Abstract
The lineage leading to modern Crocodylia has undergone dramatic evolutionary changes in morphology, ecology and locomotion over the past 200+ Myr. These functional innovations may be explained in part by morphological changes in the axial skeleton, which is an integral part of the vertebrate locomotor system. Our objective was to estimate changes in osteological range of motion (RoM) and intervertebral joint stiffness of thoracic and lumbar vertebrae with increasing aquatic adaptation in crocodylomorphs. Using three-dimensional virtual models and morphometrics, we compared the modern crocodile Crocodylus to five extinct crocodylomorphs: Terrestrisuchus, Protosuchus, Pelagosaurus, Steneosaurus and Metriorhynchus, which span the spectrum from terrestrial to fully aquatic. In Crocodylus, we also experimentally measured changes in trunk flexibility with sequential removal of osteoderms and soft tissues. Our results for the more aquatic species matched our predictions fairly well, but those for the more terrestrial early crocodylomorphs did not. A likely explanation for this lack of correspondence is the influence of other axial structures, particularly the rigid series of dorsal osteoderms in early crocodylomorphs. The most important structures for determining RoM and stiffness of the trunk in Crocodylus were different in dorsoventral versus mediolateral bending, suggesting that changes in osteoderm and rib morphology over crocodylomorph evolution would have affected movements in some directions more than others.
Keywords: crocodylomorph, vertebrae, axial skeleton, stiffness, range of motion, aquatic adaptation
1. Introduction
Crocodiles, alligators and gharials are the only extant representatives of the clade Crocodylomorpha, whose members span the continuum from terrestrial to aquatic. Early crocodylomorphs had more erect (as opposed to sprawling) limb postures and parasagittal gaits and were probably highly terrestrial, like many other archosaurs [1], whereas later crocodylomorphs diversified to inhabit both terrestrial and aquatic environments [2]. One extinct clade, Thalattosuchia, includes obligatory aquatic marine specialists. Modern crocodylians are semi-aquatic and use a range of postures and gaits between sprawling and erect; therefore, the generally more sprawling posture of modern crocodylians must have been re-acquired at some point in their evolution. One unusual attribute of the crocodylomorph lineage is the convergent evolution of erect postures and asymmetrical (bounding and galloping) gaits otherwise found only in mammals. Only small (shorter than 3 m) crocodylids and gavialids (not alligatorids) have been observed to use asymmetrical gaits; architectural properties of the limb muscles may be a limiting factor [3]. Bounding and galloping abilities in modern crocodylians may have been inherited from their more terrestrial ancestors [1], or these abilities may have originated more recently [4].
In order to understand when and how crocodylians acquired their locomotor abilities, we must reconstruct the locomotor characteristics of extinct members of the crocodylomorph lineage. It is the existence of modern crocodylians that makes Crocodylomorpha such an attractive group in which to study the locomotor evolution of vertebrates. Methods for predicting locomotion in extinct animals should, ideally, be validated in closely related extant taxa [5,6], but this is not easy to do in groups whose extant members have very different locomotor specializations from their extinct ancestors, such as non-avian dinosaurs and birds. Crocodylomorpha provides a rare opportunity to test hypotheses about form–function relationships in a group outside Mammalia whose members once filled a wide variety of ecological niches and whose extant members are capable of diverse locomotor modes (e.g. various symmetrical and asymmetrical gaits), some of which might be ancient holdovers from more terrestrial ancestors. Furthermore, current hypotheses about locomotion in extinct crocodylomorphs are supported by multiple lines of evidence, including limb proportions and joint morphology (e.g. [1,7–10]), palaeobiogeography [1] and trackways [11] (see Discussion for details).
Because the vertebral column is an important part of the locomotor system, its morphology can give essential clues about locomotion in extinct vertebrates. Studies of extant animals have shown that biomechanical properties and, to some extent, locomotor habits can be predicted from vertebral morphology, provided differences among species are taken into account [12–19]. However, few studies thus far have sought to test or apply these principles in non-mammalian tetrapods. Recently, we [20] identified several morphometric parameters that could predict intervertebral joint (IVJ) passive stiffness in modern crocodiles and described the relationship among stiffness, range of motion (RoM) and axial tissues. We also inferred that the role of the axial column in crocodylian locomotion may be functionally different from that in mammals, even during analogous gaits.
Based on analogy with mechanical structures, Salisbury & Frey [21] defined several categories of axial morphology in extant and extinct crocodylomorphs and predicted how each would have functioned during locomotion. According to that study, modern crocodylians accommodate locomotor forces on the vertebral column primarily with their procoelous (each centrum bearing a cranial socket or cotyle and a caudal ball or condyle) IVJs and horizontally oriented zygapophyses, allowing a more flexible arrangement of bony scutes (or osteoderms) along their backs and therefore greater trunk flexibility, particularly in the mediolateral direction. In contrast, extinct crocodylomorphs with amphicoelous (biconcave) IVJs relied to a greater extent on the interlocking double row of osteoderms that run along their back—known as the paravertebral shield—for support. This arrangement would have sharply limited mediolateral trunk flexibility, particularly in early crocodylomorphs, most of which had very rigid paravertebral shields. Thalattosuchians lacked both procoelous IVJs and interlocking osteoderms (although teleosaurid thalattosuchians had osteoderms, they did not interlock like those of most early crocodylomorphs), so the ability for sustained terrestrial locomotion would have been restricted to smaller animals [21].
To test whether vertebral morphology correlates with locomotor potential and associated ecological behaviours in extinct and extant crocodylomorphs, we measured osteological RoM and took morphometric measurements from three-dimensional virtual models of thoracic and lumbar vertebrae of four major groups of the Crocodylomorpha: ‘sphenosuchians’, ‘protosuchians’, Thalattosuchia and Crocodylia (table 1). Terrestrisuchus gracilis is a ‘sphenosuchian’-grade crocodylomorph, potentially near the origin of Crocodyliformes [22]; its locomotion is reconstructed as being upright, terrestrial and possibly digitigrade [8]. Protosuchus richardsoni represents a slightly more crownward ‘protosuchian’ crocodyliform with limb proportions and presumed limb postures and degrees of terrestrial locomotor ability intermediate between those of early crocodylomorphs and modern crocodylians [23]. We also included three species of thalattosuchians with increasing degrees of aquatic adaptation. Pelagosaurus typus is a small, semi-aquatic thalattosuchian that would have retained the ability to move on land [24], Steneosaurus leedsi is a large teleosaurid thalattosuchian that would have been confined to near shore environments, and Metriorhychus superciliosus is a medium-sized metriorhynchid thalattosuchian that is hypothesized to have been a pelagic predator [25–27]. Compared to Pelagosaurus and Steneosaurus, Metriorynchus is more specialized for aquatic locomotion with hydrofoil-like limbs, complete loss of osteoderms and a fishlike tail [28]. Finally, we included Crocodylus niloticus as a representative of modern semi-aquatic crocodylians, considering that this genus is also capable of bounding and galloping gaits at small body mass (in juveniles and smaller adults [29,30]).
Table 1.
Crocodylomorph specimens used for morphometric and RoM analyses. Vertebral count is the total number of thoracic and lumbar vertebrae; figures in parentheses represent number of thoracic vertebrae (first) and lumbar (second) in taxa that have distinct thoracic and lumbar regions. Column length is summed centrum length (CL), and intervertebral (IV) space was measured from joints used to estimate RoM, excluding lumbosacral joint. Voxel size (v.s.) is the same in all dimensions. ‘Average number of mesh faces’ refers to the STL files exported from Mimics. IV space in Steneosaurus is not applicable (n.a.) because it is disarticulated and we did not model joints. Institutional abbreviations: American Museum of Natural History, New York (AMNH), Natural History Museum, London (NHMUK), Royal Veterinary College, London (RVC), Stony Brook University, New York (SBU).
| taxon | type | source | scan | vertebral count | column length and range of CL (cm) | scan parameters | avg. no. mesh faces | IV space (cm) and as % of CL | |
|---|---|---|---|---|---|---|---|---|---|
| Terrestrisuchus gracilis | fossil | NHMUK PV R 7562 | μCT | NHMUK | (15a (10a,5) | unknown (0.99–1.03) | 190 kVp, 200 μA, v.s. 0.06 mm | 61K | 0.04 (4%) |
| Protosuchus richardsoni | fossil | AMNH 3024 | CT | SBU | 15 (10,5) | 13.8 (0.88–1.31) | 140 kVp, 300 mA, v.s. 0.39 mm | 23K | 0.12–0.17 (12.5%) |
| Pelagosaurus typus | fossil | NHMUK PV OR 32598 | μCT | NHMUK | 15 (10,5) | 31.7 (1.73–2.29) | 200–210 kVp, 200–220 μA, v.s. 0.08 mm | 145K | 0.07–0.12 (9%) |
| Metriorhynchus superciliosus | fossil | NHMUK R 2054 | CT | RVC | 17 | 66.3 (3.14–4.14) | 100 kVp, 200 mA, v.s. 0.36 mm | 76K | 0.42–0.63 (13.4%) |
| Steneosaurus leedsi | fossil | NHMUK R 3806 | CT | RVC | 13 | 62.7 (5.02–5.47) | 100 kVp, 200 mA, v.s. 0.32 mm | 89K | n.a. |
| Crocodylus niloticus | frozen; recent | La Ferme aux Crocodiles | CT | RVC | 15 (10,5) | 52.7 (1.64–1.94) | 120 kVp, 100 mA, v.s. 0.41 mm | 57K | 0.48–0.59 (19%) |
aThoracic vertebral number for Terrestrisuchus is reconstructed based on related taxa [8].
Based on our taxon sampling, we hypothesized that vertebral morphometric parameters known to correlate with mediolateral stiffness [20] would be more pronounced (and those correlated with dorsoventral stiffness would be less pronounced) in early crocodylomorphs (e.g. Terrestrisuchus, Protosuchus) as compared with thalattosuchians and modern crocodylians. This hypothesis is based on the idea that early crocodylomorphs were highly terrestrial and may have used asymmetrical gaits, a suite of behaviours requiring greater dorsoventral movements of the vertebral column than sprawling or swimming. Furthermore, we hypothesized that osteological RoM would be smaller in the mediolateral direction and greater in the dorsoventral direction in early crocodylomorphs, while the reverse would be evident in thalattosuchians and modern crocodylians. Finally, we hypothesized that pelagic thalattosuchians (i.e. metriorhynchids) would have morphometric parameters correlated with an increase in IVJ stiffness. The rationale for this hypothesis is that other pelagic marine reptiles, such as ichthyosaurs, sauropterygians and mosasaurs, are thought to have had relatively stiff vertebral columns (e.g. [31–34]), because such a morphology increases average swimming speed by increasing the natural frequency of body undulation at which the energetic cost of movement is minimized relative to speed [13,14,35].
2. Material and methods
2.1. Scanning, segmentation and retrodeformation
Terrestrisuchus and Pelagosaurus were micro-CT scanned at the Imaging and Analysis Centre in the Natural History Museum, London, UK (NHMUK) using a Metris X-Tek HMX ST 225 system. Scanning parameters for Terrestrisuchus were: 190 kVp, 200 μA, voxel size 0.06 mm in all dimensions. The three blocks containing Pelagosaurus were scanned separately, and the parameters ranged from: 200–210 kVp, 200–220 μA, voxel size 0.08 mm in all dimensions. The scans were reconstructed using CT PRO (Metris X-Tek, UK) and visualized using VG Studio Max v. 2.0 (Volume Graphics, Heidelberg, Germany). Dr Farah Ahmed and Mr Dan Sykes (NHMUK) and Dr Julia Molnar performed the scans, and Dr Molnar performed the reconstruction and visualization. Protosuchus was scanned by Dr Alan Turner at Stony Brook University Hospital using a LightSpeed VCT Scanner (GE Healthcare, Waukesha, WI, USA). Scan parameters were: 140 kVp, 300 mA, voxel size 0.39 mm in all dimensions. Multiple scans were taken to improve resolution. Steneosaurus, Metriorhynchus and Crocodylus were scanned by Dr John R. Hutchinson at the Royal Veterinary College using a medical CT scanner (GE Lightspeed) and reconstructed using Medview software (www.Medimage.com). Scanning parameters were, for Steneosaurus: 100 kVp, 200 mA, voxel size 0.32 mm in all dimensions; for Metriorhynchus: 100 kVp, 200 mA, voxel size 0.36 mm in all dimensions; and for Crocodylus: 120 kVp, 100 mA, voxel size 0.41 mm in all dimensions.
