Abstract:
In early 2008, surveys of active extracorporeal membrane oxygenation (ECMO) centers in North America were conducted by electronic mail regarding neonatal ECMO equipment and professional staff. Eighty of 103 (78%) North American ECMO centers listed in the Extracorporeal Life Support Organization directory as neonatal centers responded to the survey. Of the responding centers, 82.5% routinely used roller pumps for neonatal ECMO, and the remaining 17.5% used centrifugal pumps. Silicone membrane oxygenators were used by 67% of the respondents, whereas 19% used micro-porous hollow fiber oxygenators, and 14% used polymethylpentene hollow fiber oxygenators. Of the silicone membrane oxygenator users, 86% used the Medtronic Ecmotherm heat exchanger, 10% used the Gish HE-4 heat exchanger, and 4% used the Terumo Conducer device. Sixty-four percent of the responding centers used some form of in-line blood gas monitoring. Six percent of the centers used a bubble trap in the arterial line, and 5% used an arterial line filter. A bladder was used by 85% of the centers, and 4% of these used a mechanical bladder box for servo regulation; the remaining 96% used pressure servo regulation. An air bubble detector was used by 88% of the responding centers. A surface coating was used by 44% of the centers on all their neonatal ECMO patients. Thirty-one percent of the centers use an activated clotting time of 180–220 seconds. At 54% of the responding centers, perfusionists were involved with the ECMO program, registered nurses were involved at 70% of the centers, and respiratory therapists were involved at 46% of the centers. Compared with a 2002 survey, silicone membrane use is declining, and the use of centrifugal blood pumps and coated ECMO circuits is becoming more apparent. ECMO teams are still multidisciplinary, made up of combinations of registered nurses, respiratory therapists, and perfusionists.
Keywords: neonatal, extracorporeal membrane oxygenation, devices, survey, equipment
Extracorporeal membrane oxygenation (ECMO) is a modified form of cardiopulmonary bypass (CPB) that is used to treat severe pulmonary or cardiopulmonary failure. A mechanical blood pump circulates a patient’s blood volume through an artificial lung to support a failing respiratory or cardiac system. The first successful use of ECMO was reported by Hill et al. in 1972 (1). Since that time, ECMO has become a widely accepted and valued therapy for neonatal, pediatric, and adult patients in acute cardiac or respiratory distress. The Extracorporeal Life Support Organization (ELSO) Registry reports an overall survival rate of 64%, which includes 25,536 neonatal, 8420 pediatric, and 2510 adult patients that have been treated with ECMO since 1979 (2). This survey was designed to assess the current status of routinely used neonatal ECMO devices and team roles.
In 2002, when the authors first evaluated the state of ECMO devices and personnel (3), the earliest indications of change were beginning to become evident. The purpose of this survey was to examine any further shifts in ECMO devices and professional staff since 2002.
MATERIALS AND METHODS
Between January and March 2008, North American ECMO programs listed in the ELSO Directory (n = 103) were contacted by electronic mail with a survey of neonatal ECMO equipment use and personnel. The survey was sent to the ECMO coordinator of each active North American neonatal program. Thirty-five questions were asked of the ECMO coordinators in fill-in-the-blank format (Appendix). One survey response per center was recorded into a Microsoft Excel database (Microsoft, Redmond, WA).
RESULTS
Of the 103 North American ELSO programs that treat neonates, 80 institutions responded to this survey, indicating a 78% response rate.
