Abstract:
Spontaneous coronary artery dissections are an infrequent cause of myocardial infarction and have been reported in late stages of pregnancy and in the puerperium phase. We report a case of a 26-year-old post-partum woman who was diagnosed with a spiral coronary dissection of the left anterior descending artery, with a compromised diagonal branch. She underwent an emergent surgical revascularization without the use of cardiopulmonary bypass. The patient’s in-hospital clinical course, prognosis, treatment, and potential etiologies are discussed.
Keywords: spontaneous coronary artery dissection, coronary revascularization, off-pump coronary artery bypass, pregnancy, puerperium
Spontaneous coronary artery dissection (SCAD) is a rare and fatal cause of ischemic heart disease, occurring in women during or after pregnancy (1–4). Unfortunately, its etiology and pathogenesis have not been fully elucidated (3). The most frequent outcome of patients who experience SCAD is death (3). This outcome is often misinterpreted as a cause of sudden death resulting in an underestimation of the incidence of SCAD (2). Treatment modalities vary based on the clinical presentation and range from medical therapy to surgical revascularization (1–6). Because of the lack of evidence for its etiology and a potential fear of other dissections, patients undergoing surgical intervention may greatly benefit from the use of off-pump coronary artery bypass grafting (OPCABG). This technique helps alleviate the potential variations in arterial pressure and minimizes the manipulation of the aorta, thereby decreasing the risk of aortic dissection (3).
CASE REPORT
A 26-year-old black woman (gravida 3, para 2) presented to the emergency room with chest pain radiating to both arms associated with dyspnea. Her medical history was unremarkable except for an emergency cesarean section for placental abruption, 2 weeks before admission. Physical examination was unremarkable with the exception of an initial blood pressure of 174/106 mmHg. Her social history included smoking one pack of cigarettes per day and a negative history of alcohol use. She was admitted after 4 hours of chest pain, slowly decreasing in intensity with nitroglycerin, with a non-ST segment elevated myocardial infarction. Laboratory results reported were a brain natriuretic peptide (BNP) of 82 pg/mL, creatine kinase myocardial b (CKMB) of 1.4 ng/mL, creatine kinase (CK) of 90 U/L, and a troponin I (TnI) of 0.15 ng/mL. After her transfer to the intensive care unit (ICU), the patient stated that she was not experiencing any more chest pain and wanted to go home. She was monitored and symptom free with no new episodes of chest pain over a 24-hour period. The following day, the patient remained free of pain. The electrocardiogram was normal, but the laboratory results were elevated, with TnI of 0.93 ng/mL, BNP of 376 pg/mL, CKMB 6.4 ng/mL, and CK of 136 U/L. A two-dimensional transthoracic echocardiogram raised the possibility of a dissection of the proximal ascending aorta. A transesophageal echocardiogram (TEE) was performed to rule out aortic dissection, which showed the ascending and descending aorta to be normal with no apparent dissection. The left ventricular contractility was normal on a regional and global basis. On the third day, she was pain free and once again requested to go home. That afternoon, the electrocardiogram showed a T-wave depression throughout. Subsequently, she developed an acute onset of chest pain that she stated as unbearable. She was rushed to the catheterization laboratory where a spiral coronary dissection was noted at the origin of the left anterior descending artery (LAD) to the mid-LAD (99%), with an 80% ostial diagonal artery obstruction (Figure 1). She was emergently taken to the operating room for coronary revascularization. Because of her anemia (hemoglobin, 10.6 mg/dL) and current findings of spontaneous dissection, it was decided that she undergo an OPCABG of the left internal mammary artery onto the distal LAD and a saphenous vein graft to the diagonal. During surgery, a dark bluish hematoma was noted from the origin of the LAD to the mid-LAD. The Boston Scientific (Natick, MA), formerly Guidant, ACROBAT Off-Pump System was used for the operation with excellent hemodynamics. The procedure was uneventful, with no blood transfusion, and blood loss of 450 mL, of which 141 mL of packed red cells was returned to patient with use of the C.A.T.S. cell saver unit (Terumo Cardiovascular Systems, Ann Arbor, MI). The patient was transferred back to ICU, with a hemoglobin level of 10.3 mg/dL and a cardiac index of 3.1 L/min/m2, in stable condition, and her remaining post-operative course was uneventful. The patient was discharged home on post-operative day 5.
Figure 1.