The reconstructed scans were segmented in Mimics (Materialise Inc. (www.materialise.com/mimics); Leeuwen, Belgium), a commercial image processing program for segmenting CT and MRI data and constructing triangulated three-dimensional surface meshes, to separate the fossil from the matrix. The fossils were segmented semi-automatically and each vertebra was volume-rendered, then its surface was exported as a separate STL file. Virtual bones were imported into 3D Studio Max (Autodesk 3ds Max; www.autodesk.com/3dsMax) and the joints were aligned to a standard coordinate system (figure 1).
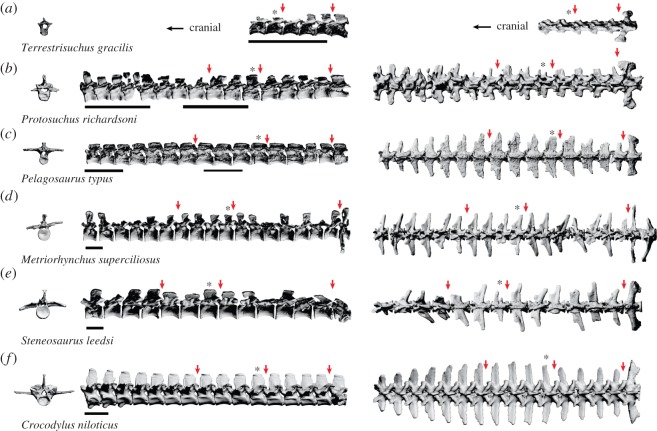
Figure 1.
Virtual thoracolumbar vertebral columns of crocodylomorphs. From left: a single vertebra (*) in cranial view, the entire column in left lateral view, and the entire column in dorsal view. Arrows indicate the positions of joints (thoracic/cranial thoracic, lumbar/caudal thoracic, lumbosacral) for which RoM was estimated. Scale bars, 5 cm.
Some degree of retrodeformation was required for Metriorhynchus, Steneosaurus and Protosuchus. The Metriorhynchus and Steneosaurus fossils were disarticulated, and each vertebra was deformed differently. Symmetry was restored using Landmark, a program with an algorithm designed for retrodeformation (Landmark; Institute for Data Analysis and Visualization, http://graphics.idav.ucdavis.edu/research/projects/EvoMorph). The Protosuchus specimen was articulated, but compressed dorsoventrally and mediolaterally. Retrodeformation using Landmark was attempted, but the software required each vertebra to be retrodeformed separately, resulting in mismatched articulations that would not fit back together. Instead, modelling tools in 3D Studio Max were used to scale the vertebrae in the direction of compression to restore symmetry and approximate the original dimensions (e.g. [36]). A sensitivity analysis showed that retrodeformation decreased RoM estimates by an average of less than 2° in each direction and that relative RoM in different directions was unaffected.
2.2. Morphometric measurements
To estimate relative joint stiffness, we compiled a list of 14 vertebral morphometric measurements linked to function through mechanical joint testing, correlation with locomotor behaviour, and/or engineering beam theory (table 2). Ten linear and four angular measurements were taken from all dorsal vertebrae. Morphometrics for Crocodylus were taken from Molnar et al. [20] and represent a single 10.1 kg juvenile specimen. For the remaining specimens, measurements were taken in ImageJ (http://rsbweb.nih.gov/ij) from orthographic projections of the virtual skeletons generated in 3D Studio Max. Linear measurements were normalized using the allometric scaling function:
where Madj is the normalized measurement, M is the original measurement, Ls is overall mean thoracolumbar length for all specimens (approximated by summing centrum length from each of the five lumbar vertebrae), L0 is lumbar length of the current specimen, and b is the slope of the regression of log10M on log10L0 for each measurement, using all specimens [19]. Using lumbar length rather than thoracolumbar length was a necessary simplification due to the incompleteness of the Terrestrisuchus specimen. Length of limb bones was not used for normalization because their lengths and proportions vary among the taxa analysed (e.g. ‘sphenosuchians’ have long slender limbs, while thalattosuchians have reduced limbs).
Table 2.
Summary of relationships between vertebral morphology and stiffness/RoM from previous studies. Long et al. [14] and Molnar et al. [20] are experimental studies that measured stiffness/RoM. Measurements identified as useful predictors of passive IVJ stiffness and/or RoM in both mammals and crocodylians are shown in italics; see §3.1 for details. DE, dorsal extension, VF, ventral flexion, LF, lateral flexion; AR, axial rotation; CL, centrum length; TPW, transverse process width; CH, centrum height; CW, centrum width; LW, lamina width; NSH, neural spine height; A-NS, neural spine angle; NSL, neural spine length; A-TPD, dorsoventral transverse process angle; A-TPC, cranio-caudal transverse process angle; A-PZ, pre-zygapophyseal angle; PZW, pre-zygapophyseal width; IVD, intervertebral disc; IZL, inter-zygapophyseal length.
| morphological feature | correlation | direction | animal(s) | study | |
|---|---|---|---|---|---|
| stiffness | CH | + | DE, VF | various mammals, Nile crocodiles | [15],[20],[37]a |
| CW | + | LF | various mammals, Nile crocodiles | [15],[20],[37]a | |
| LW | + | DE, LF | various mammals, Nile crocodiles | [20]a,[37]a | |
| A-PZ | − | LF | Nile crocodiles | [20] | |
| PZW | + | DE, VF, LF | Nile crocodiles | [20] | |
| IVD length | − | DE | dolphins | [14] | |
| IVD width | + | DE | dolphins | [14] | |
| CL | − | DE, VF | dolphins | [14] | |
| TPW | + | LF | dolphins, seals | [14],[19] | |
| NSH | + | DE, VF | aquatic mammals | [14],[15] | |
| A-NS | − | VF, LF | Nile crocodiles | [20] | |
| A-TPD | + | DE, VF, LF | Nile crocodiles | [20] | |
| RoM | A-PZ | + | LF | various mammals | [25,37a] |
| PZW | − | AR | various mammals | [17] | |
| CL | − | DE, VF | whales, seals | [16,19] | |
| NSL | − | DE | various mammals | [38] | |
| A-TPD | + | DE, VF | various mammals | [37]a | |
| A-TPC | − | DE, VF | various mammals | [37]a | |
| A-NS | − | DE, VF | various mammals | [37]a | |
| IZL | + | DE, VF | Primates | [37a,39] |
aAnd references therein.
2.3. Building virtual models
All fossil specimens used for RoM estimation, except Metriorhynchus, were preserved in articulation (both centra and zygapophyses), and the vertebral columns were fairly straight, suggesting that the intervertebral discs (IVDs) or other soft tissue between the vertebrae did not shrink extensively prior to fossilization. Therefore, the preserved space between adjoining centra probably represents a reasonable approximation of the thickness of IVDs (e.g. [40]). Vertebrae of Metriorhynchus were re-articulated by aligning the zygapophyseal facets with maximum overlap. Before manipulating the models to estimate RoM (see below), we defined a neutral pose and centre of rotation (CoR).
2.3.1. Neutral pose
In their work on sauropod necks, Stevens & Parrish [41] defined neutral poses where the pre- and post-zygapophyses overlap maximally and the margin of the cotyle of the cranial vertebra is parallel to the capsule scar of the caudal vertebra in the coronal plane. For platycoelous centra, the facets of the centra would be parallel in this model. Based upon observations of ostriches, camels and giraffes, Christian & Dzemski [42] argued that the margins of the centra are not parallel when the zygapophyses are aligned and that they often are not parallel in habitual or resting poses. We do not consider the neutral pose in a behavioural context (e.g. ‘resting pose’ or habitual posture), but only as a starting point from which to measure joint deflection. Therefore, we used a variation of the definition of Stevens & Parrish [41] because it is easy to consistently reproduce and is thought to represent an intermediate position within the joint's RoM [43]. Defining a consistent neutral pose is important in this study for two reasons: first, it allows comparison of relative RoM in ventral flexion versus dorsal extension across joints and species, and second, the joint is positioned in a ‘neutral’ dorsoventral joint angle when estimating mediolateral RoM, potentially affecting its magnitude (i.e. coupled motions).
For articulated specimens, the neutral pose was kept as similar to the preserved arrangement as possible. First, the vertebrae were linked hierarchically so that movement of one vertebra caused all vertebrae caudal to it to move along with it. Second, the cranialmost vertebra was rotated and translated to fit the following criteria:
(1) translate along the X- and Z-axes until the neural canal is centred on (0, 0) in the X–Z (coronal) plane;
(2) rotate around the X- and Z-axes until the long axis of the centrum is aligned with the global Y -axis (cranio-caudal axis); and
(3) rotate around the Y -axis until the left and right zygapophyses and transverse processes appear as mirror images in the X–Z (coronal) plane.
The next (caudally) vertebra was aligned following the same procedure after it had been linked to the CoR object (next section). Finally, that next vertebra was translated minimally along the Y -axis only if the two vertebrae were intersecting or disarticulated. The same procedure was followed for disarticulated specimens, except that translation along the Y -axis was performed until maximal zygapophyseal overlap was reached. This procedure was validated using three joints from a CT scan of a Nile crocodile cadaver: the virtual bones were duplicated and one pair was aligned visually. The average difference between the two treatments was very small: approximately 1.6% of centrum length (0.49 mm).
2.3.2. Location of centre of rotation
We placed the CoR at the centre of the condyle (cranially) for procoelous vertebrae [44] or where the centre of the IVD would have been located [45]. A sphere was fitted to the centre of the condyle of the preceding vertebra (procoelous) or an ellipsoid was fitted to the space between the two centra (amphicoelous). A more ventral CoR has been reported in some human studies (e.g. [46]), so we performed a sensitivity analysis to assess how variations in the CoR location, as well as the amount of translation allowed, affected our results. The sensitivity analysis showed that this variable produced only minor effects which did not change any of our conclusions (table 3).
Table 3.