Equipment
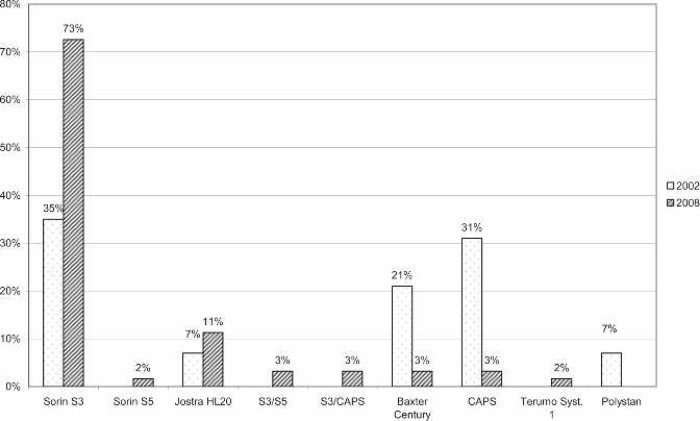
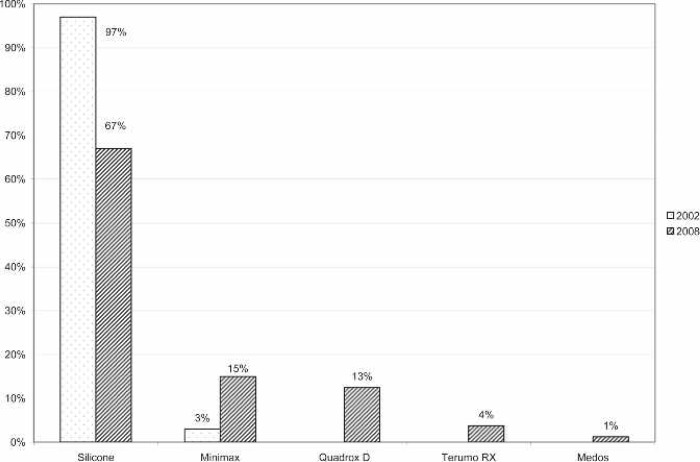
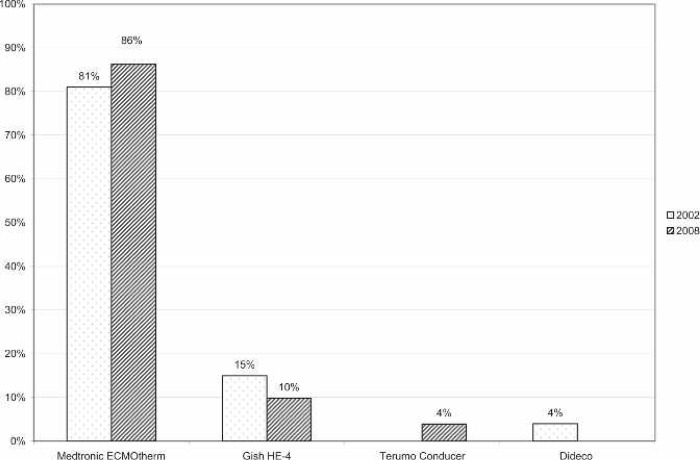
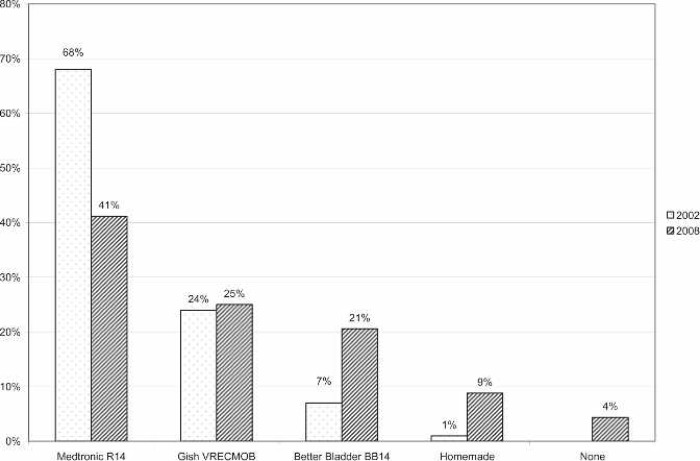
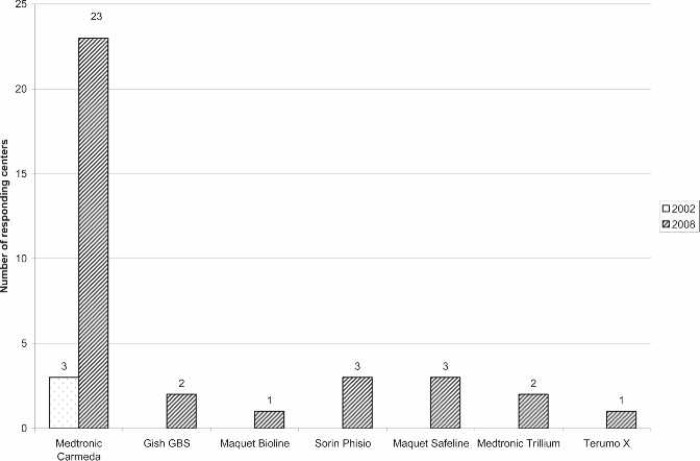
A large majority of the reporting centers used a roller pump (82.5%); of those centers, 100% used Tygon S-95-E or S-65-HL tubing in the pump raceway (Saint-Gobain Performance Plastics, Akron, OH). The majority (77%) of roller pump centers used a Sorin roller head pump console (Figure 1). Centrifugal pumps were used at 17.5% of the responding centers. The Maquet Rotaflow (Maquet Cardiovascular, Bridgewater, NJ) centrifugal pump was used by 50% of those centers. The Medtronic BP-40 (Medtronic, Minneapolis, MN) centrifugal pump was used by 36%, and the Sorin Revolution (The Sorin Group, Mirandola, Italy) was used by 14% of the centrifugal pump users. The Medtronic 0800 silicone membrane was used by 67% of the centers (Figure 2). The Medtronic Minimax micro-porous hollow fiber device was used by 15%, the Maquet Quadrox D polymethylpentene (PMP) oxygenator was used by 13%, the Terumo RX05 (Terumo Cardiovascular Systems, Tokyo, Japan) micro-porous hollow fiber device was used by 4%, and the Medos Hilite 800 LT PMP (Medos Medizintechnik, Stolberg, Germany) membrane was used by 1% of the responding centers on neonatal patients. Of the silicone membrane users, 86% used the Medtronic ECMOtherm heat exchanger. The remainder of the heat exchanger data is shown in Figure 3. All other oxygenators mentioned in this survey have integral heat exchangers. Bubble traps in the arterial line were used by 6% of responding centers. Of these five centers, four used the Terumo Capiox BT05 Bubble Trap and one used the Quest Medical (Quest Medical, Allen, TX) pediatric bubble trap. An arterial line filter (ALF) was used by 5% of responding ELSO centers. Three of the four centers using ALFs used the Medtronic Affinity pediatric ALF, and one center reported using the Maquet Quart ALF. A bladder reservoir was used