Abstract:
Aged individuals have impaired diastolic relaxation–lusitropic function. Dobutamine, a selective B1-adrenergic agonist, is used to augment systolic cardiac function at the termination of cardiopulmonary bypass (CPB). However, our question is whether dobutamine will also enhance the lusitropic function in the aged individual. The myocyte mechanism for the rate of ventricular relaxation is dependent on the velocity of calcium removal from the myocyte contractile elements by sarcoplasmic reticulum (SR) Ca2+–ATPase (SERCA2a), which is regulated by an inhibitory protein, phospholamban (PLB). Ventricular tissues harvested from young (4 month) and aged (20 months) mice were analyzed to compare the protein levels of SERCA2a and PLB with immunoblot and gene expression for PLB with reverse-transcriptase-polymerase chain reaction. The molecular analyses were compared with in vivo left ventricular function in the young and old mice before and during an intravenous infusion of dobutamine (5 μg/kg/min). The SERCA2a levels were not different between the groups; however, there was a 2-fold increase in PLB in the aged group compared with the young group (p < .05). The gene expression for PLB was increased by 5-fold in the aged group compared with the young group (p < .01). There were significant differences between the young and aged groups related to the lusitropic parameters, tau (τ) and dP/dtmin, and dobutamine infusion increased these parameters in the aged group to that of the young group. This report supports the concept that altered PLB levels correspond with the respective lusitropic function and that dobutamine administration in the aged group increased lusitropic function that was comparable with the young group. Because the patient population requiring CPB is aging, these data suggest that the use of dobutamine at the terminal phase of CPB is warranted to increase systolic and diastolic function.
Keywords: phospholamban, sarcoplasmic reticulum Ca2+−ATPase, aging, diastole, β-receptor
Weaning a patient from cardiopulmonary bypass (CPB) is dependent on restoration of cardiac function sufficiently to achieve a physiologic cardiac output. However, with increased age, there is a proportional physiologic decline in left ventricular diastolic (lusitropic) function (1,2). This diastolic dysfunction contributes to a reduction of ventricular filling and thus resulting in an inadequate cardiac output. Chronic diastolic dysfunction can progress into diastolic heart failure, defined as a decline in cardiac output with normal ventricular ejection fraction (>50%) (3), of which aging and hypertension are primary causes (4). Because aging and other risk factors such as hypertension can predispose the patient to diastolic dysfunction and diastolic heart failure, it is therefore important to understand the management of these patients in the context of inotropic agent administration used to support patients at the terminal phase of CPB.
The age-associated diastolic dysfunction in the isovolumic relaxation phase of diastole can be a result in the alteration of the calcium cycling proteins coupled with calcium sequestration into the sarcoplasmic reticulum (SR). The focus of this report is that, with increasing age, there is an alteration of the calcium cycling proteins. SR Ca2+–ATPase (SERCA2a), which is regulated by an inhibitory protein, phospholamban (PLB), controls the rate of isovolumic relaxation during diastole (5). SERCA2a serves as an ATP-dependent pump that unloads cytosolic calcium from the myocyte contractile fibers and facilitates the calcium sequestration in the sarcoplasmic reticulum. PLB is an inhibitory protein to the SERCA2a function in the non-phosphorylated state, and on phosphorylation of PLB by cAMP-dependent protein kinase (cAMP-PKA), the inhibition of SERCA2a function is relieved (6). The increase in cAMP-PKA is typically a result of increase β-receptor stimulation. Therefore, our research focus is to determine whether age-associated diastolic dysfunction can be reversed with dobutamine infusion, a selective β1-adrenergic agonist used during open heart surgery.
Much of the reported research on the pathway described above to date has been conducted in isolated heart or papillary muscle preparations and with β-agonists other than dobutamine. The report contained herein shows there are alterations of cardiac PLB gene expression and protein concentrations and that may account for the in vivo dysfunction of the isovolumic phase of diastole in the aged. Second, the in vivo administration of dobutamine, clinically used β-agonists reverses the lusitropic dysfunction in the aged animals; however, it has minimal effect in the younger animals.
