Abstract:
The Perfusion Downunder Collaboration provides research infrastructure and support to the Australian and New Zealand perfusion community, with the objective of determining best practices and producing relevant research publications. The Perfusion Downunder Collaborative Database (PDUCD) has been created for the purpose of collecting a dataset for cardiopulmonary bypass (CPB) procedures that includes integration with commercially available CPB data collection software. Initial testing of the PDUCD involved collection of data from four Australian and New Zealand hospitals from March to July 2007. Data from 513 procedures were compared with the concurrent Australasian Society of Cardiac and Thoracic Surgeons (ASCTS) database report to assess the validity of the collected data. Demographic, preoperative, and procedural variables were comparable between databases. Perfusion variables showed a median nasopharyngeal temperature of 36.7°C at separation from CPB (range, 35.3–37.5°C), which was similar to maximum nasopharyngeal temperature (median, 36.8°C). Median arterial flow and mean arterial pressure were 4.2 L/min and 57.2 mmHg, respectively. Control charts indicate a central tendency of 12.5 minutes for mean arterial pressure <50 mmHg and 3.5 minutes for arterial flow <1.6 L/min/m2 (cumulative time). There was no difference in median minimum and maximum blood glucose between diabetic and nondiabetic patients during CPB with 40% of patients receiving insulin. Median minimum and maximum activated clotting time (ACT) during CPB was 581 and 692 seconds, respectively. Outcome data for isolated coronary artery bypass grafting were similar for mortality (only) (both 1.8%). Initial data collection showed concurrent validity compared with the ASCTS database. The inclusion of a large quantity of calculated CPB variables in the dataset highlights the benefits of electronic data collection as a research tool within a collaborative research network and the potential for the evaluation of the relationships between patient risk factors, perfusion practice, and patient outcomes.
Keywords: electronic data collection, cardiopulmonary bypass
The collection of data is an integral component of the research process since data management techniques may be used both in the development of a hypothesis and in the experimental technique and methodology. Analysis and processing of the data produced from the experimental method provide a means not only to evaluate the validity of the original hypothesis but also elucidate further observations relevant to the scientific manuscript. In the development of a research project, there are various methods available for data collection, and therefore, it is important that the method is chosen based on the aims, methods, and resources of the project. To ensure validity of the collected data, the data collection process must be replicable and accurate (1).
Increasingly, research studies have used electronic means rather than paper forms for data collection, and this provides a number of benefits, including a reduction in the error associated with transcription because data are entered directly and the ability of computer software to be configured to validate the data and perform skip logic [e.g., if a angina field is entered “no,” the subsequent unstable angina field automatically appears as “no” (2)]. Given that a vast quantity of data can potentially be collected during cardiac surgery and cardiopulmonary bypass (CPB) from patient monitors and clinical devices, electronic data collection during cardiac surgery provides a useful and accurate method of data acquisition. Another important consideration in the design of a research project is an accurate determination of the sample size required to make meaningful interpretation of the results possible. The creation of a research network facilitates the recruitment of observations for inclusion in studies. Research networks are a method for collection of clinical data from geographically dispersed institutions, with the advantage being that the statistical power of a particular study can be improved through an increase in sample size. Electronic data collection has been used successfully in these networks to improve the quality of data collection and reduce secondary data entry (3).
The Perfusion Downunder Collaboration aims to improve patient outcomes through its ability to provide research infrastructure and support to the Australian and New Zealand perfusion community and to produce relevant and timely research publications (4). To support this aim, an electronic database has been created for the purpose of collecting a dataset for CPB procedures known as the Perfusion Downunder Collaborative Database (PDUCD). This report describes the development of the database and a comparison of the initially collected data with the data reported in the Australasian Society of Cardiac and Thoracic Surgeons (ASCTS) database.
