Abstract:
A hematocrit (Hct) of less than 25% during cardiopulmonary bypass (CPB) and transfusion of homologous packed red blood cells (PRBC) are each associated with an increased probability of adverse events in cardiac surgery. Although the CPB circuit is a major contributor to hemodilution intravenous (IV) fluid volume may also significantly influence the level of hemodilution. The objective of this study was to explore the influence of asanguinous IV fluid volume on CPB Hct and intraoperative PRBC transfusion. After Institutional Review Board approval, a retrospective chart review of 90 adult patients that had undergone an elective, isolated CABG with CPB was conducted. Regression analysis was used to determine if pre-CPB fluid volume was associated with the lowest CPB Hct and the incidence of an intraoperative PRBC transfusion. In separate multivariate analyses, higher pre-CPB fluid volume was associated with lower minimum CPB Hct (p < .0001), and higher minimum CPB Hct was associated with a decreased probability of PRBC transfusion (p < .0001). Compared to patients that received <1600 mL (n = 55) of pre-CPB fluid, those that received >1600 mL (n = 35) had a decreased mean low CPB Hct (22.4% vs 25.6%, p < .0001), an increased incidence of a CPB Hct <25% (74% vs. 38%, p = .0008) and PRBC transfusion (60% vs. 16%, p < .0001), and increased median PRBC units transfused (2.0 vs 1.0, p = .1446) despite no significant difference in gender, age, patient size, baseline Hct, or CPB prime volume. Patients that received a PRBC transfusion (n = 30) received a significantly higher volume of pre-CPB fluid than nontransfused patients (1800 vs. 1350 mL, p = .0039). These findings suggest that pre-CPB fluid volume can significantly contribute to hemodilutional anemia in cardiac surgery. Optimizing pre-CPB volume may preserve baseline Hct and help limit intraoperative hemodilution.
Keywords: cardiopulmonary bypass, hemodilution, hematocrit, blood transfusion, autologous priming, fluid volume
Hemodilution is a commonly used technique in cardiac surgical procedures using cardiopulmonary bypass (CPB) support. Until recently, the safe level of hemodilution and adverse effects related to its severity were not well defined. Excessive hemodilution in cardiac surgery is a major risk factor for blood transfusion and has been associated with increased morbidity and mortality in relation to the degree of hemodilution (1–7). Current evidence suggests the reduction of hemodilution and preservation of hematocrit (Hct) for best outcomes in patients undergoing routine cardiac surgery with CPB (1–14).
Although much of the focus of hemodilutional anemia in cardiac surgery has focused on perfusion practices because of the asanguinous fluid prime of the extracorporeal circuit (ECC), anesthesia intra-operative volume management practices may also contribute to the level of hemodilution. Studies have shown that a reduction in hemodilution from the ECC has a positive impact on the severity of hemodilution and requirements for packed red blood cells (PRBCs); however, the impact on hemodilution from anesthesia intravenous fluid administration has not been studied (8–14).
Hemodilution in cardiac surgery is associated with adverse outcomes that are dependent on the level of dilution. Several studies have shown that the lowest Hct during CPB is an independent risk factor for morbidity and mortality in cardiac surgery. An inverse relationship exists between Hct < 25% during CPB and mortality, stroke, return to CPB, the need for an intra-aortic balloon pump (IABP), and inotropes (1–3). In one study, patients with an Hct < 19% had a mortality rate twice as high as those with an Hct ≥ 25% (2). The risk of acute renal failure (ARF) increases as the CPB Hct falls below 24% (4,5,7). In another study, the risk of stroke, myocardial infarction, low cardiac output, renal failure, prolonged ventilator support, pulmonary edema, and post-operative bleeding necessitating re-exploration increased as CPB Hct decreased below 22% (6).
Cardiac surgery accounts for 18–20% of the total blood use in the United States (15,16). Hemodilutional anemia is a major cause of blood transfusion in cardiac surgery, although transfusing additional blood should not be considered an ideal solution because homologous blood has untoward effects in and of itself. There are risks associated with PRBC transfusions and include immunomodulation, transmission of infectious diseases, allergic reactions, hemolytic reactions, transfusion-related acute lung injury, and increased risk of infection (17,18). Blood transfusions have been linked to decreased short- (1, 6, and 12 months) and long- (5 and 10 years) term survival in patients undergoing cardiac surgery (19–21). The risk of low output failure, defined as the need for inotropes, IABP, and return to CPB, associated with an Hct < 25%, was further increased with PRBC transfusion (2). Blood transfusion during CPB also exacerbates ARF associated with severe hemodilution in coronary artery bypass grafting (CABG) patients (4). The incidence and magnitude of PRBC transfusion is also associated with an increased risk of post-operative complications and adverse events in renal, pulmonary, cardiac, and neurologic systems in CABG surgery (22).
These findings support results of a 1999 multi-center, randomized, controlled clinical trial of transfusion strategies in critical care patients that compared outcomes of a liberal vs. restrictive PRBC transfusion regimen (23). These investigators found that multi-organ dysfunction and in-hospital mortality were significantly higher in patients enrolled in the liberal vs. the restrictive transfusion group, meaning the use and magnitude of PRBC transfusion increases the risk of complications and mortality. Because outcomes become worse with anemia and get poorer with PRBC transfusion in cardiac surgery, attention needs to focus on reducing hemodilutional anemia to preserve Hct and decrease the incidence and magnitude of blood transfusions.
Recent improvements in CPB technique have attenuated the risk of significant hemodilution associated with the ECC by minimizing the amount of dilution from the ECC prime. Shortened CPB circuits with a lower prime requirement minimize hemodilution, resulting in improved CPB Hcts and decreased blood transfusion requirements (8,12). Retrograde autologous priming (RAP) of the ECC also yields higher CPB Hcts and lowers PRBC transfusion requirements (8–14). A prospective study by Cormack et al. (8) looked at the effects of varying amounts of CPB prime volumes and autologous priming on Hct, PRBC use, and outcomes. They reported a systematic decrease in incidence of Hct < 20% on CPB, magnitude of PRBC transfusions, and morbidity and mortality as CPB prime volume decreased.
