Abstract:
Wound healing impairment in the leg after removal of the saphenous vein within the framework of a coronary artery bypass graft (CABG) operation represents a clinically significant problem. Patients suffer from this complication, and treatment of the wounds is costly in terms of both time and money. No method is known to date that reliably prevents postoperative wound healing disturbances. The effect of autologous platelet gel to stimulate wound healing is known from various medical disciplines. Within a prospective randomized study, we wanted to determine whether intraoperative use of autologous platelet gel on the leg during a CABG operation could reduce the incidence of postoperative wound healing disturbances. The application group (AG) included 35 patients and was compared to a control group (CG) that also had 35 patients. The platelet gel, as well as the thrombin required to activate the platelets, was prepared from autologous patient blood during the operation. Validation of the platelet gel comprised measurement of the growth factors platelet–derived growth factor AB (PDGF AB) and epidermal growth factor (EGF), as well as the thrombocyte and leukocyte counts. Wound healing was photographically documented after surgery, and the patients were contacted by telephone on day 50 after surgery to obtain information on wound healing status. After cell separation, the platelet count was 1616 ± 845/μL, which is higher than in whole blood by a factor of 7.1 ± 2.0, with a platelet yield of 47.0% ± 13.2%. The PDGF AB concentration after activation of the platelets was raised by a median factor of 158 and EGF by a median factor of 64 compared with whole blood. During the primary clinical stay, no statistically significant differences were recorded in the number of hematomas, postoperative leg swelling, or pain level. Large-area hematomas were less frequent in the application group (AG, 29.4% vs. CG, 60%, p = .007). In the follow-up 51 ± 9 days after surgery, 17.6% (6/34) of the patients from the AG and 31.4% (11/35) of the patients from the CG showed leg wound healing disturbances (p = .184). Using the cell separation system, a biological product that contains high concentrations of platelets, leukocytes, and growth factors can be prepared reproducibly. Despite optimum application of the autologous platelet gel to the wound, no clinically relevant differences were found between the groups, either during the primary clinic stay or in the follow-up period.
Keywords: platelet-rich plasma, coronary artery bypass graft, saphenectomy, wound healing
The vena saphena magna is used in cardiac surgery to obtain autologous bypass material for bypassing of coronary stenoses as a standard procedure. Depending on the number of planned bypass anastomoses, it may be necessary to incise the thigh and lower leg along their entire length to remove the saphenous vein, potentially resulting in a wound length exceeding 1 m. Postoperative healing of such large wound areas is particularly critical in patients with additional risk factors for delayed wound healing. Such factors include, for example, insulin-dependent diabetes mellitus, obesity, advanced age, and female sex (1,2). The incidence of wound healing disturbances after open saphenectomy is reported in the literature as between 1.5% and 24% (3,4). In view of the frequency of this complication and the suffering it causes in patients, it is justified to search for preventive measures that lead to a clinically relevant reduction of postoperative wound healing impairments. In the human body, platelets play a central role in hemostasis. They also stimulate wound healing by releasing bioactive proteins and growth factors (5). Attempts have been made in a number of different medical disciplines (5–8) to stimulate wound healing in soft tissues, as well as bony regeneration after surgical procedures, by means of direct application of concentrated, activated autologous thrombocytes. The theory on which this approach is based assumes that platelet growth factors exert mitogenic and chemotactic effects on other cells, resulting in a higher rate of cell division and migration of cells into the wound area and thus in improved wound healing (9). In the past 5 years, the industry has marketed several devices that allow intraoperative cell separation to obtain platelet-rich plasma (PRP). This makes it possible to prepare a platelet concentrate from the patient’s own blood in the surgical theater and to apply it directly to the wound surface. Activation of the platelets results in release of numerous cytokines with bioactive effects on wound healing. This study investigates whether autologous platelet gel of reproducible quality can be obtained using a commercially available cell separation system and to what extent the gel is able to reduce the frequency of postoperative wound healing complications.