MATERIALS AND METHODS
The PDUCD dataset is divided into five categories: demography, clinical (medical history, etc.), perfusion (CPBrelated data), procedural (surgical data), and outcome data. The complete database was designed to be comprised of three separate Microsoft Access (Microsoft, Redmond, WA) database files: a “Tables” database that stores the collected data at each institution; A “Server” database that serves as a front-end database and contains the data collection forms; and a “Transfer database” that performs the transfer and processing of the data from automated perfusion record software.
The purpose of this design was to separate functionality and to provide a means of allowing multiple users to access the tables simultaneously through multiple copies of the server database.
The Tables database file was developed through the creation of tables for each data category, with the majority of data fields stored in the format of coded variables.
Development of the Server database file involved the creation of the data entry forms (Figure 1) and development of automation of various tasks such as opening forms or exporting of data, through visual basic programming. The server forms provided an interface for data entry that facilitated skip logic, and a data dictionary was embedded in each form to provide definitions for each field. A front-end form was created to provide various options to the user, including the ability to create a new record, view existing records, access an administration console, or export the collected data. The administration console was created for the purpose of customizing certain default entries that would be routinely entered (such as use of arterial filter, arterial pump type, etc.), creation of usernames and passwords, and defining certain variables collected in other software (such as temperature probe locations). The database is locally protected by a user login form, which allows for security measures to be incorporated because patient identification data are collected at the site of origin. The data export process, located within the Server database, was designed to transfer data that is nonidentifiable, in the format of text files, and involves assigning a new unique identifier for incorporation into the master PDUCD dataset. This creates anonymity of data within the master dataset but allows the data to be tracked at the point of origin by the data originators.
Figure 1.
Data entry workflow for the database. Step 1: Click the “create new record” button to enter the patient’s demographic data. Step 2: Click the “clinical forms” button to access the data entry forms for each category. Step 3: Clinical, perfusion, procedural, and postoperative outcomes data may be entered through forms. The clinical form is shown. Step 4: Electronic data are transferred from the electronic data management system of the heart lung machine into the database.
A third database was created as a method of transferring the data from electronic perfusion record software (Stockert Data Management System; Stockert, Munich, Germany). This process allows the integration of a large quantity of data collected by automation in the operating theater, as previously described (5). During this process, a number of calculated parameters are generated from the CPB data; for example, timed quantification of mean arterial pressure, cardiac index, or arterial outlet temperature.
The design of the database was intended to maximize flexibility of use to suit multiple institution’s ability to access electronic data collection at the point of care. Where electronic data collection is not possible or available at the point of care, a paper form of the PDUCD dataset can be used to collect data, and data can be entered manually. The data collection workflow for the electronic data collection database is shown in Figure 1.
After appropriate clinical governance and ethics committee approval, initial testing of the PDUCDB involved collection of data from four Australian and New Zealand cardiac centers from March 2007 to July 2007. All centers used the Stockert Data Management System. A site coordinator was appointed from within each center with the responsibility of installation, configuration, and coordination of the database. In a preliminary analysis, PDUCD data were collected from 513 “data eligible” procedures. Procedures defined as eligible for inclusion were isolated on pump coronary artery bypass graft (CABG), isolated valve repair, and/or replacement and valve/CABG procedures. Both first time and redo procedures were included. A total of 179 data variables were collected in the following categories: demography (12), clinical (29), perfusion (115), and outcomes (23). The PDUCD data were compared with the Australasian Society of Cardiac and Thoracic Surgeons (ASCTS) database to assess the concurrent validity of the initial dataset. A subset of variables was selected from the PDUCD dataset to facilitate comparison with the most recently published ASCTS database data, which reported data collected from 2795 procedures in six cardiac surgical centers in Victoria, Australia (6). The most recent electronically available ASCTS database report was for the period July 2005–June 2006. The data in the ASCTS database are manually entered by medical staff into a purpose-designed database and stored electronically. The dataset has been reported annually since 2002. Continuous physiological and perfusion variables are not collected in the ASCTS database; only minimal operative nonprocedural data are collected.