Although RAP can reduce the severity of hemodilution resulting from the ECC, it may not be appropriate in all cardiac operations, and it may not work for every patient; its safety and efficacy may be limited by certain patient pathologies, clinical situations, patient size, and patient tolerance to the procedure. Patients with small blood volumes in relation to the ECC prime volume, as well as those with labile hemodynamics, are often less tolerant to RAP, ultimately limiting the prime volume removed. Although fluid may be removed and blood hemoconcentrated during a CPB procedure by the perfusionist, literature supports that the single lowest Hct the patient experiences at any point during CPB is the value that matters the most (1,3,9). This value is often times the lowest after the start of CPB, because the Hct during CPB often increases concomitantly with urine output and redistribution of fluid into interstitial spaces (24).
Intra-operative fluid administration by anesthesia personnel is needed to replace pre-operative volume deficit, maintain and support intravascular volume and hemodynamics after anesthesia induction, replace intra-operative volume loss, and preserve urine output. Volume administration may be variable and may be in excess of what is required by the patient or procedure (25). Excessive asanguinous volume infusion in the pre-CPB period may exacerbate the hemodilution experienced with the ECC and lead to a lower Hct on CPB than would have otherwise been observed. It may negate the benefits of RAP if this technique is used. Last, it may predispose a patient to receiving a PRBC transfusion in the CPB prime or shortly after initiation of CPB.
The objectives of this study were to determine the influence of intra-operative asanguinous fluid volume on CPB Hct and intra-operative homologous PRBC transfusions in patients that had undergone CABG surgery with CPB support. A retrospective chart review was conducted to determine whether intra-operative fluid volume administration pre-CPB affected the CPB Hct and the incidence and magnitude of intra-operative PRBC transfusion(s).
MATERIALS AND METHODS
Study Design and Patient Selection
After Institutional Review Board (IRB) approval, University of Toledo Health Science Center IRB 105284, patient data were collected by retrospective medical chart review. Adult patients that had undergone elective, first-time, isolated CABG surgery with internal mammary artery harvest and CPB support with the same surgeon at the University of Toledo Medical Center (UTMC) were identified from a prospectively collected database for inclusion in this study. A waiver of patient consent for use and disclosure of protected health information was requested and granted because the research was determined to be of minimal risk, and a signed consent and authorization form would be the only record linking collected data to a subject. Patient charts were reviewed sequentially by date in a retrospective manner beginning December 31, 2005. Pre-study statistical analysis by NCSS Power Analysis and Sample Size (PCSS) software determined that a minimum sample size of 90 patients would provide adequate power for this study based on a range of possible correlation coefficients, over a range of 85–90% power, and holding the type I error at 0.05.
With the objective of isolating the variability and influence of fluid volume from anesthesia personnel to examine its influence on CPB Hct and PRBC transfusions, inclusion and exclusion criteria were chosen in an attempt to control for fluid changes from perfusion technique. Select patients would not have a fluid restriction or require acute volume resuscitation, and they would have PRBC transfusions resulting primarily from hemodilution and not bleeding. Patients that were Jehovah’s Witnesses, on platelet glycoprotein IIb/IIIa inhibitors, had an ejection fraction of <40%, had pre-operative renal insufficiency (creatinine >2.0 mg/dL), or had renal failure were not included. Patients that had retrograde autologous priming of the CPB circuit, ultrafiltration, fluid additions on CPB (including medications) exceeding 500 mL, CPB time exceeding 120 minutes, intra-operative renal insufficiency requiring diuretic support (intra-operative urine output <1 mL/kg/h), intra-aortic balloon or ventricular support, documented central venous pressures >25 mmHg, or documented intra-operative acute blood loss requiring acute fluid resuscitation of >1000 mL of fluids were excluded. Any patient charts meeting the exclusion criteria were rejected and replaced with the next retrospective, sequential patient. This process continued until 90 patients for the study were obtained. A total of 148 medical charts were reviewed. Fifty-eight medical charts were excluded as a result of meeting exclusion criteria or lack of specific data documentation. Medical charts reviewed included procedures performed between July 2001 and December 2005.
Patient characteristic data collected consisted of patient sex, age, weight, body surface area (BSA), and estimated blood volume (EBV), which was calculated based on patient height and weight. Procedural data collected consisted of the type and volume of fluid administered by anesthesia pre-CPB, perfusion fluid administration volume, incidence and magnitude of homologous PRBC transfusions with time event and most recent Hct value, and Hct measurements at six time points as defined in Table 1. The Hct values were measured at procedure time events as part of our routine arterial blood gas analysis procedure in cardiac surgery using the IRMA Trupoint blood analysis system (International Technidyne Corp., Edison, NJ), with all CPB samples measured with the “on bypass” mode of the analyzer activated. Pre-CPB anesthesia volume was the non-heme volume administered by anesthesia personnel before start of CPB. This volume was requested and recorded by the perfusionist either at the time of post-heparinization or start of CPB. All patient data obtained from these chart reviews were collected, stored, handled, and secured in compliance with the Federal Health Insurance Portability and Accountability Act (HIPAA) and UTMC federal assurance and institutional policies.
Table 1.
Definitions of procedural hematocrit measurements by event.
| Variable | Time Point | Description |
|---|---|---|
| Baseline OR Hct | Initially in OR | Initial hematocrit measured in the operating room, generally drawn shortly before or shortly after anesthesia induction |
| Pre-CPB Hct | Before CPB initiation | Hematocrit measured post-heparinization for CPB, generally drawn with the post heparinization/pre-CPB ACT sample |
| On-CPB Hct | Initially On-CPB | Initial hematocrit measured after commencement of CPB, generally drawn within 5 minutes after delivery of cardioplegic arrest |
| End CPB Hct | End of CPB | Final hematocrit measured on-CPB, generally drawn after removal of aortic cross clamp using a single cross-clamp surgical technique |
| Low CPB Hct | During CPB | Lowest hematocrit measured during the CPB procedure |
| Post-CPB Hct | After CPB termination | Initial hematocrit measured post-CPB/post-protamine, generally drawn within 10 minutes after administration of protamine |
| ICU Hct | On ICU admission | Hematocrit measured on admission to the ICU, generally drawn within 5 minutes after arriving in the ICU |
ACT, activated clotting time; CPB, cardiopulmonary bypass; Hct, hematocrit; OR, operating room; ICU, intensive care unit.