MATERIALS AND METHODS
Patient Selection
The study protocol was approved by the University Ethics Committee responsible for the hospital. All of the patients signed an informed consent to participate in the study discussed with them by a physician. The prospective, block-randomized study included patients for whom coronary artery bypass graft (CABG) surgery using the saphenous vein was planned. The patients were assigned to groups according to a randomization list, i.e., either to the application group (AG, n = 35) or the control group (CG, n = 35). Neither the patients nor the physicians who assessed postoperative wound healing were informed as to which group the patients had been assigned to. Additionally, the following laboratory parameters were defined as inclusion criteria: platelet count > 130 × 109/L; leukocyte count < 10 × 109/L; C-reactive protein (CRP) < 2.0 mg/dL; and hemoglobin (Hb) > 10 g/dL.
Blood Sampling
One hundred seventy grams of whole blood was taken from the patients in the AG during induction of anesthesia through a routinely used 9-Fr access port in the jugular vein. This blood was put into a blood bag containing 23 mL of ACD-A stabilizer. The total volume was 180 mL.
Cell Separation
Using a fully automated cell separation system (Angel; Dideco, Mirandola, Italy), the whole blood taken from the patients was separated by centrifuge into three components: platelet-poor plasma (PPP), thrombocyte-rich plasma (TRP), and erythrocytes. The TRP thus obtained was re-diluted with PPP to obtain a total volume of 12 mL. This final product was designated as PRP. Both the separated erythrocytes and the PPP left over from preparation were re-transfused to the patients through the heart-lung machine.
Thrombin Preparations
Autologous thrombin was used to activate the platelets. A set of sterile single-use products (Activat; Dideco) was used for this purpose. Twelve milliliters of the PPP from cell separation was drawn into a transfer syringe. A solution of calcium chloride and ethanol was injected in this syringe. Ethanol in a concentration of 17% by volume denatures certain proteins, including antithrombin III. The plasma, now re-calcified, was transferred into a process syringe containing a glass microbead powder. After 30–45 minutes of reaction time, the remaining thrombin-rich serum was pressed out manually through a filter medium integrated in the syringe. Using this method, 3–5mL of thrombin serum was obtained, with an activity level of at least 50 international units according to the manufacturer.
Sampling Times
Samples were taken at various times to determine the cell counts and growth factor concentrations. The sampling times were defined as follows:
P0: preoperative values on admission day before surgery.
P1: whole blood sample, taken while inducing anesthesia, without ACD-A
P2: whole blood sample, taken before cell separation to determine the initial plasma growth factor concentration. Immediately after it was collected, the blood sample was centrifuged for 20 minutes at 6000 rpm and 2000g, whereupon the plasmatic aliquot was preserved by freezing at −24°C until final analysis.
P3: This sample was taken from the PRP for cell count determination.
P4: This sample was used to determine concentrations of the growth factors after activation of the platelets. This was done by adding 100 μL autologous thrombin to 1 mL PRP and allowing them to stand for 90 minutes at room temperature. This was followed by centrifugation for 20 minutes at 6000 rpm and 2000g. The aliquot was preserved by freezing at −24°C until final analysis.
Cell Counts
Cell counting in samples P0 and P2 was carried out with a standard Hematology Analyzer (Coulter LH 750; Beckman Coulter, Krefeld, Germany). The P2 samples were also measured at various dilution levels to increase measurement accuracy.
Growth Factor Assay
The concentrations of platelet–derived growth factor AB (PDGF AB) and epidermal growth factor (EGF) were determined using the sandwich ELISA technique. This technique uses monoclonal and polyclonal antibodies (Quantikine; R&D Systems, Wiesbaden, Germany) to bind the growth factors, followed by photometric concentration measurement at a light wavelength of 450 nm.
Application of the PRP to the Wound Surface
After removal of the saphenous vein, the PRP together with the autologous thrombin, in a ratio of 10:1, were sprayed directly onto the wound surface. This was done with a spray applicator (Duploject Spray Set; Baxter, Vienna, Austria) that distributed the two components mixed to a fine mist with sterile compressed air over the entire wound area. Application was made in several layers during wound closure. Within a few seconds of application, a gel-like layer formed on the wound, which is designated as autologous platelet gel. Figure 1 shows application of PRP and thrombin to the partially adapted wound surface. The autologous platelet gel is visible as a shiny layer on the skin in the proximal portion of the lower leg.