Sensitivity analysis: differences in mean RoM for joint L1–2 by CoR location and translation. Mean RoM in degrees estimated from virtual models. For CoR location condyle: CoR is located at the condyle of the cranial vertebra for procoelous vertebrae or the centre of the IVD, where present. For CoR location NC: CoR is located at the anterior (ventral) margin of the spinal (neural) canal. NC, neural canal; ML, mediolateral; DE, dorsal extension; VF, ventral flexion; AR, axial rotation; 50% Z.O., minimum 50% zygapophyseal overlap.
| translation allowed |
CoR location |
|||||
|---|---|---|---|---|---|---|
| 0% | 1.50% | 3% | condyle | NC | 50% Z.O. | |
| Terrestrisuchus | ||||||
| ML | 6.23 | 11.18 | 17.71 | 11.71 | 11.71 | 8.6 |
| DE | 0.42 | 3.79 | 7.69 | 3.79 | 4.13 | 3.79 |
| VF | 2.12 | 6.14 | 9.26 | 5.67 | 6.02 | 3.25 |
| AR | 0.34 | 1.41 | 2.51 | 1.26 | 1.59 | 1.26 |
| average | 2.28 | 5.63 | 9.29 | 5.61 | 5.86 | 4.23 |
| Protosuchus | ||||||
| ML | 8.45 | 16.02 | 21.45 | 15.31 | 15.31 | 13.08 |
| DE | 2.44 | 5.55 | 8.59 | 5.19 | 5.87 | 5.19 |
| VF | 4.77 | 9.94 | 11.87 | 9.08 | 8.64 | 2.22 |
| AR | 1.23 | 2.2 | 3.1 | 2.06 | 2.29 | 2.06 |
| average | 4.22 | 8.43 | 11.25 | 7.91 | 8.03 | 5.64 |
| Pelagosaurus | ||||||
| ML | 10.68 | 15.06 | 19.31 | 15.02 | 15.02 | 12.9 |
| DE | 4.17 | 5.46 | 7.21 | 4.2 | 7.03 | 4.2 |
| VF | 10.68 | 12.36 | 13.21 | 15.37 | 8.8 | 1.64 |
| AR | 1.71 | 2.54 | 3.34 | 2.19 | 2.86 | 2.19 |
| average | 6.81 | 8.85 | 10.77 | 9.19 | 8.43 | 5.23 |
| Metriorhynchus | ||||||
| ML | 14.53 | 18.78 | 8.17 | 16.33 | ||
| DE | 1.12 | 4.12 | 4.12 | 1.12 | ||
| VF | 10.54 | 11.34 | 3.97 | 3.97 | ||
| AR | 3.64 | 7.44 | 7.44 | 7.44 | ||
| average | 7.46 | 10.42 | 5.93 | 7.22 | ||
| Crocodylus | ||||||
| ML | 16.98 | 20.19 | 21.53 | 19.56 | 19.56 | 7.92 |
| DE | 11.69 | 12.7 | 13.75 | 9.43 | 16 | 9.43 |
| VF | 7.49 | 11.45 | 13.35 | 17.47 | 4.05 | 2.47 |
| AR | 2.45 | 2.87 | 3.24 | 2.63 | 3.08 | 2.63 |
| average | 9.65 | 11.8 | 12.97 | 12.27 | 10.67 | 5.61 |
2.3.3. Estimating osteological range of motion
RoM in ventral flexion, dorsal extension and lateral flexion was estimated by manipulating the virtual fossils in 3d Studio Max. The preceding vertebra was held stationary, while the vertebra caudal to it was rotated about its CoR until it reached an osteological stop (i.e. the bones intersected) or the joint disarticulated (i.e. zygapophyseal facets no longer overlapped). Contacts between bones were detected visually, checking that the vertebrae did not touch or penetrate each other in three dimensions. We examined the effect of setting zygapophyseal overlap minima of both 0% (no overlap) and 50% to cover the range found in previous studies [41,42,47] (table 3). Additionally, we did not allow separation of zygapophyseal facets in the dorsoventral direction because disarticulation of zygapophyses would cause instability and would be unlikely to occur during normal locomotion. A small amount of translation between vertebrae (1.5% centrum length) and up to 3° of rotation about the long axis of the column (torsion) were allowed. Three degrees of torsion was chosen as a conservative value to allow the maximum possible flexion/extension; torsion during walking in extant alligators averages only about 10° across the entire trunk [48]. Some translation almost certainly occurs at IVJs, because the soft tissues of the joint deform under pressure. In human lumbar IVJs, an average of 0.3–2.0 mm translation was measured in concert with mediolateral and dorsoventral bending [49,50]. For comparison, 1 mm is about 3.3% of centrum length in the human lumbar vertebrae used by Xia et al. [50]. A sensitivity analysis was conducted to assess the effects of amounts of translation on RoM estimates (table 3). We were not able to include osteoderms in this study because only two of the fossil specimens had osteoderms preserved.
2.4. Sequential removal of axial tissues in Crocodylus
Deflection and stiffness of the whole trunk were measured, both with and without soft tissues, for two juvenile C. niloticus specimens with body masses of 1.6 and 2.2 kg. The specimens were provided by the conservation centre La Ferme aux Crocodiles (Pierrelatte, France), had died of natural causes and were sealed in airtight plastic bags and frozen (−20°C) immediately thereafter. They were thawed at room temperature for approximately 24 h before dissection. The tail, head and limbs were removed, leaving the last cervical and first caudal vertebrae intact. These two vertebrae were stripped of flesh and holes were drilled through them in the mediolateral and dorsoventral directions to accommodate rods used for suspension. Sewing pins were inserted between the osteoderms and into the neural spines of each vertebra to track its movement. The tenth thoracic (i.e. dorsal) vertebra was located by counting rows of osteoderms, and its position was marked on the skin to approximate the midpoint of the trunk. Each specimen was weighed. Thin metal rods were passed through the drilled holes on either end of the specimen, and the rods were balanced on two larger rods running parallel to the length of the specimen (figure 2). The diameter of the rods was smaller than the drilled holes, so the vertebrae were able to rotate freely. Also, the thinner rods were able to slide along the length of the thicker rods, meaning that the apparatus did not impede flexion of the specimen or rotation about either end. A length of fishing line was looped around the midpoint of the trunk, and metric weights were suspended from the fishing line, causing the trunk to flex and its ends to move closer to each other.
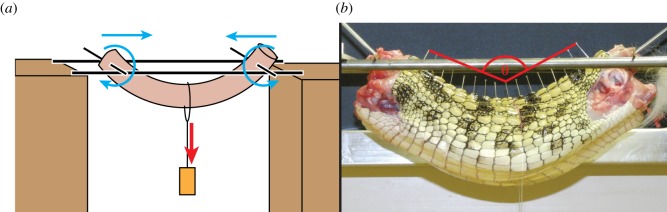
Figure 2.
Experimental set-up for whole trunk bending in Crocodylus (a) and photograph of whole trunk in dorsal extension (b). Red arrow shows the application of force, and blue arrows show the direction of movement of the two ends of the specimen; θ is trunk angle measured from position of pins.
The specimen was loaded and deflections were recorded as described in Molnar et al. [20]. Ten different weights were used: 0.02, 0.05, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7 and 0.8 kg. The entire procedure was performed three times for each specimen in ventral flexion, dorsal extension and lateral flexion. Soft tissues were removed sequentially, and measurements were repeated after each step, as follows:
(1) whole trunk;
(2) skin removed (osteoderms left intact);
(3) viscera removed;
(4) ribs removed;
(5) accessory osteoderms removed;
(6) paravertebral osteoderms (two sagittal rows either side of the midline) removed; and
(7) epaxial muscles removed.
Deflection angles were measured between the cranialmost, caudalmost and central pins, the central pin having been placed in the neural spine of the tenth thoracic vertebra (figure 2b). Measurements were taken from photographs (to scale) in ImageJ software.
The applied moment (M) was then calculated using the following equation:
where the applied force is the sum of the mass of the added weight (mw) and the specimen mass in kilograms (ms) times g (=9.81 ms−2), and the moment arm of the applied force was calculated from specimen length in metres (L) and trunk angle in degrees (θ) using trigonometry. To simplify calculations, it was assumed that specimen length does not change and that forces act on the specimen at the base of the central pin.
Stiffness was calculated as described in Molnar et al. [20]. Briefly, the rotational force applied to the specimen in Newton metres (moment; M) was plotted against trunk angle in degrees, where 180° represents a flat trunk with no deflection in any direction. Because the mass of the specimen changed substantially with the removal of tissues, it was not possible to choose a range of equivalent moments across different treatments. Therefore, measured points from the approximately linear region of the moment–deflection graph with moments above 0.3 Nm were used to fit the regression lines that defined stiffness. Differences in deflection produced by equivalent moments (‘trunk deflection’) are represented by the x-intercept of the regression line, but they are not equivalent to neutral zones because very small moments were not measured in most cases.
3. Results
3.1. Estimates of thoracolumbar intervertebral joint stiffness based on morphometrics
Within each of the three crocodylomorph groups examined (early crocodylomorphs, thalattosuchians and modern crocodylian), the vertebrae share many morphological characteristics. The early crocodylomorph vertebrae are characterized by relatively long centra, short vertebral processes (neural spines and transverse processes) and medium-sized zygapophyseal joints, qualitatively similar to the ancestral archosaurian state (JR Molnar & JR Hutchinson 2015, personal observation). The thalattosuchian vertebrae have shorter centra (except Pelagosaurus), long, slender vertebral processes, and small zygapophyses. The modern crocodylian vertebrae have short, procoelous centra that are wider than they are tall, long, robust vertebral processes, and large, widely spaced zygapophyses (figures 1 and 3).
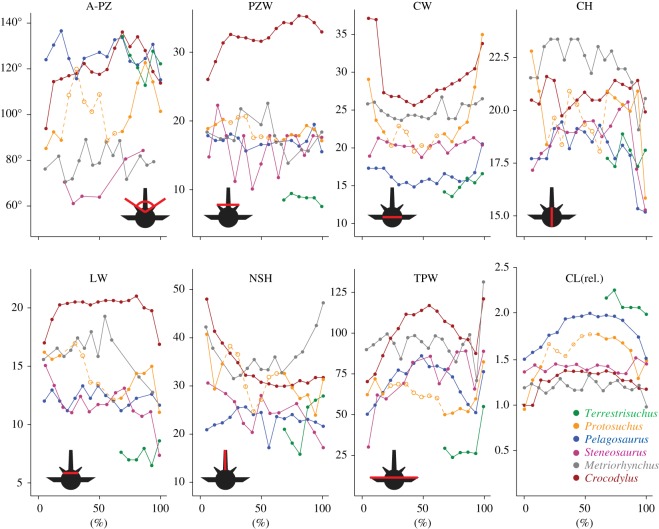
Figure 3.
Normalized morphometric measurements from thoracic and lumbar vertebrae. Length units not shown because measurements were normalized; see electronic supplementary material, table S2. The x-axis shows percentage of trunk length: 0% is the first thoracic, 100% is the first sacral. Dashed lines represent regions of poor preservation. Diagrams show vertebra from cranial view, and red lines show how each measurement was taken. A-PZ, pre-zygapophyseal angle (not normalized); PZW, pre-zygapophyseal width, i.e. mediolateral spacing of pre-zygapophyses; CW, centrum width; CH, centrum height; LW, lamina width; NSH, neural spine height; TPW, transverse process width; CL(rel.) relative centrum length = 2×length/(centrum width+height).
Based on previous studies, five morphometric measurements appear to be the most useful predictors of passive IVJ stiffness and/or RoM in both mammals and crocodylians (italic font in table 2). Correlates of high mediolateral stiffness include vertically oriented zygapophyses (smaller pre-zygapophyseal angles), widely spaced zygapophyses and mediolaterally wide centra and laminae. Correlates of high dorsoventral stiffness include horizontally oriented zygapophyses (larger pre-zygapophyseal angles) and dorsoventrally tall centra [20]. In addition, the length of neural spines and transverse processes give an idea of the cross-sectional areas and leverages of axial muscles, which would increase passive stiffness in the intact animal [19].