MATERIALS AND METHODS
Study Design
To determine changes in SERCA2a and PLB protein levels and gene expression during aging, protein and RNA were extracted from the ventricles of young (4 months old) and aged mice (20 months old). Immunoblot analysis was used to quantify protein levels of SERCA2a and PLB, and reverse transcriptase-polymerase chain reaction (RTPCR) was used for the gene expression of PLB. Hemodynamic data were collected in vivo using the Millar Conductance Catheter System (Millar Corporation, Houston, TX) with six young mice (4 months old) and six aged mice (20 months old). Dobutamine was infused at a rate of 5 μg/kg/min, and hemodynamic data were collected at baseline and after dobutamine infusion.
Mice
Female C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME). During the study, the mice were maintained in the facility on an NIH-31-modified mouse sterilized diet (mouse diet 7001; Teklad, Madison, WI) and water ad libitum. This study conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Immunoblot
Hearts were individually homogenized at 4°C in a 1:5 weight per volume ratio using a protein extraction buffer consisting of 0.05 mol/L TBS (pH 8.0), 0.5 mmol/L EDTA, 26% vol/vol protease inhibitor cocktail (Sigma, St. Louis, MO), 13% vol/vol phosphatase inhibitor cocktail (Sigma), 1 mmol/L sodium orthovanadate (Calbiochem, San Diego, CA), 0.128 mmol/L α-naphthyl acid phosphate (Calbiochem), and 300 mmol/L sucrose (Sigma). Homogenate was spun at 3000 rpm at 4°C for 30 minutes, and the supernatant was collected. After addition of 100 μL of 10% SDS (Gibco, Carlsbad, CA), the supernatant was agitated for 30 minutes at room temperature. Samples were re-cooled on ice for 10 minutes and spun at 4°C at 15,000 rpm for 25 minutes, and the supernatant was collected. An aliquot from each sample was analyzed by BCA (Pierce, Rockford, IL) to determine total protein concentration, and the remaining aliquots were stored at −80°C until needed.
Gel Electrophoresis and Western Blot Analysis
To determine SERCA2a protein expression, 15 μg of each sample was loaded and run on a 0.75-mm 7.5% SDSPAGE gel, transferred 1 hour at 100 V to a PVDF membrane (BIO-RAD, Hercules, CA), and blocked overnight at 4°C with 3% milk-TBS buffer. The membrane was incubated an additional 24 hours at 4°C in anti-SERCA2a monoclonal antibody diluted 2:1000 (Affinity Bioreagents, Golden, CO) and washed three times with PBS-Tween. Next, the membrane was incubated in goat anti-mouse IgG secondary (1:10,000; Santa Cruz Biotechnology, Santa Cruz, CA) for 45 minutes and washed three times, and finally proteins were detected using ECL reagents (Amersham, Piscataway, NJ).
For PLB protein expression, 15 μg of total protein from each sample was loaded and run on an 18% SDS-PAGE gel, transferred 1 hour at 100 V to PVDF, and incubated 2 hours in anti-phospholamban monoclonal antibody (1: 1000; Affinity Bioreagents). The PVDF membrane was washed three times with PBS-Tween, incubated in goatanti-mouse IgG secondary (1:10,000) for 1 hour, and washed an additional three times, and the enhanced chemiluminescent protein signals were exposed. Optical densities of each Western blot were quantified using a densitometer (GS-800; BIO-RAD).
RT-PCR
The method of RT-PCR was used to determine the gene expression of cardiac PLB as described by our previous report (7). PLB primer design was (sense) 5′-GCTATCAGGAGAGCCTCCACTA-3′ and (anti-sense) 5′ATGTGAGGACCCAGTGAGCTAT-3′, and the results were normalized with 18s rRNA QuantumRNA (Ambion, Austin, TX).