Figure 1.
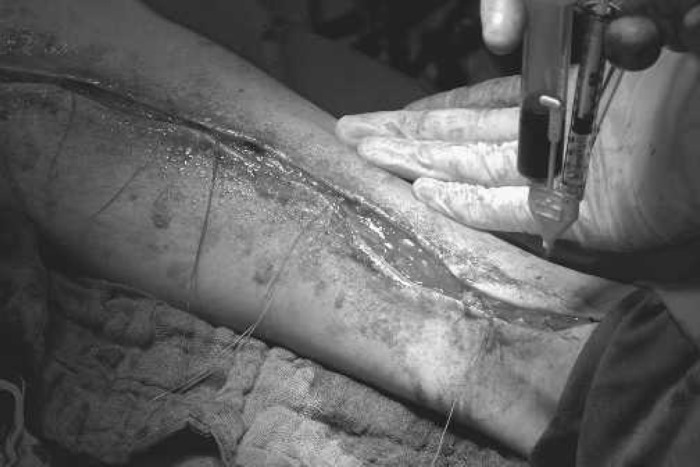
Application of PRP and thrombin after saphenectomy.
Postoperative Assessment of Wound Healing and Follow-up on Day 50 after Surgery
During the clinical stay, numerous digital photographs of the legs were taken over the postoperative course (EOS 10D; Canon, Krefeld, Germany), and a specially designed wound healing evaluation protocol was filled out by a physician. The following criteria were taken into account in the evaluation: size and number of hematomas; swelling of the extremity; number of non-adapted wound areas; pain perception on a visual pain scale; presence of necrotic areas; reddening of wound margins; wound secretion.
On the basis of these criteria, each wound was assessed as normal or abnormal and as to whether a wound healing impairment was already present.
On day 50 after surgery, the patients were contacted by telephone and asked several questions regarding the wound healing on the leg.
Statistical Evaluations
The program Statistica 8.0 (Statsoft Europe, Hamburg, Germany) was used for the evaluations. Statistical significance was assumed for p < .05. The calculations were based on the chi-square test for independent groups and on the t test for independent and paired random samples.
Calculation of the Platelet and Leukocyte Yield
The cell separation procedure cannot completely separate all of the cells contained in the initial volume. The “yield” is therefore defined as the ratio of the total cell count in PRP to the total cell count in whole blood.
| VPRP | = volume of PRP |
| nT,PRP | = thrombocyte count in PRP |
| VE | = volume of whole blood drawn from the patient |
| nT,E | = thrombocyte count in whole blood |
RESULTS
The preoperative demographic data for these two patient groups did not show statistically significant differences for any of the parameters (Table 1). However, the AG included a considerably larger proportion of insulin-dependent diabetics.
Table 1.
Group characteristics.
| Variable | Unit | AG (n = 35) | CG (n = 35) | p | Significance |
|---|---|---|---|---|---|
| Age | years | 65.3 ± 8.2 | 66.6 ± 9.7 | 0.541 | NS |
| BMI | kg/m2 | 31.1 ± 5.7 | 29.3 ± 4.2 | 0.134 | NS |
| IDDM | % | 28.6% | 11.4% | 0.073 | NS |
| Sex female | % | 25.7% | 25.7% | 1.00 | NS |
| nT,P | ×109/L | 248 ± 80 | 227 ± 58 | 0.193 | NS |
| nL,P | ×109/L | 7.6 ± 2.0 | 7.3 ± 1.9 | 0.513 | NS |
| CRP | mg/dL | 1.2 ± 1.4 | 1.6 ± 2.8 | 0.415 | NS |
| Quick | % | 94 ± 13 | 95 ± 8 | 0.753 | NS |
| PTT | s | 35 ± 16 | 42 ± 22 | 0.174 | NS |
| HbP | g/dL | 14.0 ± 1.4 | 13.8 ± 1.9 | 0.773 | NS |
| HkP | L/L | 0.41 ± 0.04 | 0.41 ± 0.05 | 1.00 | NS |
Values are means ± SD.