by 85% of responding centers. Figure 4 shows that the Medtronic R-14 ECMO bladder was reportedly being used most frequently by the responding centers. Pressure servo regulation was reported by 96% of responding ECMO centers. Bladder Box venous reservoir servo regulation was reported by 4% of all respondents. Of those centers using a Bladder Box, all used the Origen (Origen Biomedical, Austin, TX) device. An air bubble detector was reportedly used by 87.5% of the centers. Some type of surface coating was used on ECMO circuits at 44% of the responding centers. The Carmeda BioActive Surface by Medtronic was cited by 66% of the centers who use surface coating on their ECMO circuits. Figure 5 shows the different types of surface coatings and the number of centers using them compared with 2002 survey results.
Figure 1.
Percentage of responding centers using each type of roller pump console for neonatal ECMO applications compared with survey results from 2002. CAPS, Sorin Computer-Assisted Perfusion System.
Figure 2.
Percentage of responding centers using each type of oxygenation membrane for neonatal ECMO applications compared with survey results from 2002. Silicone, Medtronic 0800; Minimax, Medtronic Minimax Plus; Quadrox D, Maquet Quadrox D; Terumo RX, Terumo RX05, Baby RX; Medos, Medos Hilite 800 LT.
Figure 3.
Percentage of responding centers using each type of heat exchange device in conjunction with the Medtronic 0800 silicone membrane for neonatal ECMO applications compared with survey results from 2002.
Figure 4.
Percentage of responding centers using each type of compliance bladder for neonatal ECMO applications compared with survey results from 2002.
Figure 5.
Number of responding centers using each type of surface coating compared with survey results from 2002. The Medtronic Carmeda, Gish GBS, and Maquet Bioline coatings are heparin based. The Maquet Safeline coating is albumin based. The Sorin Phisio, Medtronic Trillium, and Terumo X coatings are non–heparin-based hydrophilic surface coatings.