Anesthesia Technique
A femoral arterial line and Swanz-Ganz catheter with continuous cardiac output monitoring were placed in all patients shortly after arriving in the operating room. Anesthesia was induced with high-dose narcotic induction and maintained with inhalational anesthetics, supplemented with narcotic as dictated by patient heart rate and blood pressure. All patients were monitored with intraoperative transesophageal echocardiography. All cases were performed with a resident anesthesiologist with an attending anesthesiologist readily available throughout the case and present for critical portions of each case to include placement of monitors and catheters, induction of anesthesia, initiation and termination of CPB, and management in the immediate post-CPB period.
Perfusion Technique
A standard perfusion circuit was used for all patients, which included an open system with centrifugal pump (Biomedicus, Minneapolis, MN), Trillium-coated hollow fiber membrane oxygenator (Medtronic, Minneapolis, MN), arterial line filter, and uncoated ECC tubing. Whole blood micro-cardioplegia was administered by a Quest MPS cardioplegia system (Quest Medical, Allen, TX). Active aortic root venting was used, as was cardiotomy suction on all patients. CPB circuit prime included 1000–1350 mL Plasmalyte-A solution, 0.5 g/kg 20% osmitrol, 100 mL 25% albumin, 10,000 Units sodium heparin, and 50 mEq 8.4% sodium bicarbonate. The addition of homologous PRBCs (∼300 mL/unit) to the prime with removal of the same aliquot of clear priming fluid was indicated in patients that had an estimated on-CPB Hct < 20%, calculated based on the patient’s EBV, pre-CPB Hct, pre-CPB volume given, and CPB prime volume. The mean pre-CPB Hct at which this occurred was 27.3% ± 0.52%. The standard mean total volume of clear priming fluid in the circuit transfused to the patient on start of CPB was 1463 ± 143 mL (range, 1000–1750 mL). The generally acceptable Hct during CPB was >20%. The mean Hct at which initial homologous PRBC transfusion occurred during CPB was 19.6% ± 1.2%. Body temperature was maintained at normothermia. Standard autotransfusion technique was used on all cases using the Cobe BRAT 2 autotransfusion system (Cobe Cardiovascular, Arvada, CO) with a 250-mL bowl processing kit and manufacturer set cardiac protocol settings of 400 mL/min fill rate and wash volume of 1000 mL at a rate of 800 mL/min. No autologous cell saver blood was given during CPB in any of the cases because of the lack of salvaged blood. Residual CPB circuit blood was processed with the autotransfusion device, and washed autologous cells (250 mL/unit) were given to anesthesia and transfused to the patient post-CPB before leaving the operating room.
Surgical Technique
All cases were performed by the same surgeon. All patients received an internal mammary graft. A single cross-clamp technique was used on all cases. Cardioplegic arrest and maintenance was accomplished with cold whole blood microplegia with a standard arrest dose of 1000 mL. The generally accepted minimum Hct before the decision for a homologous blood transfusion during CPB and post-CPB was 20% and 22%, respectively, in the absence of available autologous cell saver blood; however, thresholds may have varied depending on physician, patient, and clinical situation.
Statistical Analysis
Patient and procedure characteristics for all subjects are described by mean and SD or n (%). In all comparison analyses, descriptive statistics (mean and SD) or (median and 25th, 75th percent interquartile range) were performed, where appropriate based on normality of the data, for all continuous variables.
Regression analysis was performed on variables deemed as feasible for being associated with the endpoints low CPB Hct and receipt of a PRBC transfusion. The primary independent variable of interest was pre-CPB volume. Several univariate simple regression and logistic regression models were fit to examine possible predictors of the response variables low CPB Hct and receipt of a PRBC transfusion, respectively. Multivariate simple regression analyses were performed with all variables significant in the univariate analyses. Backward and stepwise model reduction techniques were used to determine which variables provided a good fit and were significant at the p = .05 level with the response variables.
Data were analyzed to divide patients into groups that received a different amount of pre-CPB volume for com-parison. A breakpoint of <1600 and ≥1600 mL pre-CPB volume was determined for comparison analysis.
In all comparison analyses, following tests for normality, independent t test and Wilcoxon signed-rank test were used, where appropriate, to compare the means and medians, respectively, for all continuous variables between comparison groups. The χ2 test or Fisher exact test was used to compare categorical data between groups. In the analysis of the magnitude of homologous PRBC transfusions, all possible pair-wise comparisons between PRBC transfusion categories (0, 1, 2, 3+) were made, and the p values were adjusted using the Bonferroni adjustment for multiple comparisons.
All statistical analyses were performed using PC SAS (version 9.1.3; SAS, Cary, NC); p < .05 was considered statistically significant in all analyses.
RESULTS
Patient and procedure characteristics of all patients are shown in Table 2. Pre-CPB volume administered to the 90 patients ranged from 500 to 3100 mL and 6.9 to 42.3 mL/kg indexed to patient weight.
Table 2.
Patient and procedure characteristics for all subjects (n = 90).
| Variable | n (%) or Mean (SD) |
|---|---|
| Sex | |
| Male | 58 (64%) |
| Female | 32 (36%) |
| Age (yr) | 62.5 (11.8) |
| Weight (kg) | 87.7 (15.9) |
| BSA (m2) | 1.99 (0.21) |
| EBV (mL) | 5588 (1208) |
| No. of distal anastomoses (%) | |
| (1), (2), (3), (4), (5) | 1%, 7%, 30%, 55%, 7% |
| Pre-CPB time (min) | 123 (26) |
| CPB time (min) | 90 (17) |
| Pre-CPB prime volume (mL) | 1562 (557) |
| (mL/kg) | 18.4 (7.2) |
| CPB prime volume (mL) | 1463 (143) |
| Hematocrit (%) | |
| Baseline OR | 39.0 (4.0) |
| Pre-CPB | 36.1 (4.3) |
| On CPB | 24.7 (3.8) |
| Low CPB | 24.4 (3.8) |
| End CPB | 26.0 (3.5) |
| Post CPB | 25.3 (3.6) |
| ICU | 29.5 (4.4) |
BSA, body surface area; CPB, cardiopulmonary bypass; EBV, estimated blood volume; ICU, intensive care unit.