BMI, body mass index; IDDM, insulin-dependent diabetes mellitus; nT,p, preoperative thrombocyte count; nL,p, preoperative leukocyte count; CRP; C-reactive protein; PTT, partial thromboplastin time; Hbp, preoperative hemoglobin; Hkp, preoperative hematocrit; NS, not significant.
Validation of the Autologous Platelet Gel
Table 2 lists the thrombocyte and leukocyte counts before and after cell separation.
Table 2.
Thrombocyte and leukocyte counts: yield and increase over baseline.
| Unit | No. of Probes | Mean ± SD | p | |
|---|---|---|---|---|
| Thrombocytes | ||||
| nT,E | ×109/L | 35 | 220 ± 72 | <.005 |
| nT,PRP | ×109/L | 33 | 1613 ± 845 | significant |
| Thrombocyte yield | % | 33 | 47.0 ± 13.2 | |
| Thrombocyte increase over preoperative baseline | 1 | 33 | 7.1 ± 2.0 | |
| Leukocytes | ||||
| nL,E | ×109/L | 35 | 5.6 ± 1.6 | <.005 |
| nL,PRP | ×109/L | 33 | 26.8 ± 13.7 | significant |
| Leukocyte yield | % | 33 | 31.8 ± 14.5 | |
| Leukocyte increase over preoperative baseline | 1 | 33 | 4.8 ± 2.2 |
nT,E, thrombocyte count in probe P1; nT,PRP, thrombocyte count in probe P3; nL,E, leukocyte count in probe P1; nL,PRP, leukocyte count in probe P3.
As was to be expected, both cell types were present in the PRP in significantly higher concentrations. Less than one half of the platelets and about one third of the leukocytes were separated out of the whole blood.
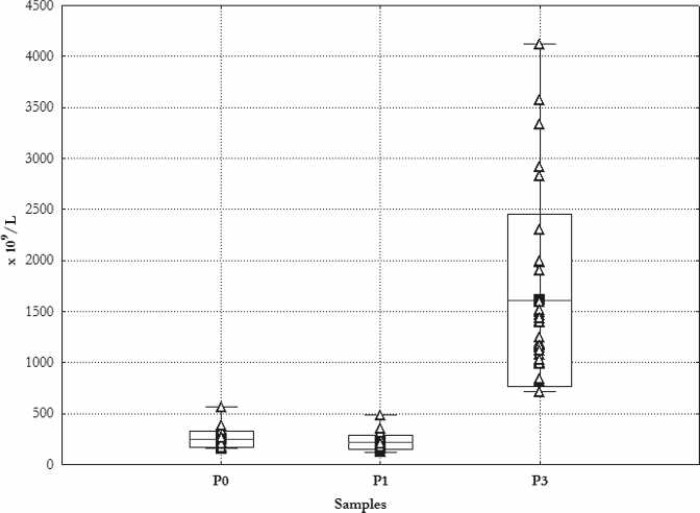
Figure 2 shows the results of the individual platelet counts. The highest measured platelet cell count was 4120 × 109/L, corresponding to concentration by a factor of 8.5 at a yield level of 57%. The highest single measured yield was 81% and the lowest was 27%.
Figure 2.
Thrombocyte count. Mean ± SD. _Min, Max, Δ single measurements. P1 preoperative day; P2 during induction of anesthesia; P3 cell count in PRP.