Quantification of Left Ventricular Function
Left ventricular pressure-volume loops were measured with the Millar Conductance Catheter System (Millar Corp., Houston, TX), as previously described by our laboratory (8,9). An external jugular was also cannulated with a 23-gauge butterfly for infusion of vehicle or dobutamine. Pressure-volume loops were recorded for each age group at baseline and after dobutamine infusion at a rate of 5 μg/kg/min into the internal jugular vein using a dual syringe microdialysis pump (SP101i; World Precision Instruments, Sarasota, FL). Hemodynamic parameters of chronotropic, inotropic, and lusitropic function were derived from the pressure-volume loops as previously described (8,10,11).
Statistics
Data are reported as mean ± SEM. Values obtained from the different age groups were compared using the unpaired Student t test. A level of 0.05 and a power of 0.8 were used for statistical significance calculations.
RESULTS
Immunoblot and Gene Expression
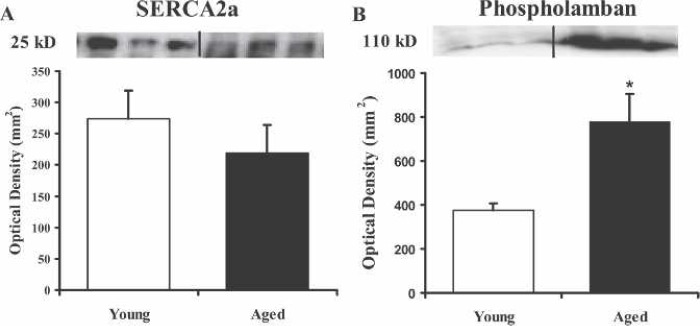
Immunoblot analysis was performed to define the age-related differences in myocyte concentrations of SERCA2a and PLB. The comparison of ventricular SERCA2a protein levels of the two groups exhibited no significant differences (n = 3 each; Figure 1A). The immunoblot (Figure 1B) of the ventricular PLB protein levels showed a 2-fold increase in PLB expression of aged mice compared with the young mice (p < .05). Using RT-PCR, Figure 2 shows that there is a 5-fold increase in the gene expression of PLB in the aged mice compared with the young (p < .01). These data are in good agreement with the immunoblot data indicating that the increased gene expression is most likely responsible for the increased PBL protein levels.
Figure 1.
Immunoblots for SERCA2a and PLB compare the levels protein in young and aged hearts. Identical amounts of ventricular homogenate (15 μg) were separated on a 7.5% gel by SDS-PAGE, transferred to polyvinylidene diflouride membranes, incubated with antibody against SERCA2a (A) or phospholamban (B), and visualized with chemiluminescence. Protein levels for each group were quantified by optical density (mm2) and reported as mean ± SEM. *p < .05 compared with the young group.
Figure 2.
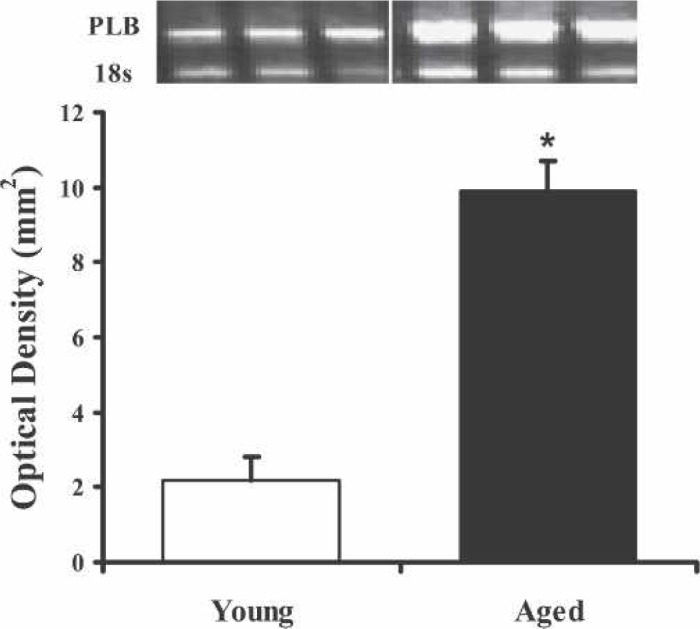
PLB mRNA expression amplified by RT-PCR shows increased gene expression in the aged compared with younger mice. The PCR product was resolved on a 1% agarose gel and stained with ethidium bromide. PLB mRNA expression quantified by optical density (mm2) was normalized against 18s and reported as mean ± SEM. *p < .01 compared with young group.