Demographic and procedural variables included patient age distribution, procedure type, conduits used [left internal mammary artery (LIMA), right internal mammary artery (RIMA) radial artery (RADG), gastroepiploic artery (GEPA)], and average number of grafts in CABG. Clinical variables included preoperative risk factors (smoking, recent myocardial infarction, hypertension, peripheral vascular disease, cerebrovascular disease, diabetes). A further subset of data to compare outcomes data in isolated CABG was comprised of mortality, new renal failure, stroke, cerebrovascular complication, return to theater, and red blood cell transfusion. Definitions for each variable were equivalent other than for recent myocardial infarction (MI), which is defined in the current dataset as a MI within 90 days before surgery compared with 21 days in the ASCTS data. Off-pump CABG procedures are included in the isolated CABG group in the ASCTS data; however, they are grouped into the “other” category in the PDUCD. A small subset of variables related to CPB management was also included (activated clotting times and blood glucose concentrations both manually collected, and, temperature, pressure, and flow management all collected continuously electronically). Because these data have not previously been reported as part of a registry, comparative data were not available. Statistical control charts were used to display the data obtained for CPB quality indicator variables mean arterial pressure (MAP) <50 mmHg and cardiac index <1.6 L/min/m2 (both cumulative time). Data for these two variables were collected every 20 or 30 seconds during CPB using the Data Management System. Pressure artefacts and periods of partial CPB were automatically excluded from analysis by programming techniques. Control charts of individual case data were created using SPCXL (Air Academy Associates, Colorado Springs, CO) with Shewhart control limits set at ±3 SD.
RESULTS
Comparison of PDUCD and ASCTS Database
Demographic and procedural variables for the PDUCD data eligible patients (CABG, isolated valve, and valve/CABG) were closely comparable to the ASCTS database for procedure type and age distribution (Table 1). Internal thoracic arterial grafts for isolated CABG were similar for both datasets, but use of radial artery grafts was markedly different. Preoperative risk factors for both datasets were also comparable (Table 2). Preliminary outcome data collected to date for isolated CABG was similar to ASCTS for mortality only (Table 3).
Table 1.
Demographic and procedural data.
| Procedure Types | n | PDUCD (%) | ASCTS (6) (%) |
|---|---|---|---|
| Isolated CABG | 339 | 50 | 63 |
| Isolated valve | 107 | 15 | 13 |
| Valve CABG | 67 | 10 | 10 |
| Other | 162 | 24 | 14 |
| Total | 675 | ||
| Eligible | 513 | ||
| Age distribution | |||
| <40 yrs | 4 | 4 | |
| 40–49 yrs | 7 | 6 | |
| 50–59 yrs | 17 | 18 | |
| 60–69 yrs | 30 | 30 | |
| 70–79 yrs | 31 | 34 | |
| 80+ yrs | 11 | 7 | |
| Conduit use (CABG) | |||
| LIMA | 93 | 85 | |
| RIMA | 3 | 1 | |
| RADG | 14 | 54 | |
| GEPA | 0 | 0 | |
| Mean no. grafts | 2.7 | 3.4 |
The PDUCD data have been compared with the published data from the ASCTS database for procedure type, age, and conduit use. The term “Eligible” relates to whether a case met the inclusion criteria for the PDUCD data collection.
PDUCD, Perfusion Downunder collaborative database; ASCTS, Australasian Society of Cardiac and Thoracic Surgeons; CABG, coronary artery bypass graft; LIMA left internal mammary artery; RIMA, right internal mammary artery; GEPA, gastroepiploic artery.
Table 2.
Patient preoperative risk factors.
| PDUCD (%) | ASCTS (6) (%) | |
|---|---|---|
| Current smoker | 13 | 13 |
| Recent myocardial infarct | 26 | 19 |
| Hypertension | 65 | 71 |
| Peripheral vascular disease | 11 | 13 |
| Cerebrovascular disease | 7 | 12 |
| Diabetes | 27 | 29 |
PDUCD, Perfusion Downunder collaborative database; ASCTS, Australasian Society of Cardiac and Thoracic Surgeons.
Table 3.