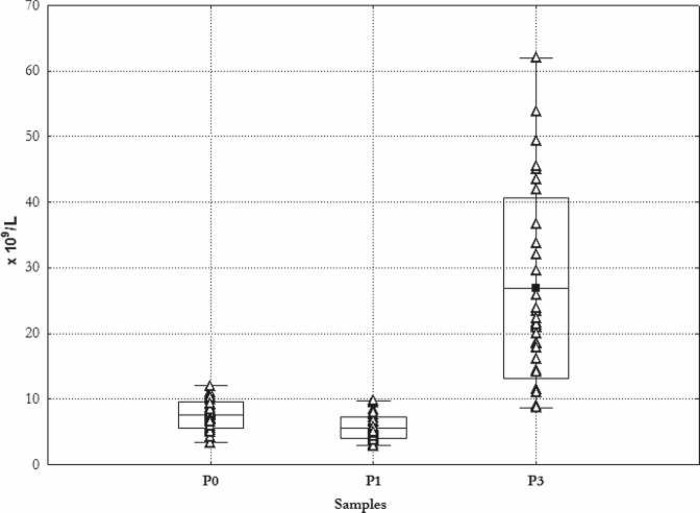
Figure 3 shows the results of the individual measurements of leukocytes. The range of cell count results measured in the PRP was from 8.7 × 109/L to 62 × 109/L, whereby the starting value in all cases was ≤ 10 × 109/L as stipulated by the inclusion criteria.
Figure 3.
Leukocyte count. Mean ± SD. _Min, –Max, Δ single measurements. P1 preoperative day; P2 during induction of anesthesia; P3 cell count in PRP.
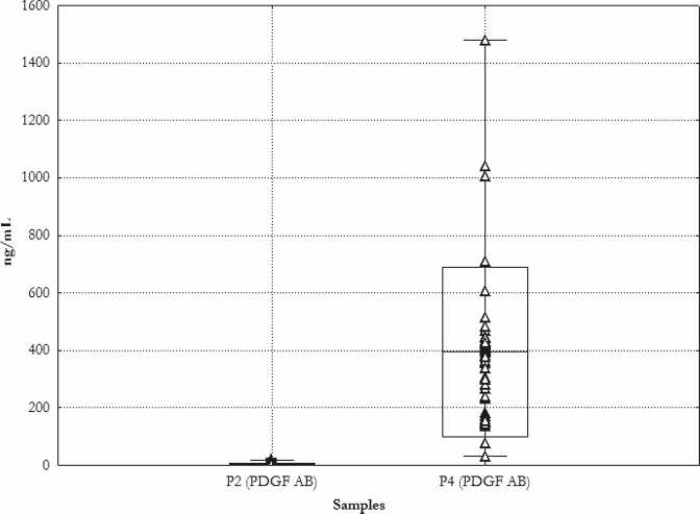
The average PDGF AB concentration in the whole blood before cell separation was 4.1 ± 4.7 ng/mL, with a range of .7–20 ng/mL. After a 90-minute activation of the thrombocytes with thrombin, average PDGF AB concentrations of 394.2 ± 295.5 ng/mL were measured, with a range of 31.9–1480 ng/mL. Figure 4 shows the results of the individual measurements.
Figure 4.
Concentration of PDGF AB. Mean ± SD. _Min, –Max, Δ single measurements. P2 during induction of anesthesia; P4 probe of thrombocyte gel.
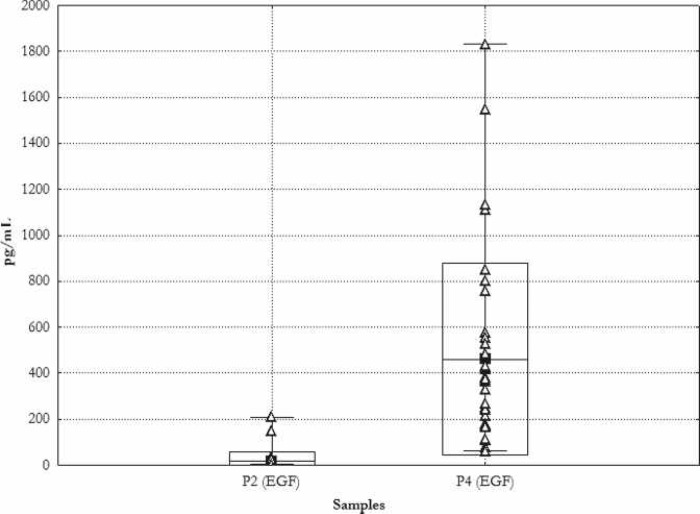
The concentration of the growth factor EGF in sample P4 was higher than in the sample P2 by a median factor of 64. (P2: 16.6 ± 41.4 pg/mL; median, 4.0 pg/mL vs. P4: 461.3 ± 417.2 pg/mL; median, 345.5 pg/mL). Figure 5 is a graphic presentation of the individual measured values along with the relevant average, SD, and range.