CPB Hematocrit
The lowest Hct observed during CPB was the initial on CPB Hct in 67 (74%) of the 90 patients. The mean difference between the initial on and low CPB Hct values in the other 23 (26%) patients was 1.23% ± 0.90%.
Variables statistically significant for being associated with low CPB Hct by univariate and multivariate analysis are listed in order of statistical significance in Tables 3 and 4, respectively. Slope estimates for the variables showed that as age and pre-CPB volume increased, the low CPB Hct decreased, and as weight, baseline OR Hct, and CPB prime volume increased, the low CPB Hct increased. Male sex was associated with an increased low CPB Hct. The direct relationship shown between CPB prime volume and low CPB Hct more than likely resulted from the patients that received a PRBC in the ECC prime with displacement of the same aliquot of crystalloid prime. Patients that received a PRBC in the ECC prime had lower mean crystalloid prime volume of 1162 vs. 1477 mL for those that did not. The lower crystalloid prime volume along with the addition of the PRBCs lessened the hemodilution incurred from CPB.
Table 3.
Univariate simple regression results for factors associated with low CPB Hct.
| Variable | Slope Estimate | SE | p |
|---|---|---|---|
| Sex (male) | 3.76789 | .73634 | <.0001 |
| Hct baseline OR (%) | 0.72272 | .06387 | <.0001 |
| Weight (kg) | 0.10401 | .02278 | <.0001 |
| CPB prime (mL/100)* | 0.96210 | .26272 | .0004 |
| Age (yr) | −0.08900 | .03291 | .0082 |
| Pre-CPB volume (mL/100)* | −0.14482 | .07081 | .0438 |
This variable was divided by 100 before fitting the univariate model; therefore, a unit increase for this variable is an increase of 100 mL.
Table 4.
Multivariate simple regression results for factors associated with low CPB Hct.
| Variable | Slope Estimate | SE | p |
|---|---|---|---|
| Hct baseline OR(%) | 0.65286 | .05447 | <.0001 |
| Pre-CPB volume (mL/100)* | −0.19768 | .03555 | <.0001 |
| Weight (kg) | 0.06050 | .01283 | <.0001 |
| Sex (male) | 1.02734 | .45811 | .0275 |
This variable was divided by 100 before fitting the univariate model; therefore, a unit increase for this variable is an increase of 100 mL.
A comparison of patients that received <1600 mL to those that received ≥1600 mL of pre-CPB volume is shown in Table 5. The breakpoint of 1600 mL was close to the mean for the entire data set, which was 1562 mL. The two groups were well matched because there was no statistically significant difference in sex, age, weight, BSA, baseline OR Hct, and CPB prime volume between the groups. The incidence of patients experiencing mild (Hct > 25%), moderate (21% ≤ Hct < 25%), and severe (Hct < 21%) hemodilution in the <1600 mL group vs. ≥ the 1600 mL group was 60% vs. 26% (p = .0015), 31% vs. 29% (p = .8135), and 9% vs. 46% (p < .0001), respectively. The mean Hct values at each event between these two groups of patients are displayed in Figure 1. The initial drop in Hct in the ≥1600 mL group (Figure 1), which occurred from baseline to pre-CPB period, remained relatively the same throughout the procedure until a transfusion occurred on or after CPB.
Table 5.
Comparison of patients that received <1600 mL pre-CPB fluid to those that received 21600 mL.
| Variable | Received <1600 mL Mean (SD) (or Median and Interquartile Range) (n = 55) | Received ≥1600 mL Mean (SD) (or Median and Interquartile Range) (n = 35) |
t Test p Value |
Wilcoxon p Value |
χ2 p Value |
|---|---|---|---|---|---|
| Pre-CPB volume | |||||
| mL* | 1200 (1100, 1400) | 2000 (1800, 2400) | <.0001 | ||
| mL/kg* | 14.3 (11.5, 17.1) | 24.7 (19.9, 29.6) | <.0001 | ||
| Sex | .2483 | ||||
| Male | 38 (69%) | 20 (57%) | |||
| Female | 17 (31%) | 15 (43%) | |||
| Age (yr)* | 58 (52, 73) | 62 (54, 74) | .3536 | ||
| Weight (kg) | 88.6 (17.2) | 86.2 (13.9) | .4949 | ||
| BSA (m2) | 2.00 (0.21) | 1.97 (.20) | .4754 | ||
| CPB prime (mL)* | 1550 (1350, 1600) | 1400 (1350, 1550) | .1132 | ||
| Hematocrit (%) | |||||
| Baseline O* | 39.6 (36.6, 42.3) | 39.9 (34.5, 42) | .3349 | ||
| On CPB | 25.9 (3.4) | 22.8 (3.8) | <.0001 | ||
| Low CPB | 25.6 (3.3) | 22.4 (3.7) | <.0001 | ||
| ICU | 29.1 (4.0) | 30.2 (4.9) | .2529 | ||
| CPB Hct < 25% | .0008 | ||||
| Yes | 21 (38%) | 26 (74%) | |||
| No | 34 (62%) | 9 (26%) | |||
| PRBC transfusion | <.0001 | ||||
| Yes | 9 (16%) | 21 (60%) | |||
| No | 46 (84%) | 14 (40%) | |||
| Transfused units† | 1 (1, 2) | 2 (1, 2) | .1446 | ||
| Autotransfusion units* | 3 (3, 4) | 3 (3, 4) | .1384 |
Median (25th percentile, 75th percentile) are presented because of skewness of the data.
Only those who received units of blood included in comparison.
