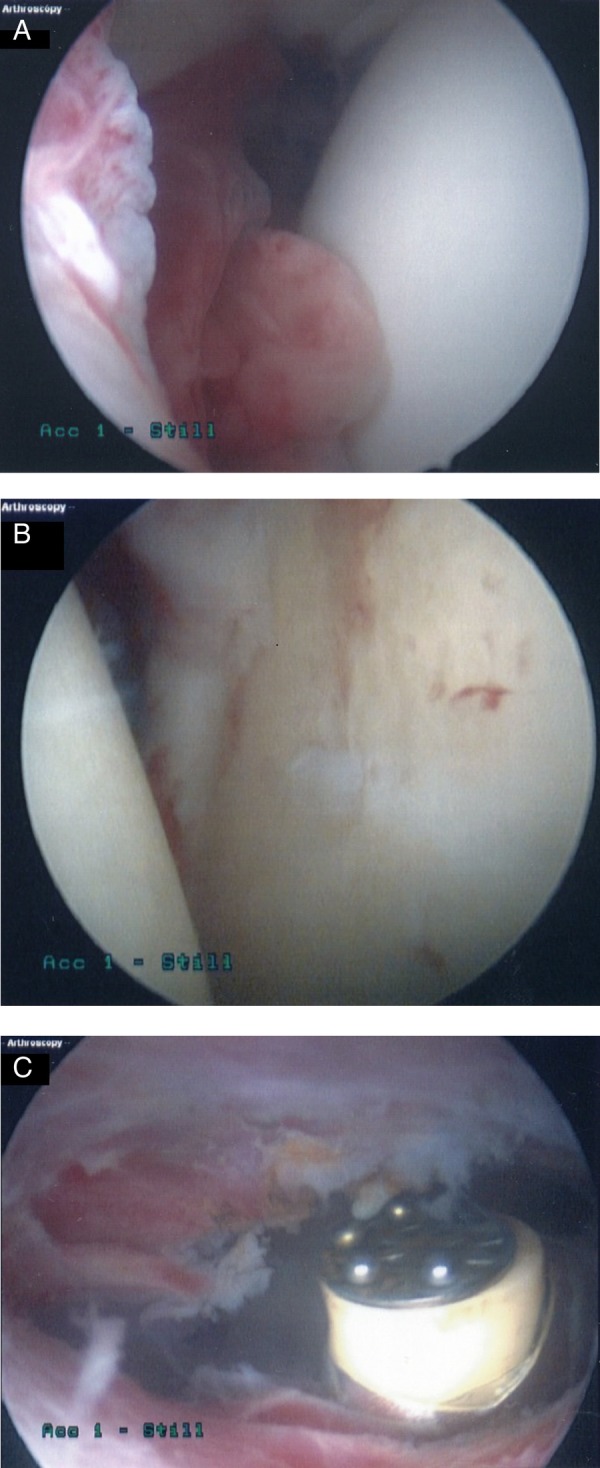
(A) Intra-articular image of right shoulder showing humeral head synovitis over the anterior aspect of glenoid. (B) Left glenohumeral joint showing inflammation, fibrin deposits and early fibrillation of articular cartilage of humeral head and glenoid due to infection. (C) Left shoulder subacromial bursoscopy showing clearance of infected bursa by ablation.
Forty-eight hours following the first washout, the patient still had painful, erythematous, hot shoulders, with no improvement in joint function. He was taken for a further arthroscopic evaluation, and was found to have bilaterally inflamed, infected subacromial bursae. Fortunately, he had no rotator cuff damage. He thus went on to have a washout, debridement and complete subacromial bursectomy of both shoulders during this second surgical procedure (figure 2C).