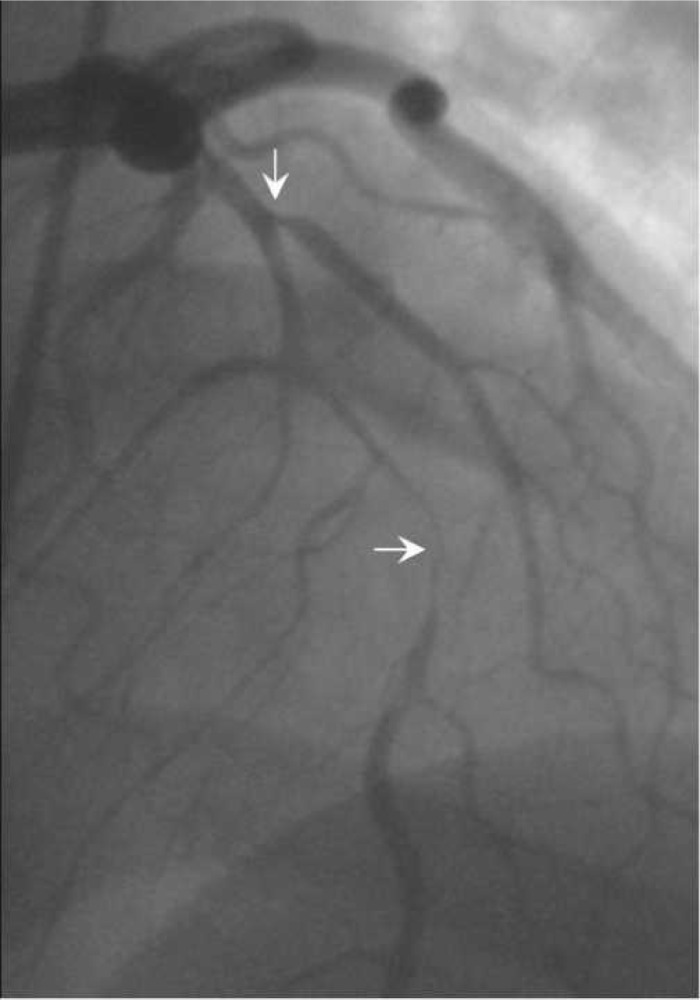
Left anterior oblique coronary angiogram showing the spiral dissection, compromising 99% of the left anterior descending artery and 80% ostial diagonal branch.
DISCUSSION
Acute myocardial infarction (AMI) in women in child-bearing age is rare (.7%) and even more uncommon is an infarction caused by a spontaneous coronary artery dissection (1). It has been reported that mortality for AMI in women during and after pregnancy is estimated as high as 37% (7). The immediate mortality in SCAD is reported to be as high as 50%, decreasing to 20% over the hours after dissection (3). In addition, up to 75% of SCAD cases are diagnosed post-mortem. It has also been reported that only .1% of SCADs are observed during angiography (2). SCAD is more common in the later stages of pregnancy and in the early puerperium phase, with 80% of dissections involving the left main and LAD (1).
The etiologies of SCAD in post-partum women is un-known; however, postulated theories have been reported in literature including abnormalities in collagen synthesis together with eosinophilic infiltration, hormonal changes during and after pregnancy, oral contraceptive use, structural changes of the vessel wall, lytic enzyme secretions, and presence of antiphospholipid antibodies (1,3,4). Treatment modality and management for SCAD patients remain controversial and are reported in the literature to be from minimal drug therapy to heart transplantation. There have been both successful and detrimental outcomes with the use of fibrinolytics in SCAD patients (8). Other practitioners have been successful in treating patients pharmacologically with aspirin and β-blockers (4). As in our case, surgical revascularization was also a proven modality for patients that continue to have persistent chest pain resulting in ischemia.
Even though the incidence of SCAD during and after pregnancy is uncommon; the diagnosis of SCAD should be considered by today’s practitioner for prompt treatment of such a high mortality condition. It has been reported that SCAD has been initially suspected with the use of TEE, which did not prove to be a valuable tool in our patient (4,9). A speedy diagnosis of a true SCAD is essential for survival, and coronary angiography should be strongly and quickly considered. Surgical intervention with or without the use of cardiopulmonary bypass has been shown to be beneficial for emergency revascularization (2–4). Surgical revascularization in our patient was conducted using off-pump technique because of her underlying pathophysiology and to minimize the risk of aortic dissection at the site of aortic cannulation.
REFERENCES
- 1.Krishnamurthy M, Desai R, Patel H.. Spontaneous coronary artery dissection in the postpartum period: association with antiphospholipid antibody. Heart. 2004;90:e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thistlethwaite PA, Tarazi RY, Giordano FJ, et al. Surgical management of spontaneous left main coronary artery dissection. Ann Thorac Surg. 1998;66:258–60. [DOI] [PubMed] [Google Scholar]
- 3.Carmi D, Touati G, Barry M, et al. Spontaneous coronary artery dissection: Value of beating heart myocardial revascularization. Interact Cardiovasc Thorac Surg. 2003;4:694–96. [DOI] [PubMed] [Google Scholar]
- 4.Terrovitis JV, Kanakakis J, Nanas JN.. Spontaneous coronary artery dissection as a cause of acute myocardial infarction in the postpartum period. Cardiol Rev. 2005;13:211–3. [DOI] [PubMed] [Google Scholar]
- 5.Chabrot P, Motreff P, Boyer L.. Postpartum spontaneous coronary artery dissection: a case of pseudoaneurysm evolution detected on MDCT. AJR Am J Roentgenol. 2006;187:W660. [DOI] [PubMed] [Google Scholar]
- 6.Lee FH, Yeung AC, Fowler MB, et al. Spontaneous postpartum coronary dissection. Circulation. 1999;99:721. [DOI] [PubMed] [Google Scholar]
- 7.Ladner HE, Danielsen B, Gilbert WM.. Acute myocardial infarction in pregnancy and the puerperium: a population-based study. Obstet Gynecol. 2005;105:480–4. [DOI] [PubMed] [Google Scholar]
- 8.Buys EM, Suttorp MJ, Morshuis WJ, et al. Extension of a sponta-neous coronary artery dissection due to thrombolytic therapy. Cathet Cardiovasc Diagn. 1994;33:157–60. [DOI] [PubMed] [Google Scholar]
- 9.Lerakis S, Manoukian S, Martin RP.. Transesophageal echo detection of postpartum coronary artery dissection. J Am Soc Echocardiogr. 2001;14:1132–3. [DOI] [PubMed] [Google Scholar]