Figure 1.
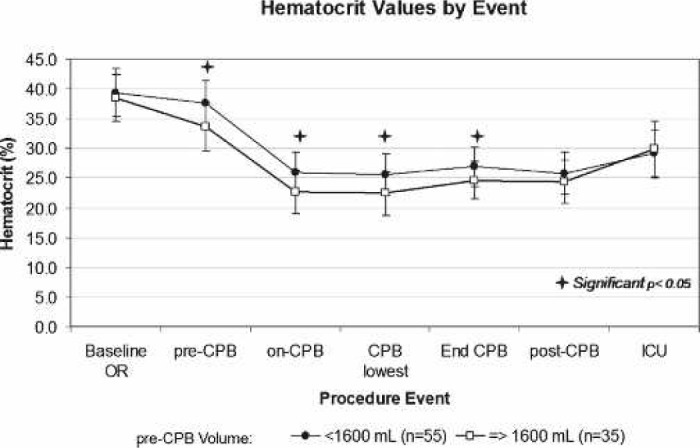
Mean procedural hematocrit values in patients that received <1600 mL pre-CPB fluid to those that received ≥1600 mL. Despite having similar mean baseline Hct values, patients that received 21600 mL of fluid pre-CPB consistently had lower intra-operative Hct values compared with patients that received <1600 mL (bars represent ±SD, +p < .05).
Homologous PRBC Transfusions
An intra-operative homologous PRBC transfusion occurred in 30 (33%) of the 90 patients. The magnitude of homologous blood transfusions ranged from 1 to 4 units, with a mean of 1.8 ± 1 units. The breakdown of initial PRBC transfusion by event was 13% (n = 4) in the CPB prime, 50% (n = 15) during CPB, and 37% (n = 11) in the immediate post-CPB period. The mean Hct values at which initial PRBC transfusion occurred in the CPB prime, during CPB, and post CPB were 27.3%, 19.6%, and 21.8%, respectively. The low CPB Hct was the lowest Hct observed during the surgical procedure in 80% of the patients who received a PRBC transfusion.
A comparison of patients that received a homologous blood transfusion to those that did not is shown in Table 6. Although transfused patients had a lower mean baseline Hct than non-transfused patients, they received higher pre-CPB volume and had a greater mean drop in Hct despite receiving a lower CPB prime volume. This trend was also present in the analysis of the magnitude of transfusions (Table 7); however, the value difference in variables between the different transfusion categories (1 vs. 2 vs. 3+ units) did not reach statistical significance. Transfused patients received a similar number of autotransfusion units and were transfused to a similar ICU Hct as non-transfused patients.
Table 6.
Comparison of patients that received a PRBC transfusion to those did not.
| Variable | Received PRBC Transfusion Mean (SD) (or Median and Interquartile Range) (n = 30) | Did Not Receive PRBC Transfusion Mean (SD) (or Median and Interquartile Range) (n = 60) |
t Test p Value |
Wilcoxon p Value |
Fisher Exact Test p Value |
|---|---|---|---|---|---|
| Sex | <.0001 | ||||
| Male | 10 (33%) | 48 (80%) | |||
| Female | 20 (66%) | 12 (20%) | |||
| Age (yr)* | 67 (57, 76) | 58.0 (52.0, 70.5) | .0075 | ||
| Weight (kg)* | 78.6 (13.48) | 92.3 (15.17) | <.0001 | ||
| BSA (m2) | 1.86 (0.17) | 2.06 (0.19) | <.0001 | ||
| CPB Prime (mL)* | 1350 (1300, 1550) | 1550 (1375, 1600) | .0003 | ||
| Pre-CPB volume | |||||
| mL* | 1800 (1400, 2000) | 1350 (1100, 1500) | .0039 | ||
| mL/kg | 22.90 (7.81) | 16.15 (5.68) | <.0001 | ||
| Hematocrit (%) | |||||
| Baseline | 36.3 (3.8) | 40.4 (3.4) | <.0001 | ||
| On-CPB | 21.2 (2.4) | 26.5 (3.2) | <.0001 | ||
| Low CPB | 20.8 (2.3) | 26.2 (3.0) | <.0001 | ||
| ICU | 29.5 (5.1) | 29.5 (4.0) | .9946 | ||
| Autotransfusion units* | 3 (3, 3) | 3 (3, 4) | .0678 |
Median (25th percentile, 75th percentile) are presented because of skewness of the data.
Table 7.
Comparison of patients that received 0, 1, 2, and 3+ units of PRBCs.
| Did Not Receive PRBC Transfusion |
Received PRBC Transfusion |
|||
|---|---|---|---|---|
| Variable | 0 Unit Mean (SD) (or Median and Interquartile Range) (n = 60) | 1 Unit Mean (SD) (or Median and Interquartile Range) (n = 15) | 2 Units Mean (SD) (or Median and Interquartile Range) (n = 10) | 3+ Units Mean (SD) (or Median and Interquartile Range) (n = 5) |
| Sex | ||||
| Male | 48 (80%) | 5 (33%) | 4 (40%) | 1 (20%) |
| Female | 12 (20%) | 10 (67%) | 6 (60%) | 4 (80%) |
| Age (yr)* | 58 (52, 70.5) | 60 (53, 78) | 65.5 (62, 74) | 76 (73, 80) |
| Weight (kg) | 92.3 (15.2) | 81.3 (15.0) | 77.2 (13.4) | 73.2 (7.8) |
| BSA (m2) | 2.06 (0.19) | 1.88 (0.17) | 1.85 (0.20) | 1.80 (0.12) |
| CPB Prime (mL)* | 1550 (1375, 1600) | 1400 (1350, 1550) | 1325 (1200, 1350) | 1350 (1350, 1400) |
| Pre-CPB volume | ||||
| mL* | 1350 (1100, 1500) | 1700 (1200, 1900) | 1800 (1500, 2100) | 2000 (2000, 2300) |
| mL/kg | 16.1 (5.7) | 19.7 (6.3) | 24.9 (8.9) | 28.5 (6.0) |
| Hematocrit (%) | ||||
| Baseline | 40.4 (3.4) | 37.1 (3.1) | 34.7 (4.5) | 37.3 (4.0) |
| On-CPB | 26.5 (3.2) | 22.0 (2.8) | 20.7 (1.5) | 19.7 (1.7) |
| Low CPB | 26.2 (3.0) | 21.8 (2.7) | 20.1 (1.5) | 19.2 (1.2) |
| ICU | 29.5 (4.0) | 28.3 (3.9) | 31.2 (5.6) | 30.1 (7.3) |
| Autotransfusion units* | 3 (3, 4) | 3 (3, 3) | 3 (3, 4) | 3 (3, 3) |
Median (25th percentile, 75th percentile) are presented because of skewness of the data.