Monitoring
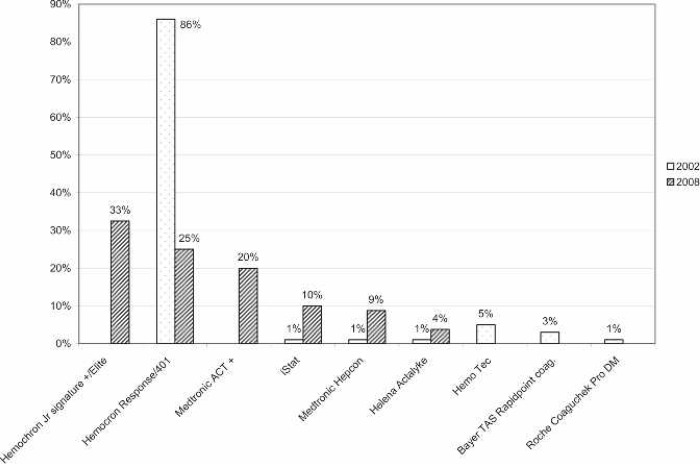
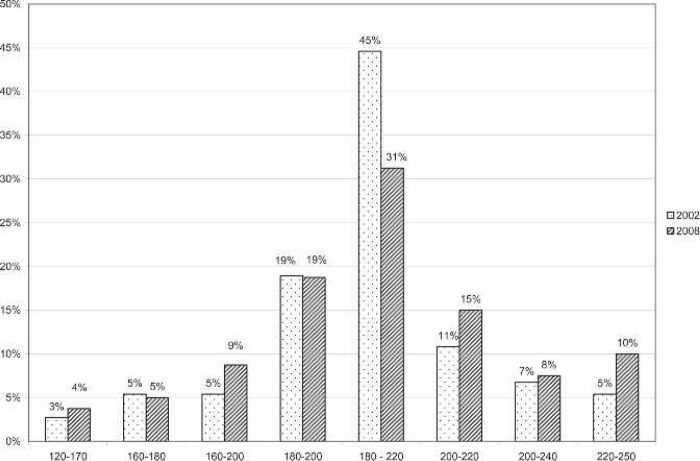
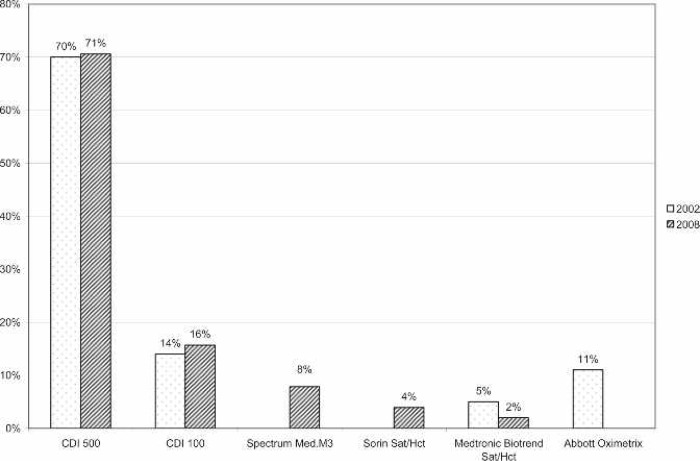
All of the responding centers monitored activated clotting time (ACT) during ECMO support. The majority (57%) used a Hemochron device (International Technidyne, Edison, NJ). The remaining devices used for anti-coagulation monitoring are shown in Figure 6. The most commonly used ACT range was 180–220 seconds (Figure 7). Thirty-six percent of responding centers did not use in-line blood gas monitoring. Of the centers using in-line technology, the Terumo CDI 500/100 system was used most often (71%). The remaining centers using in-line technology used venous side blood hematocrit/saturation monitoring only (Figure 8).
Figure 6.
Percentage of responding centers using each type of ACT monitoring device for neonatal ECMO applications compared with survey results from 2002.
Figure 7.
ACT ranges of responding centers for neonatal ECMO applications compared with survey results from 2002. Range units, seconds.
Figure 8.
Percentage of responding centers using each type of in-line monitoring devices for neonatal ECMO applications compared with survey results from 2002. CDI 500, Terumo Cardiovascular CDI 500; CDI 100, Terumo Cardiovascular CDI 100, Spectrum Med; M3, Spectrum Medical M3.