Clinical outcomes.
| PDUCD (%) | ASCTS (6) (%) | |
|---|---|---|
| Mortality | 1.8 | 1.8 |
| New renal failure | 2.1 | 5.1 |
| Stroke | 2.1 | 0.7 |
| Encephalopathy | 4.7 | 1.4 |
| Return to theatre | 10.6 | 5.1 |
| Red blood cell transfusion | 33.0 | 43.4 |
PDUCD, Perfusion Downunder collaborative database; ASCTS, Australasian Society of Cardiac and Thoracic Surgeons.
CPB Variables
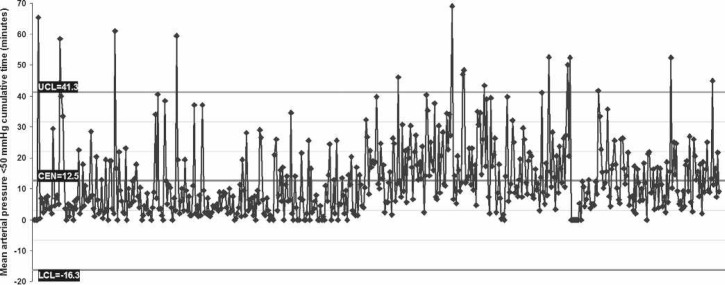
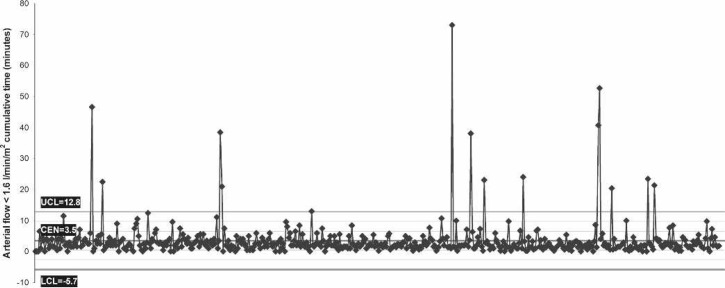
Perfusion variables showed a median nasopharyngeal temperature of 36.7°C at separation from CPB (range, 35.3–37.5°C), which corresponds with the maximum nasopharyngeal temperature (median, 36.8°C; range, 35.3–37.6°C). Median MAP was 57.2 mmHg and arterial flow was 4.2 L/min (Table 4). Control charts indicate a central tendency of 12.5 minutes for MAP <50 mmHg (Figure 2) and 3.5 minutes for arterial flow <1.6 L/min/m2 (Figure 3). There was no difference in median minimum and maximum glucose between diabetic and nondiabetic patients during CPB, despite 40% of patients having glucose management with insulin (Figure 4). Median minimum and maximum ACT during CPB was 581 and 692 seconds, respectively (Figure 5).
Table 4.
Perfusion variables.
| Temperature Management | Median | Range |
|---|---|---|
| Maximum nasopharyngeal (°C) | 36.8 | 35.3—37.6 |
| CPB separation nasopharyngeal (°C) | 36.7 | 34.8—37.5 |
| Maximum arterial outlet (°C) | 37.3 | 35.3—38.5 |
| Pressure/flow | ||
| Average mean arterial pressure (mmHg) | 57.2 | 17.1—77.7 |
| Average arterial flow (L/min/m2) | 4.2 | 2.5—5.9 |
CPB, cardiopulmonary bypass.
Figure 2.
Control chart of individual case data (horizontal axis) for the amount of cumulative time that the mean arterial pressure was <50 mmHg (vertical axis). CEN, central tendency (mean); UCL, upper control limit; LCL, lower control limit. Cases are ordered chronologically.
Figure 3.
Control chart of individual case data (horizontal axis) for the amount of cumulative time that the arterial flow <1.6 L/min/m2 (vertical axis). CEN, central tendency (mean); UCL, upper control limit; LCL, lower control limit. Cases are ordered chronologically.
Figure 4.