The morphometrics of the lumbar region in Terrestrisuchus (figure 3) are consistent with low stiffness, particularly in the mediolateral direction. Its narrowly spaced zygapophyses and narrow laminae imply low mediolateral stiffness, and its pre-zygapophyseal angles well over 90° suggest greater dorsoventral than mediolateral stiffness. Protosuchus is intermediate in almost every measurement, although the taphonomic distortion of the fossil and the low resolution of the scans make the morphometrics somewhat difficult to interpret. Its transverse processes are relatively short, suggesting that it lacked the muscle mass and leverage for powerful lateral flexion. The relative centrum dimensions of Protosuchus were most similar to Steneosaurus, but the zygapophyses of the former were closer to horizontal (suggesting greater dorsoventral stiffness) and its transverse processes did not become broader in the middle of the column. Pelagosaurus seems to have low mediolateral and intermediate dorsoventral stiffness: its zygapophyses are sub-horizontal, suggesting greater dorsoventral than mediolateral stiffness, and the centra are spool-shaped with a small diameter relative to their length, suggesting low overall stiffness. In contrast, Steneosaurus has more vertical zygapophyses and shorter, slightly wider centra, suggesting greater mediolateral than dorsoventral stiffness; other morphometrics are similar to Pelagosaurus. Metriorhynchus also has more vertical zygapophyses, but it has much taller, wider centra suggesting higher overall stiffness. Crocodylus has widely spaced, sub-horizontal zygapophyses, broad laminae and very short, wide centra, suggesting high mediolateral and dorsoventral stiffness.
3.2. Osteological range of motion estimated from virtual skeletons
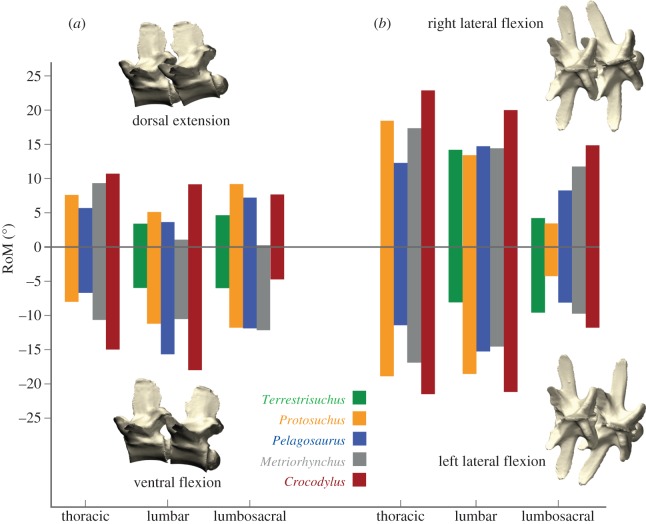
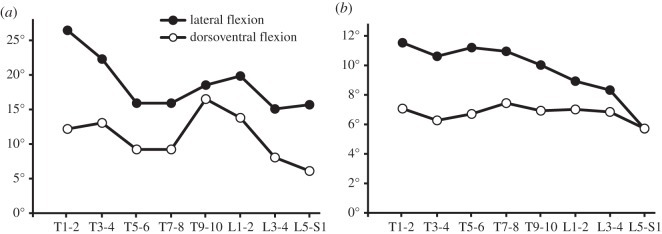
Despite their morphological differences, estimated RoM was surprisingly similar across the five taxa. No specimen stood out as being remarkably different from the others in overall RoM, osteological limitations to RoM, or the effects of variation in CoR and allowed translation amounts (tables 3 and 4). However, some taxa clearly had greater RoM than others in certain directions, and in many cases RoM was substantially different between the lumbosacral joint and the other two joints chosen to represent different regions of the trunk (figure 4). Crocodylushad the greatest RoM on average in both planes of movement for all three joints tested (figure 4), but the most restricted RoM in ventral flexion at the lumbosacral joint. Based on the caudalmost two joints, Terrestrisuchus had the smallest average RoM in every direction except dorsal extension, where Metriorhynchus had the smallest RoM. In most cases, RoM was greater in lateral flexion than dorsal or ventral flexion. However, the average RoM in ventral flexion for Pelagosaurus was similar to average lateral flexion RoM (11.4° versus 11.7°), and Metriorhynchus, Pelagosaurus and Protosuchus had greater RoM in ventral than lateral flexion at the lumbosacral joint. RoM in lateral flexion was generally fairly symmetrical, except in Terrestrisuchus where asymmetrical preservation caused the joints to reach osteological stops at different degrees of flexion to the left and right. The lumbosacral joints of all taxa had notably lower lateral RoM than the lumbar joints (3.9–13.4° versus 11.2–20.2°). RoM was similar between the thoracic and lumbar joints except in Metriorhynchus, where dorsal extension was greater in the thoracic joint. See electronic supplementary material, table S2, for non-normalized measurements.
Figure 4.
Maximum osteological RoM estimated by manipulation of virtual models. Estimates shown in (a) dorsoventral and (b) mediolateral flexion for three dorsal IVJs (except Terrestrisuchus, which lacked a thoracic region). Steneosaurus was not included because the zygapophyses were deformed and could not be articulated. Crocodylus vertebrae are shown.
The amount of space between adjacent centra relative to centrum length was substantially greater in Crocodylus than the other taxa, and substantially smaller in Terrestrisuchus (table 1). This difference might be related to preservation (in the case of Terrestrisuchus), or it might reflect different amounts of soft tissue within the joints (more likely in the case of Crocodylus because it is the only studied taxon with procoelous IVJs). If it is an artefact of preservation, the small amount of intervertebral space in Terrestrisuchus might cause our RoM estimates to be too low, particularly in lateral flexion because this movement was limited by contact between the centra (table 4).
Table 4.
Osteological RoM and structures that limit RoM. Summary of estimated RoM values. Values for ML and AR are averages of left and right RoM. Mean RoM in degrees and limitations on RoM (in italics) in each taxon, joint and direction. ZI, zygapophyses intersect; ZDl, zygapophyses disarticulate in the mediolateral, dorsoventral (ZDd) or cranio-caudal (ZDc) direction; CI, centra intersect.
| lateral flexion | dorsal extension | ventral flexion | ||||
|---|---|---|---|---|---|---|
| Terrestrisuchus | ||||||
| lumbar | 11.2° | CI | 3.5° | ZI | 6.0° | ZD(d) |
| lumbosacral | 6.9° | CI | 4.7° | ZI | 6.0° | ZD(d), CI |
| Protosuchus | ||||||
| thoracic | 18.7° | CI, ZD(l) | 7.7° | ZI | 8.0° | ZD(d) |
| lumbar | 16.0° | CI, ZD(l) | 5.2° | ZI | 11.2° | ZD(d) |
| lumbosacral | 3.9° | CI | 9.3° | ZI | 11.8° | ZD(d), CI |
| Pelagosaurus | ||||||
| cranial thoracic | 11.9° | CI | 5.8° | ZI, CI | 6.7° | CI |
| caudal thoracic | 15.1° | ZI, ZD(d) | 3.7° | ZI | 15.7° | CI, ZD(c) |
| lumbosacral | 8.2° | CI | 7.3° | ZI, CI | 11.9° | CI |
| Metriorhynchus | ||||||
| cranial thoracic | 17.2° | CI | 9.4° | ZI | 10.7° | ZD(d,l) |
| caudal thoracic | 14.5° | ZI, CI | 1.1° | ZI | 10.5° | ZD(d,l) |
| lumbosacral | 10.8° | ZI, CI | 0.3° | ZI | 12.2° | ZI, CI |
| Crocodylus | ||||||
| thoracic | 22.3° | ZD(l), ZI | 11.0° | ZI | 15.1° | ZD(d) |
| lumbar | 20.2° | ZD(l), ZI | 9.4° | CI | 18.2° | ZD(d, l), CI |
| lumbosacral | 13.4° | ZD(l), ZI | 7.9° | ZI, CI | 4.7° | ZD(d) |
| average | 18.6° | 9.4° | 12.7° | |||
Comparison of our virtual RoM Crocodylus data with those data recovered experimentally in cadaveric specimens showed that the virtual RoM estimation method successfully captured much of the variation between bending directions and along the vertebral column (figure 5).
Figure 5.
Validation of RoM estimation method by qualitative comparison with experimental data. Patterns of RoM along the thoracolumbar vertebral column in Crocodylus in dorsoventral and mediolateral flexion estimated from manipulation of virtual skeletons of Crocodylus allowing 0.45 mm translation (a) and experimental measurements from individual cadaveric joints taken from Molnar et al. [20] (b). The x-axis shows joints (e.g. T1–2 is the joint between the first and second thoracic vertebrae), and the y-axis shows flexion in degrees. CoR was located at the condyle of the cranial vertebra and minimum zygapophyseal facet overlap was 1%. Both sets of results showed greater average RoM in lateral flexion than dorsoventral flexion; RoM in both directions decreased along the vertebral column—lateral flexion more steeply—whereas RoM in dorsoventral flexion peaked in the mid-trunk. The data from the virtual model were noisier and did not capture the more subtle differences, which was expected considering that only one specimen was scanned for the virtual model, whereas the experimental data represent averages from seven specimens.
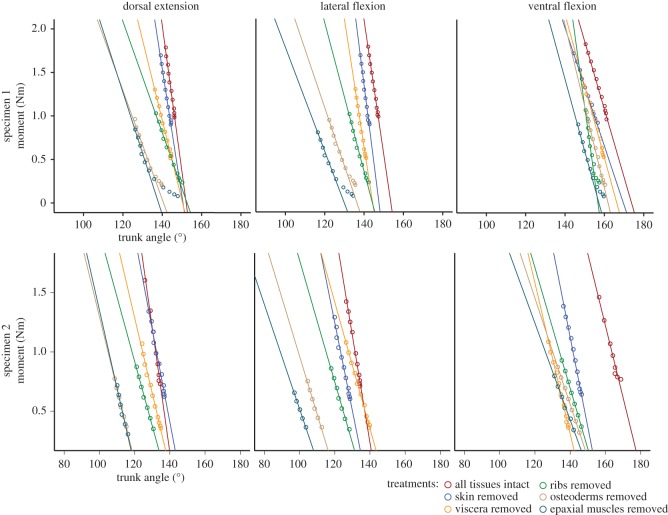
3.3. Effects of other axial tissues in Crocodylus
Removal of osteoderms, ribs and soft tissues substantially increased RoM and decreased stiffness of the trunk, and these effects varied across different bending directions. Removal of tissues had the greatest effect upon trunk deflection in lateral flexion, increasing it by a total of 12.3° versus 8.3° in ventral flexion and 9.8° in dorsal extension. Removal of tissues decreased stiffness by 58–61% in dorsal extension and lateral flexion, but only 6% in ventral flexion (table 5 and figure 6).
Table 5.
Change in stiffness with sequential removal of axial tissues in Crocodylus. Coefficients (stiffness) and x-intercepts of linear regressions with trunk deflection (Crocodylus) in degrees as the independent variable and applied moment in Newton metres as the dependent variable. Only moments greater than 0.3 Nm were used in the regression.
| dorsal extension |
lateral flexion |
ventral flexion |
|||||
|---|---|---|---|---|---|---|---|
| treatment | specimen mass (kg) | stiffness | x-intercept | stiffness | x-intercept | stiffness | x-intercept |
| whole trunk | 0.95 | 0.168 | 29.7 | 0.154 | 35.8 | 0.078 | 13.6 |
| skin removed | 0.875 | 0.146 | 26.8 | 0.179 | 35.4 | 0.068 | 21.9 |
| viscera removed | 0.50 | 0.092 | 31.2 | 0.147 | 41.9 | 0.082 | 14.8 |
| ribs removed | 0.225 | 0.064 | 37.5 | 0.085 | 41.7 | 0.165 | 17.7 |
| accessory osteoderms removed | 0.20 | 0.083 | 33.6 | 0.076 | 45.8 | 0.139 | 20.2 |
| paravertebral osteoderms removed | 0.20 | 0.063 | 30.9 | 0.067 | 37.1 | 0.094 | 14.9 |
| epaxial muscles removed | 0.075 | 0.070 | 180 | 0.060 | 180 | 0.083 | 180 |
Figure 6.