Figure 5.
Concentration of EGF. Mean ± SD. _Min, –Max, Δ single measurements. P2 during induction of anesthesia; P4 probe of thrombocyte gel.
Evaluation of Postoperative Wound Healing
In total, 1454 photographs were taken of the legs of the 70 patients, i.e., ∼21 photographs per patient during hospitalization. One study participant from the AG died of cardiac causes on day 2 after surgery. Twenty-two of 34 patients in the AG (64.7%), and 16 of 35 patients from the CG (45.7%) showed completely normal, i.e., unproblematic, courses of leg wound healing during their hospital stay.
Six of 34 patients from the AG and 5 of 35 patients from the CG showed an abnormal course of leg wound healing during their hospital stay and developed wound healing disturbances by day 50 after surgery.
In one patient from each study group, wound healing impairments occurred during hospitalization in the form of suture dehiscences, increased secretion, and necroses.
In the CG, 21/35 patients developed large-area hematomas, which was true of only 10/34 (p = .007) patients in the AG. There were no differences between the groups regarding the number of hematomas (CG, 12/34; AG, 15/35; p = .683).
Pronounced development of extremity edemas was observed in 5/34 patients in the AG and 8/35 patients in the CG, with no significant difference (p = .683).
Postoperative pain levels, assessed using a horizontal visual analog scale with a range of 0–10, were very low in both groups [mean pain value ± SD (range): AG, .083 ± .69 (0–7); CG, .11 ± 0.59 (0–5)]. The patients with clinically abnormal wound healing courses also reported no significant leg pains.
Follow-up on Day 50 after Surgery
A follow-up examination was carried out on an average of 52 ± 9 days after surgery. Twenty-eight of 34 patients from the AG (82.3%) were satisfied with both wound healing and the resulting scar. On the other hand, 6/34 patients showed wound healing disturbances. In the CG, 24/35 patients (68.6%) were satisfied with the wound healing process, whereas 3 patients were not satisfied with the scar. Manifest wound healing impairments were observed in 11/35 patients (Figure 6).
Figure 6.
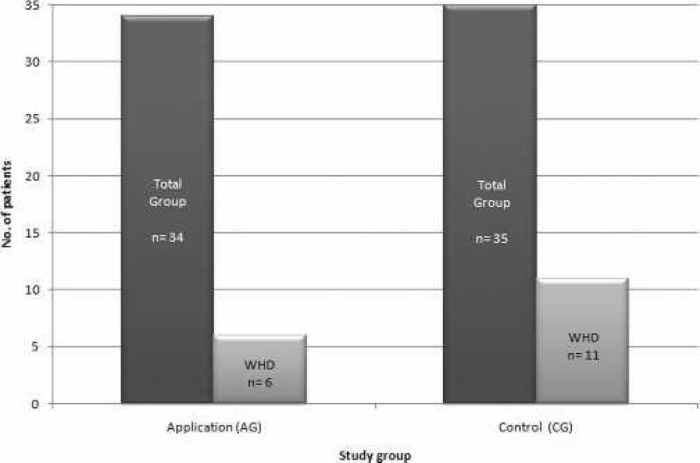
Wound healing disturbances in the follow-up period. AG: 6 of 34 patients (17.6%). CG: 35 patients (31.4%).
Table 3 lists the details of the wound healing impairments observed in both groups.
Table 3.