Personnel
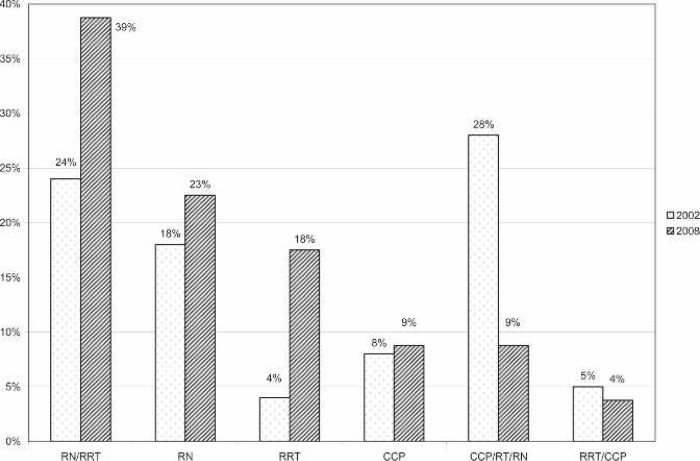
ECMO specialists often come from a variety of departments to form a multidisciplinary team. A team composed of registered nurses (RNs) and registered respiratory therapists (RRTs) made up 39% of the responding centers’ ECMO specialists. A team of RNs, RRTs, and certified clinical perfusionists (CCPs) made up 9%, and a team of RRTs and CCPs made up 4%. A complete list of ECMO specialists is shown in Figure 9. At 24% of the responding centers, perfusionists were responsible for ECMO circuitry setup, priming, initiation, and troubleshooting. Additionally, at 9% of the ELSO centers, perfusionists were listed among the ECMO specialists. Overall, perfusionists were involved in the ECMO program, in some capacity, at 54% of the responding neonatal ELSO centers.
Figure 9.
Percentage of Allied Health Professionals who make up the ECMO Specialist teams at responding centers compared with survey results from 2002. RN, registered nurse; RRT, registered respiratory therapist; CCP, certified clinical perfusionist.
DISCUSSION
ECMO is a widely used and valuable therapy to many hospitals. ELSO has reported that, to date, >36,000 patients have been placed on ECMO, with an overall 64% survival rate (2). Improvements in medical therapies such as surfactant, high-frequency ventilation, inhaled NO, and selective antibiotic prophylaxis for mothers and babies have decreased the overall numbers of infants who require ECMO (4). This means that the patients who do progress to the point of requiring ECMO support are likely acutely ill. This may account for the slight decrease in overall survival of ECMO patients from 67% in 2002 to the current 64% reported by ELSO (2,5). This survey examined the current state of North American ELSO centers’ ECMO systems and assessed the progress of new technology as it is integrated into common practice.
The large majority of ELSO centers in North America use roller pumps to generate arterial flow (82.5%) for ECMO systems (Figure 1). There is a growing use of centrifugal pumps and hollow fiber oxygenators, because 17.5% of the respondents reported routinely using centrifugal pumps and 23% reported using a hollow fiber oxygenator. Additionally, another 10% of respondents reported using centrifugal pumps and hollow fiber oxygenators only for cardiac patients and/or extracorporeal cardiopulmonary resuscitation. A further 5% of the respondents stated that they were in the process of transitioning to centrifugal pumps and hollow fiber oxygenators. This survey showed that roller pumps are still the standard but also showed a 12.5% decrease in use because the authors reported 95% use in 2002 (3). In 2004, Searles et al. (6) reported that 65% of the respondents of that survey reported using roller head pumps, 12% reported using centrifugal pumps, and 23% used both for routine use on neonatal cardiac patients. Groom et al. (7), in 2005, reported the same frequency of use for these devices. These two surveys dealt with cardiac neonatal ECMO, which may reflect a higher percentage of perfusionist involvement who may be more knowledgeable about emerging technology. This survey data suggests that perfusionists have led the way towards incorporation of newer centrifugal devices into the ECMO field.