Hemodynamics
To assess the mechanical in vivo left ventricular function of the young and aged mice, the Millar Conductance Catheter System was used to acquire in vivo pressure-volume loops from which systolic and diastolic functional parameters were derived. Table 1 shows significant differences in heart rate, ejection fraction, end-diastolic volume, dP/dtmax, dP/dtmax − Ved, and pre-load recruitable stroke work (PRSW) in baseline heart function between young and old mice (p < .05). Dobutamine infusion at 5 μg/kg/min increased stroke volume, cardiac index, and dP/dtmax in the younger group and heart rate, stroke volume, ejection fraction, cardiac index, dP/dtmax, and dP/dtmax − Ved in the aged group (p < .05). These parameters suggest that dobutamine increases cardiac output and systolic function in both age groups; however, the increase in these functions from baseline measurements was generally greater in the aged group.
Table 1.
Hemodynamic parameters of young and aged mice in response to dobutamine infusion.
| Young (4 Months) |
Aged (20 Months) |
|||
|---|---|---|---|---|
| 0 | Dobutamine | 0 | Dobutamine | |
| Hemodynamics | ||||
| Heart rate (bpm) | 657 ± 13 | 673 ± 9* | 476 ± 21* | 531 ± 15†‡ |
| Stroke volume (μL) | 7.7 ± 0.8 | 8.6 ± 0.9* | 10.3 ± 0.9 | 14.2 ± 0.4†‡ |
| Ejection fraction (%) | 77.6 ± 3.3 | 71.9 ± 8.1 | 60.2 ± 4.8* | 86.8 ± 2.9† |
| Cardiac index (μL/g) | 153 ± 11 | 174 ± 15* | 138 ± 13 | 212 ± 3† |
| Ved (μL) | 9.9 ± 1.1 | 12.9 ± 2.9 | 16.5 ± 2.0* | 15.9 ± 0.8 |
| Systolic function | ||||
| dP/dtmax (mmHg/s) | 12060 ± 474 | 13676 ± 610* | 4411 ± 450* | 9966 ± 598†‡ |
| dP/dtmax − Ved(mmHg/s/μL) | 814 ± 102 | 1301 ± 278 | 454 ± 118* | 763 ± 142† |
| PRSW (mmHg) | 102 ± 7.4 | 114 ± 11.9 | 59.2 ± 9.5* | 87.1 ± 3.3 |
Ved, ventricular end-diastolic volume; dP/dtmax, maximum derivative of change in systolic pressure over time; the parameter of isovolumic contraction, dP/dtmax − Ved, volume-independent maximum derivative of change in systolic pressure over time normalized against Ved; PRSW, preload recruitable stroke work that is the stroke work normalized against Ved.
n = 6.
p < .05 vs. young controls.
p < .05 vs. aged control.
p < .05 vs. young dobutamine.