Results of univariate and multivariate regression analysis to examine possible predictors of a PRBC transfusion are summarized in Tables 8 and 9 in order of significance. The probability of a PRBC transfusion increased with female sex, increasing age and pre-CPB volume, decreasing weight and baseline OR Hct, and low CPB Hct and CPB prime. The low CPB Hct remained as the only variable significantly associated with receipt of a PRBC transfusion by multivariate analysis.
Table 8.
Univariate logistic regression results for factors associated with receipt of a PRBC transfusion.*
| Variable | OR [95% CI] | p |
|---|---|---|
| Sex (female) | 8.000 [2.978; 21.489] | <.0001 |
| Hct baseline OR (%) | 0.743 [0.645; 0.856] | <.0001 |
| Hct CPB low (%) | 0.512 [0.391; 0.670] | <.0001 |
| Weight (kg) | 0.936 [0.903; 0.971] | .0003 |
| CPB prime (mL/100)† | 0.505 [0.346; 0.739] | .0004 |
| Age (yr) | 1.058 [1.016; 1.102] | .0064 |
| Pre-CPB volume (mL/100)† | 1.095 [1.010; 1.188] | .0281 |
Probability modeled was PRBC use = yes (subject received PRBC).
This variable was divided by 100 before fitting the univariate model; therefore, a unit increase for this variable is an increase of 100 mL. OR, odds ratio; CI, confidence interval.
Table 9.
Multivariate logistic regression results for factors associated with receipt of a PRBC transfusion.*
| Variable | OR [95% CI] | p |
|---|---|---|
| Hct CPB low | 0.512 [0.391; 0.670] | <.0001 |
Probability modeled was PRBC use = yes (subject received a PRBC). OR, odds ratio; CI, confidence interval.
The incidence of an intra-operative homologous PRBC transfusion in patients that received ≥1600 mL of pre-CPB volume was significantly higher than that seen in patients that received <1600 mL (Table 5). Of the patients transfused, the mean number of PRBC units transfused was higher in the ≥1600 mL group, but the difference was not significant. The trend of higher pre-CPB volume and lower CPB Hct levels was also present in the analysis of the incidence (Table 6) and magnitude (Table 7) of PRBC transfusions.
DISCUSSION
The findings of this study showed that the degree of the low CPB Hct observed was inversely related to the volume of pre-CPB fluid administered. Additionally, female sex, lower weight, and lower baseline Hct were found to be significantly associated with a decreased low CPB Hct. The association of sex, weight, and baseline Hct on the low CPB Hct is in agreement with previous studies (1,6,26).
In the comparison of patients that received <1600 mL of pre-CPB volume to those that received ≥1600 mL, both groups of patients ended up being well matched, because sex, age, patient size, baseline Hct values, and CPB prime volume were similar and not statistically different between the groups. The significant difference in the CPB Hct values and incidence of a CPB Hct < 25% showed that, even when the other variables significant in the multivariate analysis for a lower CPB Hct were relatively equal, lower CPB Hct values resulted from increased hemodilution secondary to higher pre-CPB fluid volume. This infers the hemodilution incurred pre-CPB directly contributed to the severity of hemodilution observed during the procedure. This is plausible and correlates with previous studies on autologous CPB priming, which have shown increased CPB Hcts result from decreased CPB prime volumes (8). Although fluid administration from anesthesia is not as “acute” as that infused on start of CPB from the ECC prime, fluids given pre-CPB still significantly influence and contribute to the overall level of hemodilution.
A significant association between the low CPB Hct and the incidence of an intra-operative PRBC transfusion was also identified in this study, where the higher the low CPB Hct the decreased probability of a PRBC transfusion. Because 50% of the transfusions occurred during CPB and 37% occurred shortly after, the low CPB Hct was the trigger or set the trend toward a PRBC transfusion, so this association is logical. The low CPB Hct is a value that presents later in the procedure, closer to when the majority of the transfusions occurred. Its value is a product of the baseline Hct and the level of hemodilution, which itself is dependent on patient sex and size and fluid volume administered. Patient size, patient sex, and baseline Hct were also factors identified as independent predictors of a PRBC transfusion by univariate analysis. The low CPB Hct is therefore the most sensitive and best predictor for a PRBC transfusion in patients receiving CPB support.
Patients that received a PRBC transfusion did so as a result of a procedural Hct value being at or near a transfusion trigger as a result of hemodilution. These patients were generally older, smaller, and predominantly women. The fact that transfused patients were predominantly women may account for some of the smaller size and lower baseline Hcts seen in this subset of patients, although transfused patients received significantly more pre-CPB volume than non-transfused patients. This pre-CPB hemodilution contributed to this group’s lower CPB Hct levels, leading to a PRBC transfusion (Tables 6 and 7). The trend of increased pre-CPB volume and PRBC transfusion was also present in the comparison of patients that received <1600 mL of pre-CPB volume to those that received ≥1600 mL.
Patients that received homologous PRBCs received a similar number of autotransfusion units and were transfused to a similar ICU Hct as non-transfused patients. For any given volume, lower patient Hct generally yields less autologous PRBC volume generated by autotransfusion. Lower autotransfusion volume could have accounted for the increased incidence and magnitude of homologous PRBC transfusions; however, the median number of autotransfusion units was identical for all comparison groups. The higher transfusion rate seen was strictly related to the increased hemodilution.