Outcome and follow-up
The patient improved markedly following 17 days of intravenous antibiotics and physiotherapy. As mentioned above, he was initially started on intravenous flucloxacillin, but developed deranged liver function secondary to this. He was thus changed to oral linezolid (600 mg two times a day), as recommended by the consultant microbiologist. He was discharged home with a 3-week course of oral cotrimoxazole (960 mg two times a day). He continued to have physiotherapy as an outpatient. Shoulder movements 2 months following initial presentation were greatly improved, with bilateral forward flexion of 90°, lateral abduction of 90° and external rotation of 40°.
At his last outpatient visit 5 months after his initial presentation, he had regained an almost full range of movement in both shoulders; the only persisting abnormality was a lag in forward flexion of approximately 5°.
Discussion
Septic arthritis of the glenohumeral joint is rare, and there is no literature on the management and outcomes of patients with bilateral shoulder septic arthritis. The rarity of this condition often leads to difficulty in diagnosis. The criterion for the diagnosis of septic arthritis was first outlined by Newman in his 1976 paper.6 He suggests that the patient must satisfy one of the four following diagnostic parameters: isolation of a pathogenic organism from a joint; isolation of pathogenic organism from another source (eg, blood) in context of an erythematous, hot joint; typical clinical features and turbid fluid; and, finally, postmortem or pathological features suspicious of septic arthritis.
Leslie et al,7 in their 1989 study, followed up 18 patients with glenohumeral joint septic arthritis over a year, and found only one patient to have rapid diagnosis and initiation of treatment. The main reason identified for delay in diagnosis was that most patients did not present clinically septic; 75% were without fever and had only mildly raised inflammatory markers. Other studies have reported up to 61% of patients presenting without clinical signs of sepsis, and biochemical or inflammatory markers non-indicative of infection. Pfeiffenberger et al,4 in their 1995 review of 10 patients with shoulder septic arthritis, reported a mean time to diagnosis of 11 days, with the longest delay being 20 days. It is widely recognised that a key prognostic factor in septic arthritis is early diagnosis and intervention.2 4 The prompt diagnosis of our patient was greatly aided by his typical septic presentation.
It has long been recognised that certain comorbidities predispose individuals to development of septic arthritis.5 8 For unilateral glenohumeral joint septic arthritis, comorbidities have been reported in as many as 87% of patients.4 The most common comorbidities reported in the literature are diabetes, cardiac abnormalities, intravenous drug use, immunosuppressive illnesses and rheumatoid arthritis.3 4 In this case, our patient was entirely fit and well. Furthermore, screens for risk factors, which included both cardiac imaging and immunological testing, yielded no positive results. There have only been two other such cases in the literature of shoulder septic arthritis in patients with no risk factors. The existence of such patients can prove to be a diagnostic challenge, and can lead to delays in management. In addition to this, polyarticular septic arthritis of the upper limbs is incredibly rare.1 It is thus an important differential to consider in the patient presenting with painful, restricted range of joint motion with no other apparent cause.
The management of septic arthritis consists of fluid resuscitation, removal of infected material through arthroscopic washout or repeated needle aspiration, adequate antibiotic therapy and physiotherapy.4 8 The majority of cases of septic arthritis are caused by S. aureus, however, atypical or resistant organisms have been seen in intravenous drug abusers and in immunosuppressed patients.5 Interestingly, Neisseria gonorrhoeae has been known to cause polyarticular septic arthritis in fit, young individuals; the majority of cases, however, occur in the knee or ankle joints.2 Microbiology results from our patient yielded a S. aureus strain sensitive to commonly used antibiotics. The current literature suggests the ideal duration of antibiotics to be 6–8 weeks.8
There have been various studies comparing the outcomes of patients treated with washout versus repeated aspiration.3 8 As Clements et al1 comment in their 2013 paper, the evidence supporting needle aspiration of the shoulder joint until dry versus arthroscopic washout is inconclusive. However, a 2009 study by Kirchoff et al3 demonstrated slightly more favourable outcomes in patients treated with early arthroscopic washout compared to delayed washout, following aspiration. Surgical intervention is routinely performed after aspiration if the patient shows no clinical improvement, or if there is underlying bone or cartilaginous damage. Despite large amounts of infected material initially aspirated from our patient's shoulder, he did not improve. He, in fact, required two washouts before demonstrating an appreciable clinical improvement.