Details of observed wound healing disturbances in the follow-up period.
| Study No. | Study Group | Postoperative Day | Findings |
|---|---|---|---|
| 38 | CG | 50 | Five open sites, 1 cm each, distributed over the entire leg |
| 45 | CG | 55 | Three open sites on leg, daily wound toilet and bandage change |
| 48 | CG | 53 | Wound exudates, is reddened, and does not heal |
| 52 | CG | 56 | Wound exudates and hurts, necrotic site already recognizable during primary clinical stay |
| 54 | CG | 50 | Wound inflamed, bandage up to knee |
| 11 | CG | 70 | Three sites inflamed, requiring medical care for longer period, wound toilet now taken care of by patient |
| 22 | CG | 54 | Wound on right leg at ankle level shows dehiscence |
| 12 | CG | 67 | Dehiscence on right lower leg with exudation of serous secretion, no signs of inflammation |
| 31 | CG | 78 | Open sites around ankle, covered with bandage, development of pronounced numbness in this area |
| 28 | CG | 45 | Wound dehiscence |
| 59 | CG | 50 | Wound dehiscence at ankle 1.5 cm long, medical treatment |
| 37 | AG | 57 | Four dehiscent sites distributed over leg. Wounds exudate and hurt. |
| 47 | AG | 53 | Wound healing impairment, 10 cm long, 3 cm wide, repeated inpatient treatment |
| 5 | AG | 30 | Readmission to hospital due to wound healing impairment on thorax and leg. 1× drainage of purulent secretion from leg. Antibiotic treatment |
| 18 | AG | 55 | Inflammatory signs with reddening and swelling after suture removal during primary clinical stay, in further course development of a wound healing impairment with medical treatment by physician |
| 1 | AG | 28 | Readmission to hospital caused by wound dehiscence. Daily Octenisept lavages and bandage changes |
| 63 | AG | 48 | At knee joint open site with 2 cm diameter, treated in rehabilitation and in internal university outpatient practice |
The evaluations show that all of the patients in the AG who had developed abnormalities during hospitalization (n = 6) also developed wound healing disturbances as shown in the follow-up. No other patients joined this group. The situation was different in the CG. In this group, n = 6 patients who had showed a normal wound healing course in the clinical setting developed wound healing impairments in the further postoperative course.
DISCUSSION
Wound healing disturbances on the legs after open saphenectomy in CABG patients represent a medical problem the impact of which is underestimated. They interfere with convalescence and require long-term treatment. They also frequently lead to unsatisfactory cosmetic results. In this study, we attempted to determine whether the incidence of postoperative wound healing impairments can be reduced by application of autologous platelet gel to the leg wound surface. Using the available cell separation system and the corresponding set used to obtain autologous thrombin, it was possible to prepare a PRP containing highly concentrated platelets and leukocytes in which, after activation with thrombin, high concentrations of the growth factors PDGF AB and EGF were measurable. The platelet yield was within the range obtained using other comparable systems (10). The autologous platelet gel was applied in several layers to completely cover the wound surfaces. Preparation and application of the PRP were thus both standardized and reproducible in all AG patients. There were, however, further intraoperative factors that could potentially influence the efficacy of the gel. These include preparation of the wound surface by means of fine surgical hemostasis. Any remaining hemorrhage source results in washing of the platelet gel out of the wound surface. The full heparinization required during cardiac surgery may have reduced the level of plasmatic coagulation, a process needed to form a platelet gel with a solid consistency. Here as well, the amount of time the gel remains on the wound surface is shortened, resulting in lower growth factor concentrations.
In the early postoperative course, we did not observe any clinically relevant differences between the two groups. On the other hand, we did observe fewer large-area hematomas in the AG, corroborating the findings reported in a paper by Englert et al. (11).
In the later postoperative course, the frequency of wound healing disturbances in the AG was lower, but the level of statistical significance that would qualify this observation as a manifest group difference was not reached. A notable observation in the CG was occurrence of wound healing impairments in six patients after they were discharged from hospital, despite normal healing courses during their hospital stays. Potential causal factors in these cases do not derive from disturbances of the wound healing phases but rather from local factors (12) such as mechanical wound traumatization or pathogen invasion after removal of the cutaneous sutures.
