Abstract:
Arterial line filters (ALFs) are arguably the most important component in the cardiopulmonary bypass circuit to protect the patient from gaseous macro-and micro-emboli (GME) originating in the perfusion circuit. The GME separating ability of 10 ALFs was ranked according to seven performance criteria. Ten ALFs rated between 20 and 43 μm were evaluated for flow resistance, the count, size, and volume of GME passed after a 10-mL room air bolus, and the ability to separate a high-count, 10-to 200-μm flowing distribution of GME. The Luna Innovations EDAC™ emboli detector was used to size, count, and sort GME. Three test trials were conducted for 3 each of the 10 filters. Performance criteria were correlated by regression analysis, statistically compared using analysis of variance, or ranked using non-parametric tests. Significance was set at 0.05. Weighting all seven test parameters equally, the most effective ALFs were the Cobe 21 and Gish 25-μm filters. The Pall LG-6 ranked more efficient than the Medtronic 20 and Dideco 27-μm filters. The Cobe 43, Terumo 40, Medtronic 38, Terumo 37, and Gish 40-μm filters were less effective as a group compared with the other filters. For the 10 filters, blood flow resistance was not correlated to rated pore size. Generally, the smaller the pore rating, the higher the GME separation ability rank, except for the leuko-reduction filter, which performed more effectively than other large pore filters.
Keywords: arterial line filter, gaseous microemboli, in vitro test
INTRODUCTION
Perhaps the single most important component in the extracorporeal circuit to protect the cardiac surgery patient from macro-and micro-gaseous microemboli (GME) is the arterial line filter (ALF). When an ALF is placed in a cardiopulmonary bypass (CPB) circuit, less neuropsychologic impairment (1,2) and GME (1,3,4) are measured. In 1995, Pugsley et al. (1), after randomly assigning patients to a filtered or non-filtered circuit, reported a reduction in cerebral injury presumably by decreasing cerebral microembolic load in the filtered group.
Borger et al. (5) showed that, when the perfusionist intervened with the CPB circuit more than ten times, this resulted in increased GME and patients exhibited significantly lower cognitive function scores after surgery. The report of Borger et al. shows that GME make it past the ALF and may be responsible for cognitive impairment. Wilcox et al. (6) reported post-filter (Bentley AF10–40D; Baxter Healthcare, Irvine, CA) arterial line GME with venous air embolism. Norman et al. (7) also documented arterial line GME during extreme settings for assisted venous drainage in an in vitro extracorporeal circuit model, especially for a low-prime circuit compared with a conventional circuit.
In 2006, Cruz et al. (8) reported on a pilot study comparing nine arterial line filters using a second-generation emboli detector (EDAC Embolus Detection and Classification Quantifier™; Luna Innovations, Blacksburg, VA). The pilot ALF project of Cruz et al. and the method used herein are modeled after the air-handling tests found in the US Food and Drug Administration guidance document (http://www.fda.gov/cdrh/ode/guidance/1622). The first use of the newly FDA-qualified EDAC™ device to measure and record GME activity in an extracorporeal circuit (ECC) in vitro test circuit was published by Dickinson et al. (9). Jones et al. (4) used a very early prototype of the EDAC and showed numerous GME passing ALFs from several manufacturers.
In addition to the air and GME handling tests, resistance to blood flow is measured and reported here. Kopp (10) showed the importance of controlling and reporting hematocrit and temperature during ALF resistance measurement. Kurusz and Butler (11) defined the role of fluid surface tension in regard to screen filtration of air emboli.
It is not known if differences in ALF microemboli separation ability will significantly affect a CPB patient’s total embolic load during cardiac surgery and therefore neurocognitive function after cardiac surgery. However, the intent of this project is to quantitatively rank 10 currently available ALFs according to physical characteristics, GME, air-separating ability, and resistance to blood flow. Information for the 10 filters is reported in Table 1. The goal is to identify the most efficient air and GME-separating arterial line filters with the lowest blood flow resistance so that clinicians will be able to construct the safest patient circuits.
Table 1.
Test filter information.
| Manufacturer | Model | Screen Pore (μm) | Coating | Prime Volume (cm3) | Filter Media (cm2) |
|---|---|---|---|---|---|
| Cobe* | Sentry | 43 | SMARxT | 178 | 785 |
| Cobe* | Sentry | 21 | PrimeGard | 178 | 785 |
| Dideco* | Micro 20 | 27 | None | 195 | 655 |
| Gish† | GAF-40-2 | 40 | GBS | 168 | 775 |
| Gish† | GAF-25 | 25 | GBS | 168 | 775 |
| Medtronic‡ | Affinity | 38 | Trillium | 212 | 545 |
| Medtronic‡ | Affinity | 20 | Trillium | 212 | 545 |
| Pall§ | LeukoGuard-6 | 40 | Proprietary | 220 | 360 |
| Terumo¶ | Capiox | 40 | X-Coating | 200 | 877 |
| Terumo¶ | Capiox | 37 | X-Coating | 125 | 877 |
Sorin Group USA, Arvada, CO (www.sorin-na.com).