The use of silicone membrane oxygenators (67%) also remains the standard for ECMO, although there was a 30% increase in hollow fiber oxygenator use from the survey in 2002 (3). This significant shift toward the use of hollow fiber oxygenators is likely because of several important factors. First, one PMP device has recently been approved by the US FDA for 6 hours of continuous use. Ten US respondents report routinely using the Maquet Quadrox D PMP oxygenator for neonatal ECMO, and one Canadian center reported using the Medos Hilite 800 LT PMP device. Centers using PMP devices made up 14% of the total group of respondents. Second, hollow fiber devices can be setup and primed very quickly for rapid-deployment life support. Third, hollow fiber devices, unlike silicone, can be coated with a bio-active material such as heparin. Micro-porous hollow fiber devices were used by 19% of the respondents. However, polypropylene hollow fiber material is suboptimal for use with ECMO because of its propensity to leak plasma over time. It is likely that the use of polypropylene micro-porous hollow fibers was prevalent because of the fact that these devices are offered in the United States in a pediatric size and with a heparin coating, whereas the only PMP device currently offered in the United States is an adult-sized device not yet available with a heparin coating. Similar survey results were reported in 2004 by cardiac ECMO centers, where 60% reported using the Medtronic 0800 silicone membrane and 40% using hollow fiber oxygenators (6).
The Medtronic ECMOtherm was the most frequently used heat exchanger (86%) by a large margin (Figure 2). A report by Darling et al. (8) showed that this heat exchanger worked well but that other commercially available devices had superior air trapping capabilities (8).
ALFs (5%) and bubble traps (BTs) (6%) are rarely used for ECMO. The use of these devices at low heparin levels can be risky and, judging from this survey response, few centers felt that they are advantageous. The use of these devices seems to be vestigial in nature, left over from older, outdated ECMO circuit designs. A survey from 2002 showed that an ALF or BT was used by 17% of centers (3). The incidence of their current use shows that they are slowly being phased out of use for ECMO applications.
The use of a venous bladder reservoir is widely reported in North American ELSO centers (85%; Figure 3). The traditional bladder is still the standard used at most centers. These bladders are the Medtronic R-14 (41%) and the Gish Biomedical (Gish Biomedical, Rancho Santa Margarita, CA) VRECMOB (25%). The Better-Bladder (Circulatory Technology, Oyster Bay, NY) BB14 was reported by 21% of responding centers. This is a dramatic increase from the 2002 survey, where 7% of responding centers reported using the BB14. The Better Bladder’s vertical design has been reported to have superior flow characteristics (9). It may be interesting to note that 9% of responding institutions use a homemade compliance chamber and that 4% of the roller head pump users reported using no compliance chamber at all.
The use of a bladder box to servo regulate pump flow was reported at 4%, which is significantly less than in earlier literature reports. In 1990, 87% of respondents used bladder box servo regulation (10). In 2002, 29% of responding North American ELSO centers used a bladder box for servo-regulation (3). This shift toward using pressure servo regulation may be because of the proliferation of newer pump consoles that offer electronic pressure monitoring. In the early days of ECMO, consoles with pressure transducing capabilities were not available, hence the advent of bladder boxes. Pressure servo regulation has the advantage of offering not only negative pressure regulation on the bladder but also positive pressure regulation to slow or stop the pump in the case of an inadvertent flow restriction.
Another major shift from the 2002 survey is that of the use of bubble detectors. Currently, 87.5% of the responding centers use a bubble detector. This is a notable increase in this safety device’s use from 65% in 2002 (3). This increase in the use of bubble detectors over the last few years may be because of greater availability of such devices because they are standard on new pump consoles. It may be interesting to note that, as in 2002, some centers who own pumps that can be configured with a bubble detector still choose not to use them (3).
The use of surface coatings on ECMO circuitry is becoming more widely used. Only 8% of responding centers used surface coatings routinely in 2002 (3). Currently, 44% of the responding institutions reported using some type of surface coating on their ECMO circuitry (Figure 4). A heparin coating was the most commonly reported use of a surface coating, with the Medtronic Carmeda Bioactive Surface being reported by 66% of the centers who use this type of technology. Two other companies also offer a heparin coating: Gish Biomedical’s GBS coating was reported by 6% and Maquet’s Bioline heparin coating was reported by 3% of responding centers. The Maquet Bioline technology is not currently available in the United States; the centers reporting its use were Canadian. Other, non–heparin-based surface coatings were also reportedly used, with Sorin’s Phisio coating and Maquet’s Safeline coating both being reported by 9% of the centers. Medtronic’s Trillium coating and Terumo’s X coating were reported by 6% and 3% of the centers, respectively. Although there is evidence showing advantages of using coated circuits during CPB (11), there are no data indicating that the use of a surface coating offsets its cost in the ECMO patient. However, the use of a heparin coating on ECMO surfaces may give the option of restricting systemic heparinization in certain situations such as post-cardiotomy patients who are experiencing significant post-operative bleeding, patients who develop a head bleed, or patients with congenital diaphragmatic hernia that can be repaired while on ECMO.