Moments versus trunk deflection for whole trunks with sequential removal of tissues in Crocodylus. Moments in Newton metres, angles in degrees. Trunk angle of 180° represents zero deflection. Lines are linear regressions on all points for which moment is greater than 0.3 Nm. Combining data for the two specimens masked patterns of variation, so results are presented separately.
4. Discussion
The crocodylomorph lineage includes species with locomotor habits ranging from fully aquatic (e.g. Metriorhynchus) to highly terrestrial (e.g. Terrestrisuchus). Sprawling and semi-aquatic locomotion have been associated with mediolateral movements of the vertebral column in crocodile-line archosaurs [51], whereas asymmetrical gaits seem be associated with dorsoventral movements [21]. For these reasons, we hypothesized that Terrestrisuchus and Protosuchus would have lower IVJ stiffness and greater RoM in the dorsoventral direction, and Pelagosaurus, Steneosaurus and Metriorhynchus would have lower stiffness and greater RoM in the mediolateral direction (Metriorhynchuswas hypothesized to have higher overall stiffness because it is reconstructed as a pelagic pursuit predator). While the results for the thalattosuchians and Crocodylus generally matched our predictions, those for the early crocodylomorphs did not. The most likely explanation for this discrepancy is the effect of other axial structures such as skin, muscles and particularly the paravertebral shields of most early crocodylomorphs on IVJ movements, which are best examined here from an evolutionary perspective (figure 7).
Figure 7.
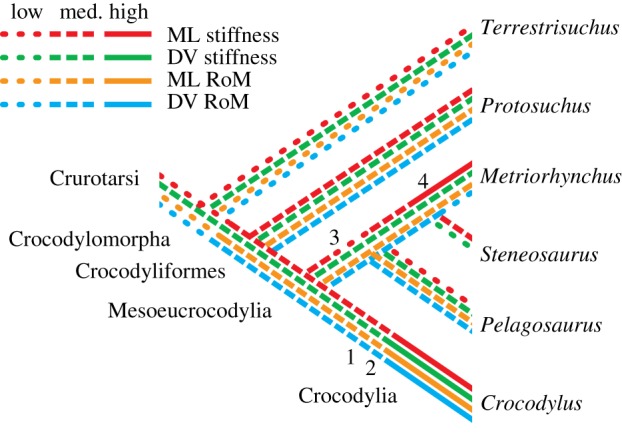
Hypothetical changes in IVJ stiffness and RoM with aquatic adaptation in Crocodylomorpha (phylogeny from [52]). Numbers show changes in other axial tissues: (1) sagittal segmentation of the paravertebral shield; (2) procoelous vertebrae; (3) ‘open’ margins of the paravertebral shield; (4) complete loss of osteoderms [21]. NB: procoelous vertebrae and/or loss of osteoderms evolved convergently in Junggarsuchus [7] and a few other crocodylomorph clades as well ([53] and references therein). Some of these hypotheses are sensitive to the phylogenetic placement of Thalattosuchia; see the electronic supplementary material for an alternative phylogeny that places Thalattosuchia outside Crocodyliformes. ML, mediolateral; DV, dorsoventral.
4.1. Evolution of vertebral functional morphology in crocodylomorphs
4.1.1. Early crocodylomorphs
We predicted that the early crocodylomorphs would have lower stiffness and greater RoM in the dorsoventral direction because members of this group are widely thought to have used parasagittal gaits based on the shapes and proportions of their limb bones and joints (e.g. [1,7–10]). In addition, analysis of pace angulation of trackways supports the inference that erect limb posture was employed by crocodile-line archosaurs in the Early Triassic [11], and early crocodylomorphs are primarily found in association with terrestrial rather than aquatic faunae [1]. Frey [54] postulated that the morphology of the trunk of Protosuchus was specialized for fast terrestrial locomotion and would not have allowed substantial lateral undulation (or adept swimming), in contrast to the morphology of modern crocodylians, which mainly permits mediolateral movements during terrestrial and aquatic locomotion. However, our estimates of IVJ stiffness and RoM do not reflect this pattern. On the contrary, average mediolateral RoM in the early crocodylomorphs was greater than dorsoventral RoM (similar to the other taxa in this study), and dorsoventral stiffness was estimated to be higher than mediolateral stiffness. These patterns were more pronounced in Terrestrisuchus: RoM in ventral flexion was sharply limited (6° maximum) by separation of the zygapophyseal facets; dorsoventral stiffness would have been increased by the near-horizontal zygapophyses; and mediolateral stiffness would have been reduced by the narrow spacing of the zygapophyses and narrow laminae and centra. The paravertebral shield would have further restricted movements and increased stiffness (see ‘Effects of other axial tissues’ below). While we only studied two early crocodylomorphs, the similar features of Terrestrisuchus and Protosuchus provide strong qualitative support that relatively greater IVJ stiffness and smaller RoM were ancestral for the crocodylomorph lineage (figure 7).
4.1.2. Thalattosuchians
Thalattosuchians were characterized by short, flat limbs; furthermore, metriorhynchid thalattosuchians, reconstructed as specialized aquatic pursuit predators [55–57], had aquatic adaptations including paddle-like limbs, a streamlined body shape, a hypocercal tail [24,28,58] and osteoporotic lightening of the skeleton [59]. In agreement with our predictions, the thalattosuchians we examined had greater mediolateral than dorsoventral osteological RoM (figure 4) and estimated stiffness was greater in the dorsoventral than mediolateral direction in Pelagosaurus and greater overall in Metriorhynchus than the other taxa. The results for Pelagosaurus fit our predictions well: mediolateral stiffness was estimated to be lower than dorsoventral stiffness and RoM was greater in mediolateral flexion, similar to the semi-aquatic Crocodylus. On the contrary, the more vertically oriented zygapophyses and relatively low centra of Steneosaurus compared to the other taxa, including Crocodylus (figure 3), suggest greater stiffness in the mediolateral direction as compared to the dorsoventral direction. Since Steneosaurus probably spent most of its time in near shore environments, stiffness of the trunk in the mediolateral direction may have stabilized the body to allow fast lateral sweeping movements of the neck during prey capture. As noted by Hua [25], the cervical vertebrae of Steneosaurus had more horizontal zygapophyses, suggesting greater mediolateral RoM and lower stiffness. Metriorhynchus had smaller than average RoM in the dorsoventral direction, particularly in dorsal extension, which was limited by the zygapophyses (although more vertical than horizontal, their facets could not slide far in this direction before contacting the neural spine of the adjacent vertebra). Similar to Steneosaurus, estimated stiffness in Metriorhynchus was greater in the mediolateral than dorsoventral direction, due primarily to the more vertical orientation of their zygapophyses. This characteristic was also noted by Hua [25], who interpreted it to mean that Steneosaurusand Metriorhynchus had stiffer bodies than modern crocodylians. While our morphometric data generally agree that Metriorhynchus would have had relatively stiff IVJs, in our virtual models it was the large, flat central articulations rather than the zygapophyses that constituted the major restriction on lateral flexion. The zygapophyses of Metriorhynchus are small and close to the midline, meaning that large degrees of IVJ flexion require only small displacements of the zygapophyseal facets. Likewise, IVJ stiffness in Metriorhynchus would be conferred by resistance to compression of the soft tissue between the centra and by the large axial muscles implied by its long vertebral processes, in addition to stretching of the zygapophyseal joint capsules. We were not able to estimate RoM for Steneosaurus, but the preserved morphology qualitatively indicates a RoM within the range observed for the other two thalattosuchians.
4.1.3. Modern crocodylians
In Crocodylus, our estimates from virtual models and morphometrics agree with experimental tests [20] showing that stiffness is lower and RoM is greater in the mediolateral direction (figure 4). This result is consistent with our expectations based on studies (cited below) showing that extant crocodylians use more mediolateral than dorsoventral flexion during locomotion. Lateral trunk flexion of 20–30° (left plus right) has been observed during terrestrial locomotion [48,60,61], and up to 90° (unilaterally) during swimming [21]. Dorsoventral flexion of about 5° (dorsal plus ventral) was recorded during a high walk [48], and some (presumably greater) dorsoventral flexion has been observed during galloping/bounding ([21] and references therein; [29]).
4.2. Implications for locomotion
Cross-sectional areas and leverages of axial muscles in crocodylomorphs, approximated by the lengths and orientations of vertebral processes, were largely consistent with presumed locomotor strategies. In more erect limb postures, such as those widely reconstructed for early crocodylomorphs, a greater component of ground reaction force acts on the vertebral column in the sagittal direction, whereas in more sprawling postures, such as those reconstructed for the remaining taxa, mediolateral components are greater (Crocodylia actually use a variety of ‘semi-erect’ postures [60]). In (more upright) mammals, axial muscles that stiffen the trunk in the dorsoventral direction are larger than in other tetrapods, and these muscles are bilaterally active during symmetrical gaits [62]. As expected, the aquatic and semi-aquatic crocodylomorph taxa had relatively broad transverse processes (figure 3), increasing the leverage of lateral flexors, and Protosuchus had relatively tall neural spines, increasing the leverage of dorsal extensors. Terrestrisuchus did not have particularly tall neural spines, but its transverse processes were even shorter. The more powerful active axial movements implied by the long vertebral processes of Crocodylus and Metriorhynchus may reflect the relative importance of axial movements, which decreases over the continuum of sprawling to erect locomotion [63] and might play a major role in undulation and/or stiffening of the trunk during swimming. The shorter vertebral processes of Terrestrisuchus and Protosuchus suggest that they relied to a greater extent on passive stabilization mechanisms such as the paravertebral shield. Alternatively or in combination, limb propulsion as opposed to trunk undulation may have contributed a greater proportion of locomotor power in these taxa; early crocodylomorphs, particularly the earlier non-crocodyliform lineages, had relatively longer (cursorial), more robust limbs than thalattosuchians and extant crocodylians [1,7].
Body size is another factor that influences the effect of axial stiffness on locomotion. Because of the scaling relationship between cross-sectional area, which determines muscle and bone strength, and volume, which determines body mass, larger animals must compensate behaviourally or structurally to avoid dangerously high stresses during locomotion [64]. In Alligator mississippiensis, the lengths of the neural spines and transverse processes increase significantly relative to body length throughout ontogeny, which may be explained by an increase in axial muscle mass [65] and thus may help compensate for size-related changes in stress on the axial column. The early crocodylomorphs were much smaller than the thalattosuchians and most extant, adult crocodylians: Terrestrisuchus was only 49–77 cm long (but may not have been fully grown), and Protosuchus was closer to 80 cm long [8,23], whereas the other species reached 3 m or more. Therefore, smaller body mass may have compensated somewhat for the low stiffness and relatively short vertebral processes of Terrestrisuchus and Protosuchus, lending them greater agility than larger animals with similar anatomy. Similarly, small mammals tend to have more flexible vertebral columns than larger ones [66]. Due to these constraints, sustained terrestrial locomotion probably was not possible for larger crocodylomorphs that lacked both rigid osteoderm articulations and procoelous IVJ articulations, such as thalattosuchians and dyrosaurids [21,67].
Apart from body size-related constraints on locomotor abilities, there is little evidence that the biomechanics of the vertebral column changes over ontogeny in crocodylians. Smaller adults, including the West African dwarf crocodile (Osteolaemus tetraspis), have been observed bounding and galloping ([3] and references therein), and measurements of limb bones [68] suggest no major departure from isometry across ontogeny. Molnar et al. [20] did not report allometry in the vertebrae of C. niloticus over the size range they studied (1.4–15.6 kg). Ikejiri [65], who examined a larger number of individuals over a greater range of sizes, did find statistically significant vertebral allometry in A. mississippiensis, but not in any of the dimensions correlated with stiffness in C. niloticus [20] which were used to estimate stiffness in this study. Given that the fossil individuals we studied appear to have been adults or sub-adults, the characters we measured probably would not have changed substantially if the animals had lived longer. Moreover, in smaller bodied taxa such as Terrestrisuchus and perhaps Protosuchus, similar to the sizes of many early Crocodylomorpha in general, this issue of large body size-related ontogenetic declines in locomotor abilities is less of a concern.