Figure 3, A–C, shows the differences of lusitropic parameters between the two age groups. Figure 3A shows that there was a 37% prolongation in the isovolumic relaxation time, Tau (τ), when comparing the 4-month-old with the 20-month-old mice (p = .0002), and dobutamine infusion enhanced τ by 31% in the old mice but had no affect on the younger mice. Correspondingly, Figure 3B shows a 55% reduction of the rate of ventricular pressure decrease over time (dP/dtmin) in the aged group compared with the younger group (p = .008) and a 63% increase in this parameter with dobutamine infusion. Figure 3C shows that the rate of diastolic filling over time (dV/dtmax) was increased 66% in the aged mice with dobutamine administration (p < .01). These data support the concept that β-adrenergic stimulation of the β1-receptor enhances lusitropic performance in aged mice but with minimal effects on the young mice.
Figure 3.
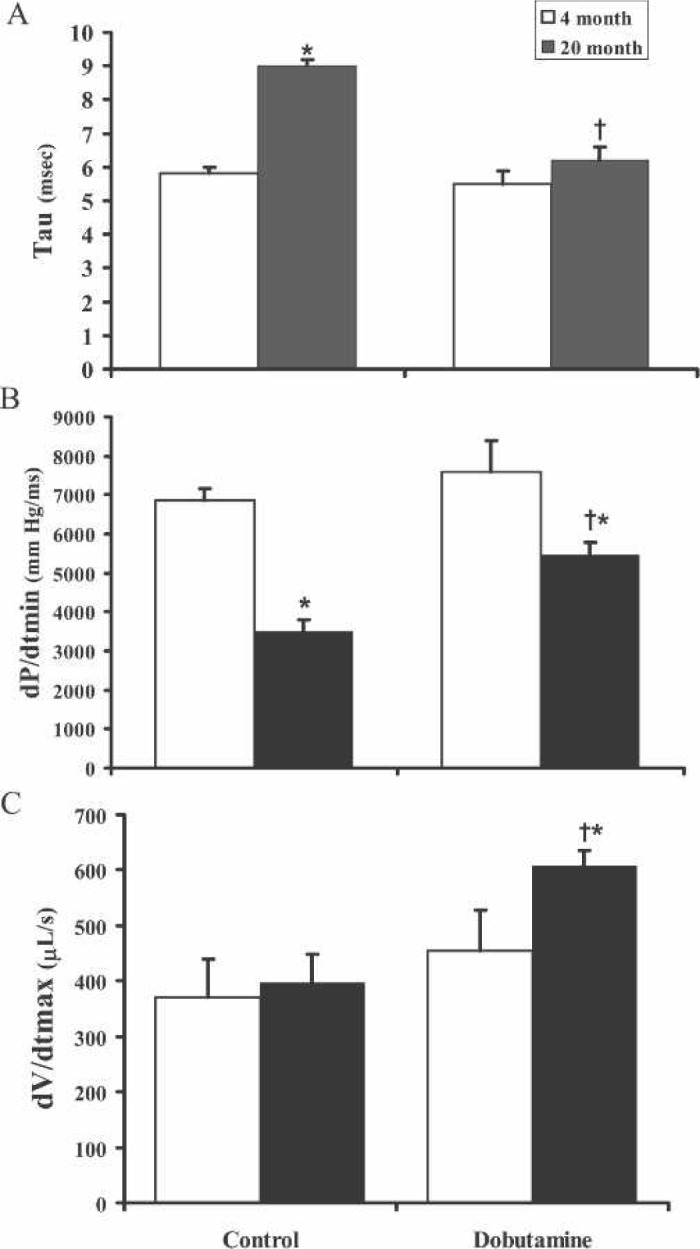
Comparison of young vs. aged isovolumic diastolic response to dobutamine infusion. A, The comparison of the time constant of isovolumic relaxation, Tau-Weiss (τ), of young and aged mice groups shows that the rate of relaxation is prolonged in the aged and is decreased with dobutamine infusion in the aged but not the young group. B, The peak derivative of change in diastolic pressure over time, dP/dtmin, shows that there is a decrease in the rate of left ventricular pressure change in the aged that is increased in the aged but not in young group with dobutamine administration. C, The derivative of the diastolic filling over time, dV/dtmax, shows that dobutamine enhances the rate of left ventricular filling in diastole. n = 6,*p < .05 compared with young control, †p < .001 compared with aged control.