Universally, ACT was used to monitor the ECMO patient’s anticoagulation status. Hemochron (International Technidyne) devices were the most frequently cited ACT monitors currently being used. Figure 5 shows the different ACT machines used and compares them with 2002 findings. A report done in 1996 by Graves et al. (12) showed the majority of ECMO centers at that time using an ACT range of 180–220. The survey by Lawson et al. (3) in 2002 showed a similar practice, and this survey showed that these ACT ranges are still the standard (Figure 6). Several respondents (24%) reported using lower ACT ranges for cardiac patients and also reported using no systemic heparinization for some period of time while on ECMO for this patient population.
The use of in-line blood gas analyzers was common, with 64% of the centers reporting their use. The Terumo CDI devices dominate the North American ECMO market. The CDI 500/100 has been reported to be a reliable device (13) and was reported to be used at 71% of the centers using in-line monitoring. In-line venous hematocrit and saturation monitoring was reported by the other 29% of the centers. Figure 7 shows the frequency of different in-line monitors and compares them with the survey from 2002.
The demographics of ECMO specialists have changed over the past 18 years (Figure 8). In 1990, perfusionists (CCPs) were cited as comprising 14% of the total ECMO specialist population (10). In 1992, CCPs were listed as 2% of the total number of specialists (14). In 2002, CCPs were mentioned to make up 27% of the number of specialists. Currently, 22% of the responding centers reported CCPs acting in the role of ECMO specialist. Additionally, CCPs are involved with ECMO at another 32% of institutions where they setup, prime, troubleshoot, and consult on ECMO cases. This 54% total involvement of CCPs with ECMO is similar to the 2002 survey, indicating that this rate of involvement may have hit a plateau at centers that report to the ELSO registry (3). The 2004 survey by Searles et al. (6) reported that 79% of the centers responding used CCPs. This significant difference is likely because of differences in the survey populations. The survey of Searles et al. (6) polled pediatric cardiac centers, which may or may not have reported to ELSO. In the 1990 article by Allison et al. (10), the following statement is made: “Our personal experience favors the use of perfusionist expertise in every ECMO program.” This statement is just as pertinent today as it was 18 years ago.
In conclusion, neonatal ECMO equipment is continuing to change as technology improves and is adopted. New oxygenator material is becoming available to US ECMO centers, which are easier to setup and prime, can be coated with bioactive materials, and will not fail prematurely because of plasma leakage. A greater percentage of responding centers are using a hollow fiber oxygenator than in 2002. More centers use pressure servo regulation than mechanical, bladder box, servo regulation. More centers are using bubble detectors. There has been a 36% increase in the use of surface coatings for neonatal ECMO circuitry from 2002. An increased frequency of centrifugal pump use is being reported as well. This study shows that CCPs remain involved with ECMO and should provide leadership in adopting and improving ECMO circuitry.
The use of survey methodology can be corrupted by errors because of incomplete sampling or because of misinterpretation of survey questions by the respondents. The authors chose to poll North American neonatal ELSO centers so that a more representative comparison could be made to the survey done in 2002 of North American ELSO centers. This practice excludes those centers that perform ECMO but do not report to the ELSO Registry. A 78% response rate indicates that these data reported in this survey are representative of a large, diverse sampling of neonatal centers in North America (Figure 10).
Figure 10.
Geographic locations of responding ELSO centers.
The respondents were sent follow-up emails or phone calls to clarify any survey answers that did not seem appropriate. In this way, the authors hoped to minimize misinterpretation errors. This survey describes trends in current ECMO device use and team roles. It should not be used to set criteria for practice.
APPENDIX
Dear Sir,
I would like to take a few moments of your time to ask a few questions regarding neonatal ECMO equipment. Please take a few minutes to answer the following questions about the equipment that you use for neonatal ECMO at your institution.
Do you use a roller pump?
If so, what brand?
Do you use Super Tygon S-65-HL or S-95-E tubing in your raceway?
Do you use centrifugal pump?
If so, what brand?
Do you monitor venous line negative pressure?
Do you use a silicone membrane?
What brand?