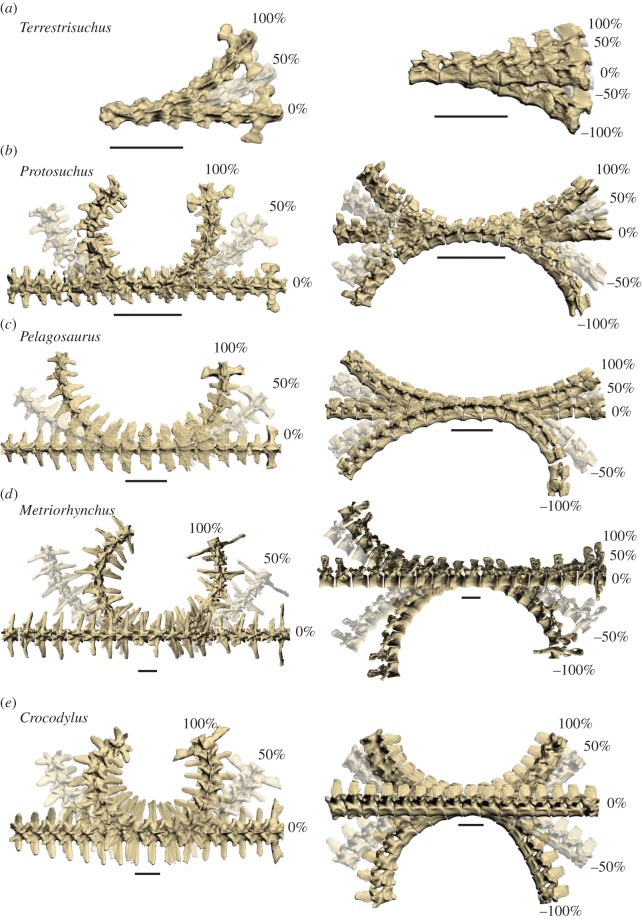
Finally, the total number of thoracolumbar vertebrae affects the behaviour of the trunk during locomotion. If RoM of individual joints remains constant, a greater number of dorsal vertebrae translates into greater RoM along the trunk [16]. Relative to Protosuchus, Pelagosaurus and Crocodylus, Metriorhynchus has two more thoracolumbar vertebrae and Steneosaurus has two fewer (table 1), presumably granting slightly greater total trunk RoM to the former and slightly less to the latter. Total trunk RoM for each taxon, extrapolated from the virtual joints we tested, is shown in figure 8.
Figure 8.
Osteological RoM estimates extrapolated to the entire thoracolumbar vertebral column. In the left column are dorsal views showing lateral flexion, and in the right column are lateral views showing dorsoventral flexion. Cranial is to the left. The RoM of each joint is equal to that of the nearest joint that we tested, disregarding the lumbosacral joint (RoM of the lumbosacral joint was applied only to that joint). Percentages are relative to maximum osteological RoM. Scale bar, 5 cm.
4.2.1. Bounding and galloping
The point at which asymmetrical gaits first evolved in the crocodylomorph lineage is not known [4]. Similarities in dorsoventral stiffness and RoM between Terrestrisuchus, Protosuchus and Crocodylus not shared by the thalattosuchians would support the idea that bounding and galloping in modern crocodylians is an ancestral trait inherited from their Triassic forebears and lost in more aquatic thalattosuchians. The lack of any such pattern in our results implies that asymmetrical gaits may be confined to Crocodylia, that they evolved more than once in this lineage, or that our methods were not able to identify vertebral features associated with these gaits. Incorporation of other axial structures (e.g. osteoderms; soft tissues) into the models might change these results.
Maximum dorsoventral RoM (9–11° per joint in Terrestrisuchus and 15–21° in Protosuchus; table 4) certainly does not preclude the use of mammal-like asymmetrical gaits in early crocodylomorphs. In a range of small mammals across different asymmetrical gaits, maximum dorsoventral flexion (mostly produced by the last seven pre-sacral vertebrae) was only 40–51° [69]. However, osteological RoM is probably much larger than flexibility of the intact trunk (i.e. ligaments, muscles and skin prevent animals from using their full osteological RoM), and dorsoventral RoM was far smaller in Terrestrisuchus than in Crocodylus (9–11° versus 12–28°), which use asymmetrical gaits infrequently [30,70] and at slower speeds than cursorial mammals [29]. Therefore, early crocodylomorphs probably employed less dorsoventral movement (and resultant increase in step length and speed) than many extant mammals that use these types of gaits. This result reinforces our point [20] that, despite their convergent evolution of asymmetrical gaits, mammals and crocodylomorphs display key differences in vertebral function—and thus locomotor dynamics.
4.2.2. Swimming
In many secondarily aquatic taxa, including other marine reptiles and cetaceans, adaptation to aquatic locomotion seems to have involved an initial decrease in stiffness of the vertebral column followed by a subsequent increase in stiffness. In cetaceans, for example, the initial decrease in stiffness is associated with adaptation for undulatory swimming with slow, high-amplitude waves of flexion followed by increase in stiffness (by different structural mechanisms) with adaptation for carangiform swimming using faster waves of smaller amplitude [71]. Our morphometric data support a similar sequence of changes in thalattosuchians (figure 7): in the semi-aquatic Pelagosaurus, relatively low mediolateral stiffness could indicate a similar locomotor pattern to basilosaurids (stem cetaceans), which swam by undulation of the lumbar and caudal regions [72], or simply a swimming style similar to extant crocodylians [24], which have a large mediolateral RoM but use axial swimming—in which the limbs are adducted, the trunk remains fairly straight, and thrust is produced by undulation of the tail—and, less often, paraxial swimming, in which the trunk also undulates [73]. Crocodylus would be analogous to amphibious archaeocetes (e.g. Ambulocetidae), which retained the stiffer lumbar vertebrae of their artiodactyl ancestors [71].
Morphometrics of the aquatic specialist Metriorhynchus are consistent with high mediolateral IVJ stiffness (relatively long vertebral processes implying large axial muscles; tall and fairly broad centra correlated with high IVJ stiffness in Crocodylus [20]), particularly in the cranial portion of the trunk, corresponding to higher frequency oscillations with amplitudes that increase towards the tail, similar to most modern whales [16]. The estimated mediolateral IVJ stiffness of Steneosaurus was intermediate between that of Pelagosaurus and Metriorhynchus, consistent with its reconstruction as an ambush predator rather than a pursuit predator like Metriorhynchus. Similar patterns of increase in vertebral stiffness with adaptation for rapid swimming have been inferred for secondarily aquatic marine reptiles, including ichthyosaurs [31], nothosaurs [34] and mosasaurs [32,33]. Thus, together, the available evidence on the evolution of vertebral form and function in aquatic tetrapods indicates widespread convergent evolution owing to common biomechanical principles and constraints.
4.3. Effects of other axial tissues
The contention of Salisbury & Frey [21] that axial mechanics depend more upon IVJs in modern crocodylians and more upon dorsal osteoderms in early crocodylomorphs provides a possible explanation for our unexpected results for Terrestrisuchus and Protosuchus. Our models showed relatively large mediolateral RoM in the thoracic vertebrae of Protosuchus (16–19°; figure 4 and table 4). However, if the paravertebral shield in early crocodylomorphs limited lateral and ventral flexion to 5–10° per joint, as Salisbury & Frey [21] predicted, they would have had the smallest mediolateral RoM of the studied taxa, consistent with primarily dorsoventral axial movements. Likewise, with a substantial increase in mediolateral IVJ stiffness incurred by the paravertebral shield in Protosuchus and Terrestrisuchus, the early crocodylomorphs would have greater mediolateral than dorsoventral stiffness, while the reverse would be true of the thalattosuchians and modern crocodylians, consistent with presumed locomotor behaviour in the extinct taxa. The ‘open’ paravertebral shield of Pelagosaurus is thought to have allowed greater lateral flexion than the ‘closed’ paravertebral shields of Terrestrisuchus and Protosuchus, but it may have restricted ventral flexion [21]. Combined with limited dorsal IVJ extension caused by the cranio-caudally elongate neural spines (table 4), this restriction would probably result in a smaller dorsoventral RoM in Pelagosaurus compared with the early crocodylomorphs, consistent with its more sprawling posture and aquatic habits. This hypothesis implies a functional trade-off between mobility and stiffness: with the use of more parasagittal kinematics in early crocodylomorphs, lateral movements of the vertebral column may have become less important than passive stiffness, and the axial column was stiffened via the paravertebral shield. In contrast, modern crocodylians, which use lateral undulation in terrestrial locomotion, have less rigid osteodermal armour and increase stiffness via large epaxial muscles and stiff IVJs (figure 7). The development of procoelous vertebrae in the recent ancestors of modern crocodylians also may have played a role in shifting the burden of gravitational support from the osteoderms to the IVJs [21], resulting in higher joint stiffness but greater RoM (figure 7). Our results showed that Crocodylus had substantially greater space between adjacent centra than the other taxa we studied (table 1), suggesting that the shift to procoelous IVJs may have involved an increase in the amount of soft tissue within the joint, increasing RoM. Future studies should investigate this speculation with a larger dataset.
By sequentially removing axial tissues in Crocodylus and measuring deflection and stiffness, we found that the most important structures for determining passive stiffness and trunk deflection varied between bending directions. In ventral flexion, IVJs were of primary importance; in dorsal extension, ribs and body wall musculature contributed to stiffness and osteoderms restricted trunk deflection. In contrast, in lateral flexion, ribs contributed to stiffness and each of the other structures restricted trunk deflection to some degree. Therefore, passive stiffness and RoM in ventral flexion in crocodylians presumably can be predicted based upon vertebral morphology alone. However, we did not test the effects of epaxial muscle activation, which probably resists ventral flexion in living crocodylians [21]. Our results emphasize that the effects of soft tissues and dermal ossifications should be considered when attempting to infer axial mechanics of the intact trunk, not just in terms of total RoM and stiffness, but also relative RoM and stiffness between bending directions.
4.4. Validity and accuracy of results
Estimates of stiffness and RoM in extinct animals should be approached with caution because mechanical properties of joints may be influenced by many factors that cannot be observed in fossils. However, our validation test of the RoM estimation method used in this study (figure 5) showed that differences in RoM between bending directions and major patterns of variation along the column could be predicted with confidence in Crocodylus. Of course, estimated RoM magnitudes are almost certainly larger than RoM in the living animal (or even isolated joints, such as those used for the validation test), and a greater sample size would be needed to predict the amount of overestimation. Our estimates of stiffness relied upon correlations with morphometric measurements found in previous studies, particularly Molnar et al. [20] because it is the only such study on non-mammalian tetrapods. However, it is possible that some of the relationships we had found are unique to extant crocodylians; similar studies on other related animals are required to test this possibility.
Some of our hypotheses concerning the evolution of vertebral function are sensitive to the phylogenetic placement of Thalattosuchia within Crocodylomorpha. Although several recent analyses support the position of Thalattosuchia within Mesoeucrocodylia (e.g. [74,75]), at least one places Thalattosuchia outside of Crocodyliformes [76], which if correct would mean that low mediolateral stiffness in Pelagosaurusmight represent retention of the ancestral condition rather than a specialization related to aquatic locomotion (figure 9).