DISCUSSION
This study hypothesized that there are age-related changes in lusitropic function resulting from alterations in the expression and/or function the lusitropic-dependent calcium cycling proteins. The goal of this study was to examine the role of the calcium-cycling proteins, SERCA2a and PLB, and their β1-receptor coupling as they relate to lusitropic function in young and aged mice. Our findings revealed that there are significant basal differences in the isovolumic relaxation parameters, τ and dP/dtmin, that are associated with alterations in expression of the calcium cycling proteins, SERCA2a and PLB, as a function of age. Also, we showed that β-receptor stimulation with dobutamine increased the lusitropic function of the aged mice toward the level of the young individuals and that the lusitropic function of the young mice was not significantly changed by dobutamine infusion.
Studies into the mechanisms of diastolic impairment with aging have implicated changes in the activity and expression of a number of calcium-cycling proteins as causes of delayed relaxation reviewed by Chakraborti et al. (12). SERCA2a and its inhibitor PLB have received the most attention because SERCA2a contributes to the re-uptake of 70%–80% of the total Ca2+ released during systole (12). Prior studies that compared the protein expression of SERCA2a and PLB in normal vs. failing or aged hearts have yielded mixed results. For example, some studies showed a decrease in SERCA2a protein expression in the failing myocardium compared to non-failing (13) and aged myocardium (14). Others found no significant differences in SERCA2a protein expression in heart failure (15,16) and aging (17). Results have also been mixed in regard to PLB expression in aged individuals. Our study found no significant change in SERCA2a protein expression with increasing age. We found a significant age-related increase in the PLB protein levels in the aged mice compared with the young mice. One difference of our data compared with that of others is that our old age group was 20 months, whereas others examined animals or tissues at senescent ages. The justification of our age selection was that we have reported a decrease of left ventricular function at 11 months and older compared with controls (18). The conclusion is that the overall cellular level of PLB in aged mice is a more significant cause of the basal lusitropic dysfunction than changes of SERCA2a levels in our selected age groups. Our results showed that in the 20-month-old mice there are increased protein levels of PLB in the aged mice that were related to increased levels of transcription.
Dobutamine is a β1-adrenergic receptor agonist. The stimulation of this receptor induces the formation of cAMP and thereafter the induction of cAMP-dependent protein kinase (PKA). PKA phosphorylates a number of calcium cycling proteins including; the L-type calcium channel, PLB, and troponin C (12). The focus of our study was PLB because phosphorylation of PLB impedes the rate of calcium reuptake into the SR by SERCA2a and thereby increases lusitropic function. Dobutamine has been used routinely at doses up to 10 μg/kg/min during open heart surgery to augment systolic function; however, dobutamine has yet to be studied as an agent to enhance diastolic function.
The effects of aging on β-receptor–mediated responses have been extensively studied in vitro and in vivo using the β1-and β2-adrenoceptor agonist isoproterenol; however, little is known regarding aging-induced changes in responses to dobutamine. In this regard, the stimulation of the β1-adrenergic receptor has been reported to be decreased with aging in myocardium of humans and rodents (19,20). However, we found significant inotropic, chronotropic, and lusitropic responses to dobutamine infusion in the aged mice. Therefore, in our mouse model of aging, β-receptor desensitization is apparently not a mechanism of diastolic dysfunction. Again this could be because of our selection of the aged mice (20 months) compared with those who have studied senescent mice >24 months of age. Interestingly, the lusitropic dysfunction of the aged mice was reversed on β-receptor stimulation with dobutamine, whereas the inotropic and chronotropic responses were not completely recovered in the aged group compared with the younger group.