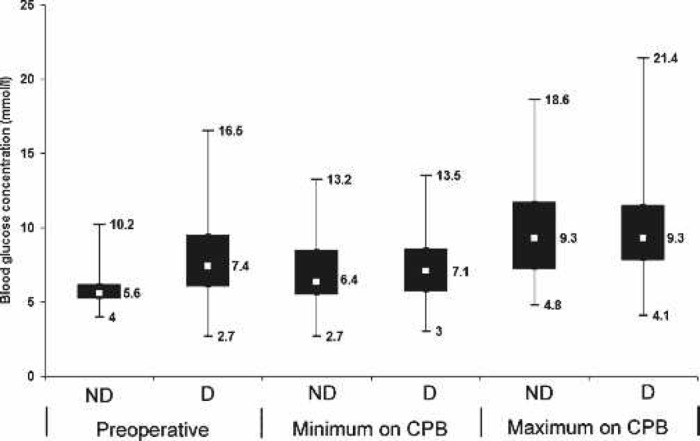
Box plot of blood glucose values comparing diabetic and nondiabetic patients, preoperatively and minimum and maximum values during CPB. ND, nondiabetic; D, diabetic. Displayed values indicate median and range.
Figure 5.
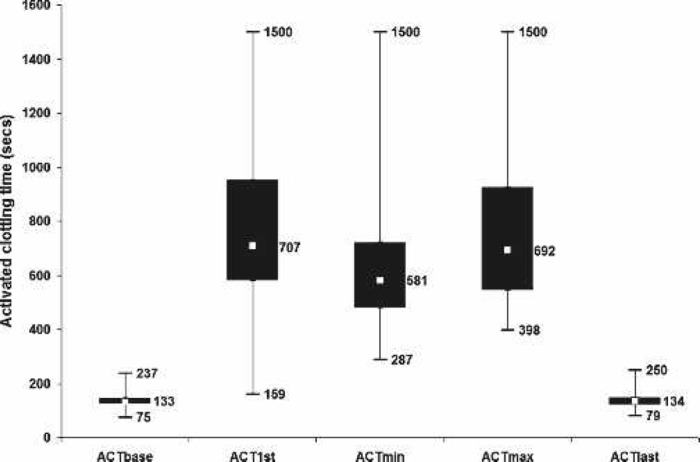
Box plot of activated clotting times. ACTbase, before heparinization; ACT1st, 1st on CPB; ACTmin, minimum on CPB; ACTmax, maximum on CPB; ACTlast, postreversal of heparin. Displayed values indicate median and range.
DISCUSSION
The task of collecting high-quality research data in clinical environments provides a number of challenges, and therefore, it is important to match the method of data collection with the objectives of the research project. When considering electronic data collection as an option, the following questions need to be addressed (2). Do the study designs lend themselves to electronic data collection? Is the expertise available to implement electronic data collection? Can the development and administration of the database be supported and assistance be provided at the point of data entry?
The aim of the Perfusion Downunder Collaboration is to generate a prospectively collected dataset for the evaluation of hypotheses relating to CPB as it is conducted in Australia and New Zealand. Electronic data collection meets the needs of this endeavor because it provides a method of transferring data from multiple sites, integration of this data into a central database, and a means to generate calculated CPB parameters and perform complex data analysis. Flinders Medical Centre has collected clinical data using this technology since 1992, and having dedicated personnel responsible for the development and administration of our database is well placed to support the PDUCD. Other centers contributing to PDUCD have found that establishment of a site coordinator role with appropriate time and resources are beneficial in the implementation, management, and coordination of the database.
The generation of a perfusion record is an integral part of CPB, and a number of electronic record systems have been developed to automate the majority of this process and improve the accuracy of the collected data (7,8). The Stockert Data Management System has been successfully integrated into a multicenter perfusion database, and the integration of alternative software is a major focus for current development to attract users of other systems. Integration has been achieved with the Jostra perfusion data collection software (Jocap XL; MAQUET Cardiopulmonary, Hirrlingen, Germany), and we look forward to incorporating data from multiple electronic perfusion data sources in the future.