Figure 9.
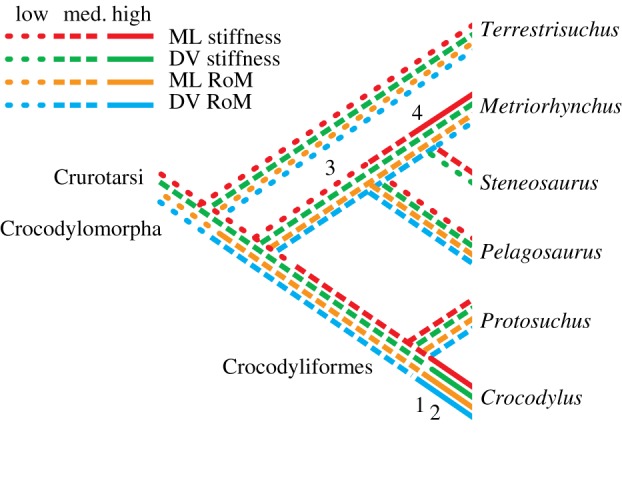
Hypothetical changes in IVJ stiffness and RoM with aquatic adaptation in Crocodylomorpha based on alternative phylogeny [76]; contrast with figure 7. Numbers show changes in other axial tissues: (1) sagittal segmentation of the paravertebral shield; (2) procoelous vertebrae; (3) ‘open’ margins of the paravertebral shield; (4) complete loss of osteoderms [21]. NB: procoelous vertebrae and/or loss of osteoderms evolved convergently in Junggarsuchus [7] and a few other crocodylomorph clades as well ([53] and references therein). ML, mediolateral; DV, dorsoventral.
5. Conclusion
Joint stiffness in mediolateral flexion tended to decrease with adaptation to aquatic locomotion in thalattosuchians, but the trend seems to have reversed somewhat in the aquatic specialist Metriorhynchus. IVJs of early crocodylomorphs were probably less stiff (based on morphometric correlates) but had smaller osteological RoM (based on virtual models) compared with those of modern crocodylians, in accord with the idea that osteoderms played a greater role in supporting the trunk in early crocodylomorphs [21]. Tissues other than vertebrae substantially influence the stiffness and RoM of the intact crocodylian trunk, and these effects vary across bending directions, so the effects of skin, osteoderms and other tissues should be taken into account when attempting to reconstruct the locomotion of extinct crocodylomorphs.
Acknowledgements
We thank Jenny Clack, Susan Evans and Paul Barrett for input on an earlier version of this manuscript, which formed part of a PhD thesis (by J.L.M.) at The Royal Veterinary College in 2013. We also thank Farah Ahmed and Dan Sykes for CT scanning assistance, and Lorna Steel (NHMUK), Mark Norell and Ruth O'Leary (AMNH) for curatorial assistance. The Crocodylus specimen was provided by La Ferme aux Crocodiles (Pierrelatte, France). We also thank our three anonymous reviewers for suggestions which greatly improved this manuscript.
Data accessibility
Non-normalized morphometric measurements have been included as part of the electronic supplementary material. Processed CT/micro-CT scan data in the form of STL files will be freely available from the 3D file repository M3 (http://morphomuseum.com): doi:10.18563/m3.1.3.e5. Raw scan data may be requested from the museums that house each specimen.
Author' contributions
J.M. planned the experiments, collected data (CT, morphometric and RoM), analysed data, interpreted the data and drafted the manuscript; S.P. conceived the study, interpreted the data and drafted the manuscript; B.B. provided access to specimens; A.T. provided access to specimens and collected CT data; J.H. conceived the study, collected CT data, interpreted the data and drafted the manuscript. All authors commented on the final manuscript and gave approval for publication.
Competing interests
The authors declare no competing interests.
Funding
This study was funded by the Natural Environment Research Council (NERC; grant nos. NE/G005877/1 and NE/K004751/1 to J.R.H. and S.E.P.).
References
- 1.Parrish JM. 1987. The origin of crocodilian locomotion. Paleobiology 13, 396–414. [Google Scholar]
- 2.Stubbs TL, Pierce SE, Rayfield EJ, Anderson PSL. 2013. Morphological and biomechanical disparity of crocodile-line archosaurs following the end-Triassic extinction. Proc. R. Soc. B 280, 20131940 (doi:10.1098/rspb.2013.1940) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen V, Molnar J, Parker W, Pollard A, Nolan G, Hutchinson JR. 2014. Comparative architectural properties of limb muscles in Crocodylidae and Alligatoridae and their relevance to divergent use of asymmetrical gaits in extant Crocodylia. J. Anat. 225, 569–582. (doi:10.1111/joa.12245) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gauthier JA, Nesbitt SJ, Schachner ER, Bever GS, Joyce WG. 2011. The bipedal stem crocodilian Poposaurus gracilis: inferring function in fossils and innovation in archosaur locomotion. Bull. Peabody Mus. Nat. Hist. 52, 107–126. (doi:10.3374/014.052.0102) [Google Scholar]
- 5.Alexander RM. 2006. Dinosaur biomechanics. Proc. R. Soc. B 273, 1849–1855. (doi:10.1098/rspb.2006.3532) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutchinson JR. 2011. On the inference of function from structure using biomechanical modelling and simulation of extinct organisms. Biol. Lett. 8, 115–118. (doi:10.1098/rsbl.2011.0399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark JM, Xu X, Forster CA, Wang Y. 2004. A Middle Jurassic ‘sphenosuchian’ from China and the origin of the crocodylian skull. Nature 430, 1021–1024. (doi:10.1038/nature02802) [DOI] [PubMed] [Google Scholar]
- 8.Crush PJ. 1984. A late Upper Triassic sphenosuchid crocodilian from Wales. Palaeontology 27, 131–157. [Google Scholar]
- 9.Walker AD. 1990. A revision of Sphenosuchus acutus Haughton, a crocodylomorph reptile from the Elliot Formation (Late Triassic or Early Jurassic) of South Africa. Phil. Trans. R. Soc. Lond. B 330, 1–120. (doi:10.1098/rstb.1990.0185) [Google Scholar]
- 10.Sereno PC. 1991. Basal Archosaurs: phylogenetic relationships and functional implications. J. Vertebr. Paleontol. 11, 1–53. (doi:10.1080/02724634.1991.10011426) [Google Scholar]
- 11.Kubo T, Benton MJ. 2009. Tetrapod postural shift estimated from Permian and Triassic trackways. Palaeontology 52, 1029–1037. (doi:10.1111/j.1475-4983.2009.00897.x) [Google Scholar]
- 12.Hebrank JH, Hebrank MR, Long JH, Block BA, Wright SA. 1990. Backbone mechanics of the blue marlin Makaira nigricans (Pisces, Istiophoridae). J. Exp. Biol. 148, 449–459. [Google Scholar]
- 13.Long JHJ. 1992. Stiffness and damping forces in the intervertebral joints of blue marlin (Makaira nigricans). J. Exp. Biol. 162, 131–155. [Google Scholar]
- 14.Long JHJ, Pabst D, Shepherd W, McLellan W. 1997. Locomotor design of dolphin vertebral columns: bending mechanics and morphology of Delphinus delphis. J. Exp. Biol. 200, 65–81. [DOI] [PubMed] [Google Scholar]
- 15.Slijper EJ. 1946. Comparative biologic-anatomical investigations on the vertebral column and spinal musculature of mammals. Amsterdam, The Netherlands: North Holland Publishing. [Google Scholar]
- 16.Buchholtz EA. 2001. Vertebral osteology and swimming style in living and fossil whales (Order: Cetacea). J. Zool. 253, 175–190. (doi:10.1017/S0952836901000164) [Google Scholar]
- 17.Boszczyk BM, Boszczyk AA, Putz R. 2001. Comparative and functional anatomy of the mammalian lumbar spine. Anat. Rec. 264, 157–168. (doi:10.1002/ar.1156) [DOI] [PubMed] [Google Scholar]
- 18.Shapiro LJ, Seiffert CVM, Godfrey LR, Jungers WL, Simons EL, Randria GFN. 2005. Morphometric analysis of lumbar vertebrae in extinct Malagasy strepsirrhines. Am. J. Phys. Anthropol. 128, 823–839. (doi:10.1002/ajpa.20122) [DOI] [PubMed] [Google Scholar]
- 19.Pierce SE, Clack JA, Hutchinson JR. 2011. Comparative axial morphology in pinnipeds and its correlation with aquatic locomotory behaviour. J. Anat. 219, 502–514. (doi:10.1111/j.1469-7580.2011.01406.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Molnar JL, Pierce SE, Hutchinson JR. 2014. An experimental and morphometric test of the relationship between vertebral morphology and joint stiffness in Nile crocodiles (Crocodylus niloticus). J. Exp. Biol. 217, 758–768. (doi:10.1242/jeb.089904) [DOI] [PubMed] [Google Scholar]
- 21.Salisbury SW, Frey EF. 2001. A biomechanical transformation model for the evolution of semi-spheroidal articulations between adjoining vertebral bodies in crocodilians. In Crocodilian biology and evolution (eds GC Grigg, F Seebacher, CE Franklin), pp. 85–134. Chipping Norton, Australia: Surry Beatty and Sons. [Google Scholar]
- 22.Nesbitt SJ. 2011. The early evolution of archosaurs: relationships and the origin of major clades. Bull. Am. Mus. Nat. Hist. 352, 1–292. (doi:10.1206/352.1) [Google Scholar]
- 23.Colbert EH, Mook CC. 1951. The ancestral crocodilian Protosuchus. Bull AMNH 97. See http://digitallibrary.amnh.org/dspace/handle/2246/413.
- 24.Pierce SE, Benton MJ. 2006. Pelagosaurus typus Bronn, 1841 (Mesoeucrocodylia: Thalattosuchia) from the Upper Lias (Toarcian, Lower Jurassic) of Somerset, England. J. Vertebr. Paleontol. 26, 621–635. (doi:10.1671/0272-4634(2006)26[621:PTBMTF]2.0.CO;2) [Google Scholar]
- 25.Hua S. 2003. Locomotion in marine mesosuchians (Crocodylia): the contribution of the ‘locomotion profiles’. N Jb Geol. Palaont. Abh. 227, 139–152. [Google Scholar]
- 26.Pierce SE, Angielczyk K, Rayfield E. 2009. Shape and mechanics in thalattosuchian (Crocodylomorpha) skulls: implications for feeding behaviour and niche partitioning. J. Anat. 215, 555–576. (doi:10.1111/j.1469-7580.2009.01137.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pierce SE, Angielczyk KD, Rayfield EJ. 2009. Morphospace occupation in thalattosuchian crocodylomorphs: skull shape variation, species delineation and temporal patterns. Palaeontology 52, 1057–1097. (doi:10.1111/j.1475-4983.2009.00904.x) [Google Scholar]
- 28.Buffetaut E. 1982. Radiation évolutive, paléoécologie et biogéographie des crocodiliens mésosuchiens. Paris, France: Société géologique de France. [Google Scholar]
- 29.Renous S, Gasc JP, Bels VL, Wicker R. 2002. Asymmetrical gaits of juvenile Crocodylus johnstoni, galloping Australian crocodiles. J. Zool. 256, 311–325. (doi:10.1017/S0952836902000353) [Google Scholar]
- 30.Webb GJW, Gans C. 1982. Galloping in Crocodylus johnstoni—a reflection of terrestrial activity? Rec. Aust. Mus. 34, 607–618. (doi:10.3853/j.0067-1975.34.1982.244) [Google Scholar]
- 31.Buchholtz EA. 2001. Swimming styles in Jurassic ichthyosaurs. J. Vertebr. Paleontol. 21, 61–73. (doi:10.1671/0272-4634(2001)021[0061:SSIJI]2.0.CO;2) [Google Scholar]
- 32.Lindgren J, Jagt JWM, Caldwell MW. 2007. A fishy mosasaur: the axial skeleton of Plotosaurus (Reptilia, Squamata) reassessed. Lethaia 40, 153–160. (doi:10.1111/j.1502-3931.2007.00009.x) [Google Scholar]
- 33.Lingham-Soliar T. 1992. A new mode of locomotion in mosasaurs: subaqueous flying in Plioplatecarpus marshi. J. Vertebr. Paleontol. 12, 405–421. (doi:10.1080/02724634.1992.10011471) [Google Scholar]
- 34.Storrs GW. 1993. Function and phylogeny in sauropterygian (Diapsida) evolution. Master's thesis, University of Texas, Austin, TX, USA.