The human population of >65 years old is marked by an increase in diastolic dysfunction that is often without the manifestation of impaired systolic function (21). Numerous factors have been implicated to play a role in the pathology of the aged heart including age-associated changes in the structure of the heart because of loss of myocytes (22), a decrease in vascular compliance contributing to an increase in cardiac fibrillar collagen content (23), a decline in endocrine-heart communication (24), and deterioration of the excitation–contraction coupling process resulting in impaired calcium reuptake into the SR (17). The study contained herein modeled the human aged condition and showed that a primary age associated cause of lusitropic dysfunction is the increase in gene expression and protein levels of PLB. This is a confirmation of what others have reported using different models and β-agonists (17). It is also possible that the phosphodiesterase III inhibitor, milrinone, which increases cytoplasmic cAMP in cardiac muscle, could provide similar efficacy to that of dobutamine in the restoration of diastolic function in the aged. Reports of milrinone did not show improvement of diastolic function post-CPB; however, patients were not stratified by age (25,26). Therefore, future studies in the aged model with milrinone could further support our findings with dobutamine. We are the first to have shown that the clinical therapeutic, dobutamine, at a clinical therapeutic dose can rescue the lusitropic function in the aged even with the potential of the above accompanying age-related cardiac pathologies. Therefore, restoration of lusitropic function at the termination of CPB with dobutamine is justified to facilitate ventricular filling and thereby augmenting cardiac output.
ACKNOWLEDGMENTS
This study was supported by NIH R01 HL079206-01, AHA Grant 0455575Z, the James and Linda Lee Heart Failure Research Award, and Steinbronn Heart Failure Research Award to DFL.
REFERENCES
- 1.Larson DF, Yang B, Shi J, Gorman M, Watson RR.. Senescent ventricular dysfunction: issues related to cardiopulmonary bypass. J Extra Corpor Technol. 2000;32:142–7. [PubMed] [Google Scholar]
- 2.Larson DF, Ingham R, Alwardt CM, Yang B.. A mechanism of diastolic filling dysfunction in the aged. J. Extra Corpor Technol. 2004;36:69–74. [PubMed] [Google Scholar]
- 3.Yturralde RF, Gaasch WH.. Diagnostic criteria for diastolic heart failure. Prog Cardiovasc Dis. 2005;47:314–9. [DOI] [PubMed] [Google Scholar]
- 4.Owan TE, Redfield MM.. Epidemiology of diastolic heart failure. Prog Cardiovasc Dis. 2005;47:320–32. [DOI] [PubMed] [Google Scholar]
- 5.Yang B, Larson DF, Watson RR.. Age-related cardiac dysfunction. In: Watson RR, Preedy VR, eds. Nutrition and Heart Disease. New York: CRC Press; 2004:321–32. [Google Scholar]
- 6.Colyer J.. Phosphorylation states of phospholamban. Ann N Y Acad Sci. 1998;853:79–91. [DOI] [PubMed] [Google Scholar]
- 7.Yu Q, Larson DF, Slayback D, Lundeen T, Baxter JH, Watson RR.. Characterization of high salt and high fat diets on cardiac and vascular function in mice. Cardiovasc Toxicol. 2004;4:37–46. [DOI] [PubMed] [Google Scholar]
- 8.Yang B, Larson DF, Watson R.. Age-related left ventricular function in the mouse: analysis based on in vivo pressure-volume relationships. Am J Physiol. 1999;277:H1906–13. [DOI] [PubMed] [Google Scholar]
- 9.Yang B, Larson DF, Beischel J, Kelley R, Shi J, Watson RR.. Validation of conductance catheter system for quantification of murine pressure-volume loops. J Invest Surg. 2001;14:341–55. [DOI] [PubMed] [Google Scholar]