Despite early adequate intervention and resuscitation, the long-term sequelae of glenohumeral joint septic arthritis can be devastating.7 9 Leslie et al7 found only 27% of patients had movement of 90° or more a year after initial presentation and management. Similar results have been found in other studies, even with long periods of physiotherapy after hospital discharge.1 4 Our patient was fortunate to have regained an almost full range of movement in both shoulders 5 months after admission. Other common complications reported in the literature are osteomyelitis, cartilaginous and soft tissue damage and death. Sadly, mortality from septic arthritis has been reported to be as high as 50%, even after aggressive management.7
Learning points.
Septic arthritis of the glenohumeral joint is rare, and is associated with high rates of morbidity and mortality.
Polyarticular septic arthritis is particularly rare, and is a differential not usually considered in the previously fit and well individual. It is easily missed, even in those with risk factors for infection.
This case illustrates how prompt diagnosis and management can lead to a favourable outcome. Septic arthritis should be considered early in the unwell patient with arthralgia and reduced range of movement.
Despite adequate management, the long-term effects of septic arthritis can be devastating and include long-term disability and death.
Acknowledgments
The authors would like to acknowledge Mr Sivaraman Balasubramanian, upper limb fellow, who contributed to the management and follow-up of our patient.
Footnotes
Contributors: SAH wrote the majority of the paper, including summary, background, case presentation and the bulk of the discussion. SK wrote part of the discussion and the learning points. RJ acted as the supervising consultant and edited the final manuscript, including providing support and advice. All three authors were involved in the management of the patient in the case report, with RJ acting as his responsible clinician.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Clements J, Dinneen A, Heilpern G. Polyarticular septic arthritis in an immunocompetent patient. Ann R Coll Surg Engl 2013;95:34–5. 10.1308/003588413X13511609955292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Griffin PL, Griffin GD, Simon EL. Spontaneous septic arthritis in a patient without trauma, coinfection or immunosuppression. Am J Emerg Med 2013;31:1623e3–4. 10.1016/j.ajem.2013.06.029 [DOI] [PubMed] [Google Scholar]
- 3.Kirchoff C, Braunstein V, Buhmann Kirchhoff S et al. Stage-dependent management of septic arthritis of the shoulder in adults. Int Orthop 2009;33:1015–24. 10.1007/s00264-008-0598-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfeiffenberger J, Meiss L. Septic conditions of the shoulder- an updating of treatment strategies. Arch Orthop Trauma Surg 1996;115:325–31. 10.1007/BF00420325 [DOI] [PubMed] [Google Scholar]
- 5.Pommering TL, Wroble RR. Septic arthritis of the shoulder: treating an atypical case. Phys Sportsmed 1996;24:74–85. 10.3810/psm.1996.05.1356 [DOI] [PubMed] [Google Scholar]
- 6.Newman JH. Review of septic arthritis throughout the antibiotic era. Ann Rheum Dis 1976;35:198–205. 10.1136/ard.35.3.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leslie BM, Harris JM, Driscoll D. Septic arthritis of the shoulder in adults. j Bone Joint Surgery Am 1989;71:1516–22. [PubMed] [Google Scholar]
- 8.Jeon IH, Choi CH, Seo JS et al. Arthroscopic management of septic arthritis of the shoulder joint. J Bone Joint Surg Am 2006;88:1802–6. 10.2106/JBJS.E.00917 [DOI] [PubMed] [Google Scholar]
- 9.Mehta P, Schnall SB, Zalavra CG. Septic arthritis of the shoulder, elbow, and wrist. Clin Orthop Relat Res 2006;451:42–5. 10.1097/01.blo.0000229322.30169.29 [DOI] [PubMed] [Google Scholar]