The effect of autologous platelet gel, in particular on the healing process in chronic wounds, has been confirmed in numerous publications (13–15). In our application model, in which the aim was to stimulate wound healing in a fresh surgical wound, the method did not show the desired effect. The confounding factors preventing recognition of a clinical benefit are difficult to eliminate.
REFERENCES
- 1.Allen KB, Heimansohn DA, Robison RJ, et al. . Risk factors for leg wound complications following endoscopic versus traditional saphenous vein harvesting. Heart Surg Forum. 2000;3:325–30. [PubMed] [Google Scholar]
- 2.Slaughter MS, Olson MM, Lee JT Jr, Ward HB.. A fifteen-year wound surveillance study after coronary artery bypass. Ann Thorac Surg. 1993;56:1063–8. [DOI] [PubMed] [Google Scholar]
- 3.Dusterhoft V, Bauer M, Buz S, Schaumann B, Hetzer R.. Wound-healing disturbances after vein harvesting for CABG: A randomized trial to compare the minimally invasive direct vision and traditional approaches. Ann Thorac Surg. 2001;72:2038–43. [DOI] [PubMed] [Google Scholar]
- 4.Avanzini MH, Reichert J, Fuchs U, Laczkovics A.. Neue aggressive Therapiestrategien zur Vermeidung von Wundheilungsstörungen bei DiabetikerInnen nach kardiochirurgischen Eingriffen. European Surgery-Acta Chirurgica Austriaca. 2001;3:138–42. [Google Scholar]
- 5.Eppley BL, Woodell JE, Higgins J.. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004;114:1502–8. [DOI] [PubMed] [Google Scholar]
- 6.Carlson NE, Roach RB Jr.. Platelet-rich plasma: clinical applications in dentistry. J Am Dent Assoc. 2002;133:1383–6. [DOI] [PubMed] [Google Scholar]
- 7.Mogan C, Larson DF.. Rationale of platelet gel to augment adaptive remodeling of the injured heart. J Extra Corpor Technol. 2004;36:191–6. [PubMed] [Google Scholar]
- 8.Rozman P, Bolta Z.. Use of platelet growth factors in treating wounds and soft-tissue injuries. Acta Dermatovenerol Alp Panonica Adriat. 2007;16:156–65. [PubMed] [Google Scholar]
- 9.Anitua E, Andia I, Ardanza B, Nurden P, Nurden AT.. Autologous platelets as a source of proteins for healing and tissue regeneration. Thromb Haemost. 2004;91:4–15. [DOI] [PubMed] [Google Scholar]
- 10.Everts PA, Brown Mahoney C, Hoffmann JJ, et al. . Platelet-rich plasma preparation using three devices: implications for platelet activation and platelet growth factor release. Growth Factors. 2006;24:165–71. [DOI] [PubMed] [Google Scholar]
- 11.Englert SJ, Estep TH, Ellis-Stoll CC.. Autologous platelet gel applications during cardiovascular surgery: effect on wound healing. J Extra Corpor Technol. 2005;37:148–52. [PMC free article] [PubMed] [Google Scholar]
- 12.Voggenreiter G, Dold C.. Wundtherapie. Stuttgart: Georg Thieme Verlag; 2004. [Google Scholar]
- 13.Mazzucco L, Medici D, Serra M, et al. . The use of autologous platelet gel to treat difficult-to-heal wounds: a pilot study. Transfusion. 2004;44:1013–8. [DOI] [PubMed] [Google Scholar]
- 14.Crovetti G, Martinelli G, Issi M, et al. . Platelet gel for healing cutaneous chronic wounds. Transfus Apher Sci. 2004;30:145–51. [DOI] [PubMed] [Google Scholar]
- 15.Driver VR, Hanft J, Fylling CP, Beriou JM.. Autologel Diabetic Foot Ulcer Study: A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manage. 2006;52:68–70, 72, 74 passim. [PubMed] [Google Scholar]