- 10.Georgakopoulos D, Mitzner WA, Chen CH, et al. In vivo murine left ventricular pressure-volume relations by miniaturized conductance micromanometry. Am J Physiol. 1998;274:H1416–22. [DOI] [PubMed] [Google Scholar]
- 11.Yang B, Larson DF, Kelley R, Beischel J, Watson RR.. Conductivity: An issue for the application of conductance catheter system in mice. Cardiovasc Eng. 2000;5:57–60. [Google Scholar]
- 12.Chakraborti S, Das S, Kar P, et al. Calcium signaling phenomena in heart diseases: a perspective. Mol Cell Biochem. 2007;298:1–40. [DOI] [PubMed] [Google Scholar]
- 13.Hasenfuss G.. Alterations of calcium-regulatory proteins in heart failure. Cardiovasc Res. 1998;37:279–89. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt U, del Monte F, Miyamoto MI, et al. Restoration of diastolic function in senescent rat hearts through adenoviral gene transfer of sarcoplasmic reticulum Ca(2+)-ATPase. Circulation. 2000;101:790–6. [DOI] [PubMed] [Google Scholar]
- 15.Linck B, Boknik P, Eschenhagen T, et al. Messenger RNA expression and immunological quantification of phospholamban and SR-Ca(2+)-ATPase in failing and nonfailing human hearts. Cardiovasc Res. 1996;31:625–32. [PubMed] [Google Scholar]
- 16.Schwinger RH, Bohm M, Schmidt U, et al. Unchanged protein levels of SERCA II and phospholamban but reduced Ca2+ uptake and Ca(2+)-ATPase activity of cardiac sarcoplasmic reticulum from dilated cardiomyopathy patients compared with patients with nonfailing hearts. Circulation. 1995;92:3220–8. [DOI] [PubMed] [Google Scholar]
- 17.Lim CC, Liao R, Varma N, Apstein CS.. Impaired lusitropyfrequency in the aging mouse: role of Ca(2+)-handling proteins and effects of isoproterenol. Am J Physiol. 1999;277:H2083–90. [DOI] [PubMed] [Google Scholar]
- 18.Yang B, Larson DF, Watson RR.. Modulation of iNOS activity in age-related cardiac dysfunction. Life Sci. 2004;75:655–67. [DOI] [PubMed] [Google Scholar]
- 19.Leenen FH, Fourney A, Coletta E, White R.. Effects of hypertension on cardiovascular responses to epinephrine in humans. Am J Physiol Heart Circ Physiol. 2007;292:H3025–31. [DOI] [PubMed] [Google Scholar]
- 20.Bazan A. Van d V, Fraeyman N.. Effect of age on beta-receptors, Gs alpha-and Gi alpha-proteins in rat heart. Biochem Pharmacol. 1994;48:479–86. [DOI] [PubMed] [Google Scholar]
- 21.Salmasi AM, Alimo A, Jepson E, Dancy M.. Age-associated changes in left ventricular diastolic function are related to increasing left ventricular mass. Am J Hypertens. 2003;16:473–7. [DOI] [PubMed] [Google Scholar]
- 22.Olivetti G, Melissari M, Capasso JM, Anversa P.. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ Res. 1991;68:1560–8. [DOI] [PubMed] [Google Scholar]
- 23.de Souza RR.. Aging of myocardial collagen. Biogerontology. 2002;3:325–35. [DOI] [PubMed] [Google Scholar]
- 24.Lakatta EG.. Age-associated cardiovascular changes in health: impact on cardiovascular disease in older persons. Heart Fail Rev. 2002;7:29–49. [DOI] [PubMed] [Google Scholar]
- 25.Couture P, Denault AY, Pellerin M, Tardif JC.. Milrinone enhances systolic, but not diastolic function during coronary artery bypass grafting surgery. Can J Anaesth. 2007;54:509–22. [DOI] [PubMed] [Google Scholar]
- 26.Shi Y, Denault AY, Couture P, Butnaru A, Carrier M, Tardif JC.. Biventricular diastolic filling patterns after coronary artery bypass graft surgery. J Thorac Cardiovasc Surg. 2006;131:1080–6. [DOI] [PubMed] [Google Scholar]