What heat exchanger do you use?
Do you use a hollow fiber membrane?
If so, what brand?
Do you use in-line blood gas monitoring?
If so, what brand?
Do you use a bubble trap?
If so, what brand?
Do you use an arterial line filter?
Is so, what brand?
Do you use a bladder?
If so, what brand?
If not, how do you servo regulate?
Do you use a bladder box?
If so, what brand?
Do you use pressure servo regulation?
If so, how?
Where? (pre-membrane/post-membrane/both)?
Do you use an air bubble detector?
If so, what brand?
Does your ECMO circuit have a surface coating?
If so, what kind/brand?
Do you ever perform ECMO without administering heparin?
What device do you use to monitor ACTs?
What ACT range do you normally use?
Who sets-up/primes and initiates your ECMO?
RNs:
RRTs:
Perfusionists:
Perfusion assistants:
Other:
Who sits the ECMO shifts 24/7?
RNs:
RRTs:
Perfusionists:
Perfusion assistants:
Other:
Who performs daily rounding and troubleshoots.
RNs:
RRTs:
Perfusionists:
Perfusion assistants:
Other:
REFERENCES
- 1.Hill J, Deleval M, Fallat R, et al. Acute respiratory insufficiency. Treatment with prolonged extracorporeal oxygenation. J Thorac Cardiovasc Surg. 1972;64:551–62. [PubMed] [Google Scholar]
- 2.ECMO Registry. Report of the Extracorporeal Life Support Organization. Ann Arbor, MI: Extracorporeal Life Support Organization; 2008. [Google Scholar]
- 3.Lawson DS, Walczak R, Lawson AF, et al. North American extra-corporeal membrane devices: 2002 survey results. J Extra Corpor Technol. 2004;36:16–21. [PubMed] [Google Scholar]
- 4.Bahrami KR, Van Meurs KP.. ECMO for neonatal respiratory failure. Semin Perinatol. 2005;29:15–23. [DOI] [PubMed] [Google Scholar]
- 5.ECMO Registry. Report of the Extracorporeal Life Support Organization. Ann Arbor, MI: Extracorporeal Life Support Organization; 2002. [Google Scholar]
- 6.Searles B, Gunst G, Terry B, Melchior R, Darling E.. 2004 Survey of ECMO in the neonate after open heart surgery: circuitry and team roles. J Extra Corpor Technol. 2005;37:351–4. [PMC free article] [PubMed] [Google Scholar]
- 7.Groom RC, Froebe S, Martin J, et al. Update on pediatric perfusion practice in North America: 2005 survey. J Extra Corpor Technol. 2005;37:343–50. [PMC free article] [PubMed] [Google Scholar]
- 8.Darling E, King C, Nanry K, Smigla G, Shearer I.. Comparison of four stainless steel heat exchangers for neonatal ECMO applications. J Extra Corpor Technol. 1994;26:68–73. [PubMed] [Google Scholar]
- 9.Tamari Y, Lee-Sensiba K, King S, Hall M.. An improved bladder for pump control during ECMO procedures. J Extra Corpor Technol. 1999;31:84–90. [PubMed] [Google Scholar]
- 10.Allison P, Kurusz M, Graves D, Zwischenberger J.. Devices and monitoring during neonatal ECMO: survey results. Perfusion. 1990;5:193–201. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds HN, Cottingham C, Mccunn M, Habashi NM, Scalea TM.. Extracorporeal lung support in a patient with traumatic brain injury: the benefit of heparin-bonded circuitry. Perfusion. 1999;14:489–93. [DOI] [PubMed] [Google Scholar]
- 12.Graves D, Chernin J, Kurusz M, Zwischenberger J.. Anticoagulation practices during neonatal extracorporeal membrane oxygenation: survey results. Perfusion. 1996;11:461–6. [DOI] [PubMed] [Google Scholar]
- 13.Fried D, Leo J, Mattioni G, et al. CDI Blood Parameter Monitoring System 500: a new tool for the clinical perfusionist. J Extra Corpor Technol. 2000;32:25–30. [PubMed] [Google Scholar]
- 14.Odell R, Erickson R, Mcewan R.. Identification and certification of extracorporeal membrane oxygenation specialists in the United States. ASAIO J. 1992;38:858–61. [PubMed] [Google Scholar]