- 35.Long JHJ, Nipper KS. 1996. The importance of body stiffness in undulatory propulsion. Am. Zool. 6, 678–694. (doi:10.1093/icb/36.6.678) [Google Scholar]
- 36.Ponce De León MS, Zollikofer CPE. 1999. New evidence from Le Moustier 1: computer-assisted reconstruction and morphometry of the skull. Anat. Rec. 254, 474–489. (doi:10.1002/(SICI)1097-0185(19990401)254:4<474::AID-AR3>3.0.CO;2-3) [DOI] [PubMed] [Google Scholar]
- 37.Shapiro LJ. 2007. Morphological and functional differentiation in the lumbar spine of lorisids and galagids. Am. J. Primatol. 69, 86–102. (doi:10.1002/ajp.20329) [DOI] [PubMed] [Google Scholar]
- 38.Gambaryan PP. 1974. How mammals run: anatomical adaptations. New York, NY: Wiley. [Google Scholar]
- 39.Jenkins F. 1974. Tree shrew locomotion and the origins of primate arborealism. In Primate locomotion (ed. FA Jenkins), pp. 85–115. New York, NY: Academic Press. [Google Scholar]
- 40.Taylor MP, Wedel MJ. 2013. The effect of intervertebral cartilage on neutral posture and range of motion in the necks of sauropod dinosaurs. PLoS ONE 8, e78214 (doi:10.1371/journal.pone.0078214) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stevens KA, Parrish JM. 2005. Digital reconstructions of sauropod dinosaurs and implications for feeding. In The sauropods: evolution and paleobiology, pp. 178–200. Berkeley, CA: University of California Press. [Google Scholar]
- 42.Christian A, Dzemski G. 2007. Reconstruction of the cervical skeleton posture of Brachiosaurus brancai Janensch, 1914 by an analysis of the intervertebral stress along the neck and a comparison with the results of different approaches. Foss. Rec. 10, 38–49. [Google Scholar]
- 43.Taylor MP, Wedel MJ, Naish D. 2009. Head and neck posture in sauropod dinosaurs inferred from extant animals. Acta Palaeontol. Pol. 54, 213–220. (doi:10.4202/app.2009.0007) [Google Scholar]
- 44.Stevens KA. 2002. DinoMorph: parametric modeling of skeletal structures. Palaeobiodivers. Palaeoenviron. 82, 23–34. [Google Scholar]
- 45.Christian A, Heinrich W-D. 1998. The neck posture of Brachiosaurus brancai. Foss. Rec. 1, 73–80. (doi:10.5194/fr-1-73-1998) [Google Scholar]
- 46.Samagh SP, Rosen CD, Otarodifard K, Kornswiet M, Palmer G, Lee TQ. 2011. New method for determining apparent axial center of rotation of lumbar and thoracic spine segments. J. Rehabil. Res. Dev. 48, 587–596. (doi:10.1682/JRRD.2010.09.0168) [DOI] [PubMed] [Google Scholar]
- 47.Stevens KA, Parrish JM. 1999. Neck posture and feeding habits of two Jurassic sauropod dinosaurs. Science 284, 798–800. (doi:10.1126/science.284.5415.798) [DOI] [PubMed] [Google Scholar]
- 48.Baier DB, Gatesy SM. 2013. Three-dimensional skeletal kinematics of the shoulder girdle and forelimb in walking Alligator. J. Anat. 223, 462–473. (doi:10.1111/joa.12102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Panjabi MM, Oxland TR, Yamamoto I, Crisco JJ. 1994. Mechanical behavior of the human lumbar and lumbosacral spine as shown by three-dimensional load-displacement curves. J. Bone Jt Surg. 76, 413–424. [DOI] [PubMed] [Google Scholar]
- 50.Xia Q, Wang S, Kozanek M, Passias P, Wood K, Li G. 2010. In-vivo motion characteristics of lumbar vertebrae in sagittal and transverse planes. J. Biomech. 43, 1905–1909. (doi:10.1016/j.jbiomech.2010.03.023) [DOI] [PubMed] [Google Scholar]
- 51.Fish FE. 1984. Kinematics of undulatory swimming in the American alligator. Copeia 4, 839–843. (doi:10.2307/1445326) [Google Scholar]
- 52.Pol D. 2003. New remains of Sphagesaurus huenei (Crocodylomorpha: Mesoeucrocodylia) from the late Cretaceous of Brazil. J. Vertebr. Paleontol. 23, 817–831. (doi:10.1671/A1015-7) [Google Scholar]
- 53.Clark JM. 2011. A new shartegosuchid crocodyliform from the Upper Jurassic Morrison Formation of western Colorado. Zool. J. Linn. Soc. 163, S152–S172. (doi:10.1111/j.1096-3642.2011.00719.x) [Google Scholar]
- 54.Frey E. 1988. The carrying system of crocodilians: a biomechanical and phylogenetic analysis. Stuttg. Beitr. Naturk. A 426, 1–60. [Google Scholar]
- 55.Massare JA. 1988. Swimming capabilities of Mesozoic marine reptiles: implications for method of predation. Paleobiology 14, 187–205. [Google Scholar]
- 56.Krebs B. 1967. DerJura-Krokodilier Machimosaurus H.v.Meyer. Paläontol. Z 41, 46–59. (doi:10.1007/BF02998548) [Google Scholar]
- 57.Krebs B. 1968. Le crocodilian Machimosaurus. GeolPortugal NovaSérie 14, 21–53. [Google Scholar]
- 58.Wilberg EW. 2015. A new metriorhynchoid (Crocodylomorpha, Thalattosuchia) from the Middle Jurassic of Oregon and the evolutionary timing of marine adaptations in thalattosuchian crocodylomorphs. J. Vertebr. Paleontol. 35, e902846 (doi:10.1080/02724634.2014.902846) [Google Scholar]
- 59.Hua S, Buffrenil VD. 1996. Bone histology as a clue in the interpretation of functional adaptations in the Thalattosuchia (Reptilia, Crocodylia). J. Vertebr. Paleontol. 16, 703–717. (doi:10.1080/02724634.1996.10011359) [Google Scholar]
- 60.Gatesy SM. 1991. Hind limb movements of the American alligator (Alligator mississippiensis) and postural grades. J. Zool. 224, 577–588. (doi:10.1111/j.1469-7998.1991.tb03786.x) [Google Scholar]
- 61.Reilly SM, Elias JA. 1998. Locomotion in Alligator mississippiensis: kinematic effects of speed and posture and their relevance to the sprawling-to-erect paradigm. J. Exp. Biol. 201, 2559–2574. [DOI] [PubMed] [Google Scholar]
- 62.Schilling N. 2011. Evolution of the axial system in craniates: morphology and function of the perivertebral musculature. Front. Zool. 8, 1–19. (doi:10.1186/1742-9994-8-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fischer MS, Witte H. 2007. Legs evolved only at the end! Phil. Trans. R. Soc. A 365, 185–198. (doi:10.1098/rsta.2006.1915) [DOI] [PubMed] [Google Scholar]
- 64.Biewener AA. 2005. Biomechanical consequences of scaling. J. Exp. Biol. 208, 1665–1676. (doi:10.1242/jeb.01520) [DOI] [PubMed] [Google Scholar]
- 65.Ikejiri T. 2015. Modes of ontogenetic allometric shifts in crocodylian vertebrae. Biol. J. Linn. Soc. 116, 649–670. (doi:10.1111/bij.12607) [Google Scholar]
- 66.Gál JM. 1993. Mammalian spinal biomechanics. II. Intervertebral lesion experiments and mechanisms of bending resistance. J. Exp. Biol. 174, 281–297. (doi:10.1016/0022-0981(93)90025-J) [DOI] [PubMed] [Google Scholar]
- 67.Schwarz-Wings D, Frey EF, Martin T. 2009. Reconstruction of the bracing system of the trunk and tail in hyposaurine dyrosaurids (Crocodylomorpha; Mesoeucrocodylia). J. Vertebr. Paleontol. 29, 453–472. (doi:10.1671/039.029.0228) [Google Scholar]
- 68.Livingston VJ, Bonnan MF, Elsey RM, Sandrik JL, Wilhite D. 2009. Differential limb scaling in the American alligator (Alligator mississippiensis) and its implications for archosaur locomotor evolution. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 292, 787–797. (doi:10.1002/ar.20912) [DOI] [PubMed] [Google Scholar]
- 69.Schilling N, Hackert R. 2006. Sagittal spine movements of small therian mammals during asymmetrical gaits. J. Exp. Biol. 209, 3925–3939. (doi:10.1242/jeb.02400) [DOI] [PubMed] [Google Scholar]
- 70.Zug GR. 1974. Crocodilian galloping: an unique gait for reptiles. Copeia. 1974, 550–552. (doi:10.2307/1442557) [Google Scholar]
- 71.Bebej RM. 2011. Functional morphology of the vertebral column in Remingtonocetus (Mammalia, Cetacea) and the evolution of aquatic locomotion in early archaeocetes. PhD dissertation, The University of Michigan, Ann Arbor, MI, USA.
- 72.Gingerich PD. 2003. Land-to-sea transition in early whales: evolution of Eocene Archaeoceti (Cetacea) in relation to skeletal proportions and locomotion of living semiaquatic mammals. Paleobiology 29, 429–454. (doi:10.1666/0094-8373(2003)029<0429:LTIEWE>2.0.CO;2) [Google Scholar]
- 73.Frey EF, Salisbury SW. 2000. The kinematics of aquatic locomotion in Osteolaemus tetraspis Cope. In Crocodilian biology and evolution (eds GC Grigg, F Seebacher, CE Franklin), pp. 165–179. Chipping Norton, Australia: Surrey Beatty and Sons. [Google Scholar]
- 74.Turner AH, Pritchard AC. 2015. The monophyly of Susisuchidae (Crocodyliformes) and its phylogenetic placement in Neosuchia. PeerJ 3, e759 (doi:10.7717/peerj.759) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pol D, Rauhut OWM, Lecuona A, Leardi JM, Xu X, Clark JM. 2013. A new fossil from the Jurassic of Patagonia reveals the early basicranial evolution and the origins of Crocodyliformes: a new Jurassic crocodylomorph. Biol. Rev. 88, 862–872. (doi:10.1111/brv.12030) [DOI] [PubMed] [Google Scholar]
- 76.Wilberg EW. 2015. What's in an outgroup? The impact of outgroup choice on the phylogenetic position of Thalattosuchia (Crocodylomorpha) and the origin of Crocodyliformes. Syst. Biol 64, 621–637. (doi:10.1093/sysbio/syv020) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Non-normalized morphometric measurements have been included as part of the electronic supplementary material. Processed CT/micro-CT scan data in the form of STL files will be freely available from the 3D file repository M3 (http://morphomuseum.com): doi:10.18563/m3.1.3.e5. Raw scan data may be requested from the museums that house each specimen.