Newland et al. (5) previously reported a technique in which the data collected by an automated CPB system can be integrated into a research database and during this process generate CPB quality indicators. However, a limitation of this process in relation to clinical practice is that measures of CPB “quality” have not been well described, and thus the definition of quality indicators may be considered subjective. It has been suggested that there is a need for the development of standards of practice for CPB obtained through the mandates of evidence-based medicine (9). One study designed to assess both the quantity and quality of the literature supporting principles currently applied to CPB concluded that the scientific data are insufficient on both counts to reliably serve as a basis for practical, evidence-based guidelines (10). One of the problems inherent in the interpretation of the clinical measures of outcomes from CPB is the low event rate of adverse events, resulting in the requirement of large cohorts to achieve adequate statistically powered studies. Amalgamation of collected data provides a means to increase cohort size and therefore reduce the confounding effects of practice changes over time. The PDU Collaboration and the PDUCD is a means to facilitate these objectives as may eventually be a recently established international consortium for evidence-based perfusion (11).
Such ventures are subject to rigorous ethical scrutiny. The most recent publication of the National Statement on Ethical Conduct in Human Research developed jointly by the National Heath and Medical Research Council, the Australian Research Council, and the Australian Vice-Chancellors’ Committee has for the first time included a chapter on databases (12). An important consideration in the ethical collection of patient information is whether data collected can be identified. The Statement has defined three levels to characterize how data are identified: individually identifiable data, where the identity of a specific individual can be ascertained; re-identifiable data, where identifiers have been removed and replaced with a code to facilitate re-identification of an individual; and nonidentifiable data, where there is no means to identify a particular individual, although it may be possible to link different datasets for the same individual. Currently, the data in the PDUCD will be individually identifiable at the site of collection, and on integration into the collaborative dataset, the data will be nonidentifiable to the custodians of the data and for research generated from the dataset. Other ethical concerns raised in the Statement include the requirement to conform to patient consent guidelines, the promotion of research through data accessibility, and that the use of the data by individual researchers must comply with any conditions relating to identification. The inclusion of this chapter in the national ethical research statement reflects the recognition of electronic data collection being increasingly adopted as a modality to facilitate the collection and dissemination of research information.
Concurrent validity may be defined as the ability of a newly developed measure to predict the results of an existing measure that represents a reference standard (13). The preliminary analysis of the initial PDUCD data collected from four centers showed concurrent validity in patient demographic and preoperative variables compared with the much larger ASCTS database from the same region. Differences in reported procedural variables could be attributed to regional surgical preferences, for example, use of the radial artery as a conduit for CABG. Future prospective collection will provide cumulative data for comparison; however, recruitment of additional contributing centers would provide a more representative dataset for comparative purposes. Some differences were observed in the outcomes data variables reported. These are likely to be related to the size of the datasets being compared. In this preliminary PDUCD report, we reported a 3-month period, whereas the ASCTS dataset is for a 12-month period from six centers. Alternatively the differences in outcome data may reflect real differences introduced caused by the geographical separation of the hospitals, or the differences may reflect differences in methods of data collection. Another factor is that off-pump cases are reported in the ASCTS data set but not in the PDUCD data, which creates a limitation in the comparison of the outcomes data.
The variables included in this report show that those routinely recorded during CPB have been successfully integrated into a multicenter database. The inclusion of a large quantity of calculated CPB variables in the PDUCD dataset highlights the potential for the creation of a multicenter registry for the evaluation of the relationships between patient risk factors, perfusion practice, and patient outcomes: the cornerstones in the evaluation of perfusion best practices.
In conclusion, the advantage of this novel perfusion database is that it provides the benefits of electronic data collection as a research tool within a collaborative research network and has the ability to perform complex data processing techniques for the analysis of CPB parameters. Use of the PDUCD and participation in the PDUC has the potential to provide a multicenter perfusion registry for the evaluation of best practices and the testing of scientific hypotheses, ultimately benefiting all the perfusion community and most importantly our patients.