This study, as well as previous studies, identified smaller size, female sex, and lower baseline Hct as risk factors for a lower CPB Hct and receipt of a blood transfusion (1,6,8,19,26). These factors characterize patients that are at an increased risk of severe hemodilution and consequentially a PRBC transfusion. A trend between these factors and the magnitude of transfusion can be seen in Table 7.
As previously discussed, older, smaller, predominantly female patients received less CPB prime volume but ironically received more pre-CPB fluid (mL and mL/kg). These are patients in whom a clinician would want to be more discreet in the fluid management to maintain or decrease the risk of a lower Hct and subsequent PRBC transfusion. This was somewhat evident in the fluid management of the CPB prime, because these patients had a lower prime volume (not including the four patients that received a PRBC unit in the prime with subsequent displacement of crystalloid prime fluid) than non-transfused patients (1415 vs. 1550 mL). The slightly lower mean prime volume in these patients may have been a result of the removal of CPB prime volume to start CPB with a near 0-mL venous reservoir level compared with a 200-to 300-mL buffer level, with the objective of minimizing CPB prime dilution.
The results of this study showed variability in pre-CPB fluid volume administered, with higher pre-CPB fluid ad-ministration volume increasing the likelihood of lower CPB Hct levels and increased probability of PRBC transfusion. The finding that the initial on CPB Hct was the lowest CPB Hct, as well as the lowest procedural Hct, in the majority of cases in this study emphasizes that provisions need to be made pre-CPB by anesthesia and perfusion to minimize the amount of hemodilution. Because the single lowest Hct value is what matters, preventing a severely low Hct is better than reversing one.
Blood conservation to include preserving Hct in cardiac surgery should be a multi-discipline, multi-modality approach, with all team members striving for the same goal. The surgical team should use methods and techniques to reduce or limit blood loss and achieve surgical hemostasis throughout the procedure. The perfusion team should use methods and techniques, such as shortened circuits and autologous priming to minimize hemodilution and preserve Hct. The findings of this study suggest the limitation of asanguinous fluids pre-CPB by the anesthesia team may have a similar influence. This may be accomplished with more use of alpha agonists instead of volume to treat arterial pressure, which is a technique used at some centers.
Because this was a retrospective chart review, this study inherently has limitations. First, their may be discrepancies in the time when the pre-CPB volume total was requested by anesthesia personnel and when CPB was initiated. The perfusionist usually asks for the pre-CPB volume total with the pre-CPB blood sample after heparinization, and CPB is usually initiated with this particular surgeon after adequate heparinization (480 seconds). There were some instances, however, where CPB was initiated ∼30 minutes after the blood draw (mean, 16.8 ± 6.8 minutes; range, 6–32 minutes). This is nearly 30 minutes of potential fluid administration possibly not recorded as pre-CPB volume. This scenario would reflect an underestimation of the actual pre-CPB volume administered. Second, the baseline Hct may not be a true baseline in some of the patients. Although our routine is to draw a baseline blood sample at or near the time of anesthesia induction, some samples were drawn as late as 45 minutes after this event (mean, 11.3 ± 10.3 minutes; range, 0–45 minutes). This is 45 minutes of fluid administration not reflected in the baseline Hct. This situation could create an underestimation of the actual baseline Hct. Third, there was no strict transfusion trigger that was adhered to in this study. Although the mean Hct values at which an initial transfusion occurred did not vary much from the acceptable thresholds of 20% for CPB and 22% after CPB in the absence of readily available autologous PRBCs, there may have been variance between clinicians and patients.
CONCLUSION
In conclusion, sex, weight, baseline OR Hct, and pre-CPB volume are the best predictors of low CPB Hct, and low CPB Hct is the best predictor of whether or not a patient receives a PRBC transfusion. Increasing pre-CPB fluid volume was associated with lower CPB Hct values. Patients that received a PRBC transfusion received more pre-CPB fluid volume and had lower CPB Hcts than non-transfused patients. Although the correlation was not as strong, this relationship was seen in the magnitude of PRBC transfusion.
Patients at an increased risk of having a lower CPB Hct are women, are smaller sized, and have lower baseline OR Hct, which are fixed variables that clinicians are given when the patient arrives in the operating room. The variables we do have some control over in the operating room are pre-CPB fluid volume and CPB prime volume. These findings, combined with previous work, suggest intraoperative fluid administration by anesthesia and perfusion personnel have a cumulative effect on the overall level of intra-operative hemodilution. To a degree, the severity of hemodilution and its effects can be controlled by clinicians and can be reduced with optimal practices. Autologous priming of the ECC can result in a reduction in hemodilution from perfusion personnel. The findings from this study suggest that closely monitoring pre-CPB fluid volume and attempting to limit this volume of fluid may be beneficial in additionally reducing the overall degree of hemodilution to the patient. This is especially warranted in patients that are female, small size, and have a low baseline Hct. This may lead to higher CPB Hcts and a reduction in PRBC transfusions.
Although a retrospective chart review is limited in its scientific merit, the information obtained from this study does show associations and trends that warrant further investigation. A prospective study, with better control of variables, would need to be conducted to further understand and confirm the associations observed, their significance, and effect on outcomes.