Current membership of the Perfusion Downunder Collaboration: Ashford Hospital, SA, Australia (J. Ottens, A. Sanderson), Auckland City Hospital, New Zealand (T.W. Willcox, K. Place), Auckland University, New Zealand (A. Merry), Flinders Medical Centre, South Australia, Australia (R.F. Newland, R.A. Baker, K. Farrar, J. Knight), Geelong Hospital Victoria, Australia (C. Morley), Royal Hobart Hospital, Tasmania, Australia (C. Fen-ton, Y.Y. Huang), Perfusion Services, Victoria, Australia (M. McDonald), Alfred Hospital, Victorial, Australia (P. Myles), University of Manitoba, Winnipeg, Canada (H.P. Grocott). Center de Cardiologie des Mascareigns, Mauritius (K.K. Jagannadham), Royal North Shore Hospital, New South Wales, Australia (K.C. Potger), St. Vincents Hospital, New South Wales, Australia (A. Dinale), The Prince Charles Hospital, Queensland, Australia (C. McDonald), Christchurch Hospital, Christchurch (J. Benner), Mater Children’s Hospital, Queensland, Australia (K. Zazulak).
REFERENCES
- 1.Polgar S, Thomas SA.. Introduction to Research in the Health Sciences. London: Harcourt Publishers; 2000. [Google Scholar]
- 2.Hulley SB, Cummings SR, Browner WS, Grady DG, Newman TB.. Designing Clinical Research. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 3.Pace WD, Staton EW.. Electronic data collection options for practice-based research networks. Ann Fam Med. 2005;3(Suppl 1): S21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perfusion Downunder Collaboration. Available at: http://www.perfusiondownunder.com/pducvision.html. Accessed April 25, 2008.
- 5.Newland RF, Baker RA, Stanley R.. Electronic data processing: the pathway to automated quality control of CPB. J Extra Corpor Technol. 2006;38:139–43. [PMC free article] [PubMed] [Google Scholar]
- 6.Dinh DT, Billah B, Shardey G, Pick A, Reid CM.. Australasian Society of Cardiac and Thoracic Surgeons (ASCTS) Victorian Cardiac Surgery Database Project Annual Report 2005–2006. Available at: http://www.ascts.org/documents/PDF/VIC_Surgeons_report_0506_Fina.pdf. Accessed June 24, 2008.
- 7.Ottens J, Baker RA, Newland RF, Mazzone A.. The future of the perfusion record: automated data collection vs. manual recording. J Extra Corpor Technol. 2005;37:355–9. [PMC free article] [PubMed] [Google Scholar]
- 8.Hollenburg JP, Pirraglia PA, Williams-Russo P, et al. Computerised data collection in the operating room during coronary artery bypass surgery: a comparison to the hand–written record. J Cardiothorac Vasc Anesth. 1997;11:545–51. [DOI] [PubMed] [Google Scholar]
- 9.Stammers AH.. Why we do, what we do, when we do it. J Extra Corpor Technol. 2005;37:250. [PubMed] [Google Scholar]
- 10.Bartels C, Gerdes A, Babin-Ebell J, et al. Working Group on Extracorporeal Circulation and Mechanical Ventricular Assist Devices of the German Society for Thoracic and Cardiovascular Surgery. Cardiopulmonary bypass: evidence or experience based? J Thorac Cardiovasc Surg. 2002;124:20–7. [DOI] [PubMed] [Google Scholar]
- 11.Likosky DS.. Integrating evidence-based perfusion into practices: the international consortium for evidence-based perfusion. J Extra Corpor Technol. 2006;38:297–301. [PMC free article] [PubMed] [Google Scholar]
- 12.National Health and Medical Research Council, Australian Research Council, Australian Vice-Chancellors’ Committee. National Statement on Ethical Conduct in Human Research. Canberra: NHMRC publications; 2007. [Google Scholar]
- 13.Ford JJ, Story I, McMeeken J.. The test-retest reliability and concurrent validity of the Subjective Complaints Questionnaire for low back pain. Man Ther. (Epub ahead of print, May 20, 2008). [DOI] [PubMed] [Google Scholar]