REFERENCES
- 1.DeFoe GR, Ross CS, Olmstead EM, et al. . Lowest hematocrit on bypass and adverse outcomes associated with coronary bypass grafting. Ann Thorac Surg. 2001;71:769–76. [DOI] [PubMed] [Google Scholar]
- 2.Surgenor SD, DeFoe GR, Fillinger MP, et al. . Intraoperative red blood cell transfusion during coronary artery bypass graft surgery increases the risk of postoperative low-output heart failure. Circulation. 2006;114(1 Suppl):I43–8. [DOI] [PubMed] [Google Scholar]
- 3.Karkouti K, Djaiani G, Borger MA, et al. . Low hematocrit during cardiopulmonary bypass is associated with increased risk of perioperative stroke in cardiac surgery. Ann Thorac Surg. 2005;80:1381–7. [DOI] [PubMed] [Google Scholar]
- 4.Habib RH, Zacharias A, Schwann TA, et al. . Role of hemodilutional anemia and transfusion during cardiopulmonary bypass in renal injury after coronary revascularization: implications on operative outcome. Crit Care Med. 2005;33:1749–56. [DOI] [PubMed] [Google Scholar]
- 5.Swaminathan M, Phillips-Bute BG, Conlon PJ, et al. . The association of lowest hematocrit during cardiopulmonary bypass with acute renal injury after coronary artery bypass surgery. Ann Thorac Surg. 2003;76:784–91. [DOI] [PubMed] [Google Scholar]
- 6.Habib RH, Zacharias A, Schwann TA, Riordan CJ, Durham SJ, Shah A.. Adverse effects of low hematocrit during cardiopulmonary bypass in the adult: should current practice be changed? J Thorac Cardiovasc Surg. 2003;125:1438–50. [DOI] [PubMed] [Google Scholar]
- 7.Karkouti K, Beattie WS, Wijeysundera DN, et al. . Hemodilution during cardiopulmonary bypass is an independent risk factor for acute renal failure in adult cardiac surgery. J Thorac Cardiovasc Surg. 2005;129:391–400. [DOI] [PubMed] [Google Scholar]
- 8.Cormack JE, Forest RJ, Groom RC, Morton J.. Size makes a difference: use of a low prime cardiopulmonary bypass circuit and autologous priming in small adults. Perfusion. 2000;15:129–35. [DOI] [PubMed] [Google Scholar]
- 9.Groom R.. High or low hematocrits during cardiopulmonary bypass for patients undergoing coronary artery bypass graft surgery? An evidence-based approach to the question. Perfusion. 2002;17:99–102. [DOI] [PubMed] [Google Scholar]
- 10.DeBois W, Sukhram Y, McVey J, et al. . Reduction in homologous blood transfusions using a low prime circuit. J Extra Corpor Technol. 1996;28:58–62. [Google Scholar]
- 11.Balachandran S, Cross MH, Karthikeyan S, Mulpur A, Hansbro SD, Hobson P.. Retrograde autologous priming of the cardiopulmonary bypass circuit reduces blood transfusion after coronary artery surgery. Ann Thorac Surg. 2002;73:1912–18. [DOI] [PubMed] [Google Scholar]
- 12.Zelinka ES, Ryan P, McDonald J, Larson J.. Retrograde autologous prime with shortened bypass circuits decreases blood transfusion in high-risk coronary artery surgery patients. J Extra Corpor Technol. 2004;36:343–7. [PubMed] [Google Scholar]
- 13.Rosengart TK, DeBois W, O’Hara M, et al. . Retrograde autologous prime for cardiopulmonary bypass: a safe and effective means of decreasing hemodilution and transfusion requirements. J Thorac Cardiovasc Surg. 1998;115:426–38. [DOI] [PubMed] [Google Scholar]
- 14.Shapira OM, Aldea GS, Treanor PR, et al. . Reduction of allogenic blood transfusions after open heart operations by lowering cardiopulmonary bypass prime volume. Ann Thorac Surg. 1998;65:724–30. [DOI] [PubMed] [Google Scholar]
- 15.Wallace E, Surgenor D, Hao HAJ, Chapman R, Churchill W.. Collection and transfusion of blood and blood components in the United States. Transfusion. 1993;33:139–44. [DOI] [PubMed] [Google Scholar]
- 16.Stover EP, Siegel LC, Parks R, et al. . Variability in transfusion practice for coronary artery bypass surgery persists despite national consensus guidelines: a 24-institution study. Institutions of the multicenter study of perioperative ischemia research group. Anesthesiology. 1998;88:327–33. [DOI] [PubMed] [Google Scholar]
- 17.Warner MA.. Infectious risks of transfusion. In: Spiess BD, Counts RB, Gould SA.. Perioperative Transfusion Medicine. Baltimore: Williams and Wilkins; 1998; 97–107. [Google Scholar]
- 18.Dailey JF.. Blood, 2nd ed. Ipswich, MA: Medical Consulting Group; 2001. [Google Scholar]
- 19.Engoren MC, Habib RH, Zacharias A, Schwann TA, Riordan CR, Durham SJ.. Effect of blood transfusion on long term survival after cardiac operation. Ann Thorac Surg. 2002;74:1180–6. [DOI] [PubMed] [Google Scholar]
- 20.Koch CG, Li L, Duncan AI, et al. . Transfusion in coronary artery bypass grafting is associated with reduced long-term survival. Ann Thorac Surg. 2006;81:1650–7. [DOI] [PubMed] [Google Scholar]
- 21.Kuduvalli M, Oo AY, Newall N, et al. . Effect of peri-operative red blood cell transfusion on 30-day and 1-year mortality following coro-nary artery bypass surgery. Eur J Cardiothorac Surg. 2005;27:592–8. [DOI] [PubMed] [Google Scholar]
- 22.Koch CG, Li L, Duncan AI, et al. . Morbidity and mortality risk associated with red blood cell and blood-component transfusion in isolated coronary artery bypass grafting. Crit Care Med. 2006;34:1608–16. [DOI] [PubMed] [Google Scholar]
- 23.Herbert PC, Wells G, Blajchman MA, et al. . A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340:409–17. [DOI] [PubMed] [Google Scholar]
- 24.Larach DL.. Anesthetic management during cardiopulmonary bypass. In: Hensley FA, Martin ME, eds. The Practice of Cardiac Anesthesia, 1st ed. Boston: Little Brown and Company; 1990; 244. [Google Scholar]
- 25.Holte K, Sharrock NE, Kehlet H.. Pathophysiology and clinical implications of perioperative fluid excess. Br J Anaesth. 2002;89:622–32. [DOI] [PubMed] [Google Scholar]
- 26.Dial S, Delabays E, Albert M, et al. . Hemodilution and surgical he-mostasis contribute significantly to transfusion requirements in patients undergoing coronary artery bypass. J Thorac Cardiovasc Surg. 2005;130:654–61. [DOI] [PubMed] [Google Scholar]