Gish Biomedical, Rancho Santa Margarita, CA (www.gishbiomedical.com).
Medtronic, Minneapolis, MN (www.medtronic.com).
Pall Corporation, East Hills, NY (www.pall.com).
Terumo Cardiovascular Systems, Ann Arbor, MI (www.terumo-cvs.com).
MATERIALS AND METHODS
In Vitro Test Circuit
The test circuit reported by Dickinson et al. (9) and Cruz et al. (8) was used (Figure 1). The EDAC Quantifier™ was used to measure GME and consisted of three 5-mHz sonar-based transducers connected to a computer operating proprietary Windows-based software (Windows; Microsoft, Redmond, WA; Quantitative Ultrasound; Luna Innovations). The EDAC Quantifier™ is calibrated by the manufacturer to simultaneously monitor up to three ECC locations, detect individual GME at rates up to at least 1000 per second between 10 μm to 2.7 mm in diameter, and instantly report the counts, size, and volume per unit time.
Figure 1.
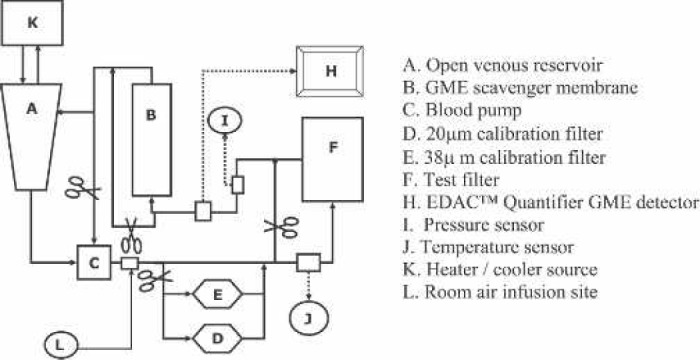
Arterial line filter in vitro test circuit.
Three each of the 10 filters listed in Table 1 were randomized and subjected to three trials each for air-separating performance by two different air challenges: separation of flowing GME and room air bolus, and blood flow resistance was measured. Circuit bovine blood hematocrit, flow rate, and temperature were maintained constant. Test filter afterload was maintained constant during all tests at 100 ± 5 mmHg.
Separation of Flowing GMEs
A consistent distribution of numerous GME of various sizes between 10 and 250 μm were created by mismanaging 4.5 L/min, 20% hematocrit bovine blood flow through a blood reservoir at 32°C. In the flowing GME challenge, total emboli count between 10 and 500 μm were recorded at 30-second intervals with the test filter in and out of the test circuit over several minutes. Figure 2 presents an example step wave for the GME measured during the flowing GME test. The fraction GME removal at various GME sizes was calculated three times for three each of the test ALFs, statistically analyzed, and plotted graphically. Fraction removal consisted of outlet count minus inlet count divided by inlet count for a specific GME size range and is reported as a decimal.
Figure 2.
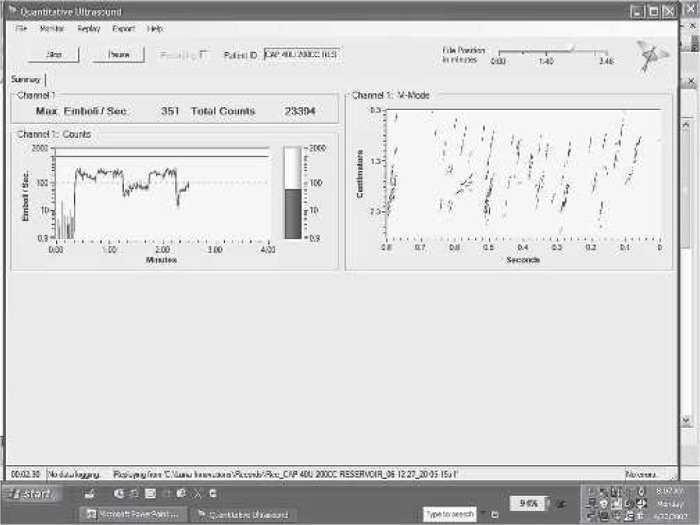
An example filter step-wave profile for the GME measured during the flowing GME test. The figure is a screen capture of the filter GME cut-off size test. The square wave effect on the left screen is created by clamping the arterial line filter into and out of the arterial line while a high count of a wide distribution of bubble size emboli are flowed into the filter.
Room Air Bolus Test
In the room air bolus challenge, GME count and size were measured continuously at the outlet of the test ALF after a 10-mL room air bolus by hand and syringe into the filter inlet line. Three each of the 10 test filters were challenged three times with a bolus. Figure 3 shows a typical Quantifier GME profile resulting from GME escaping from the ALF after the inlet room air injection. The length of time to stop passing GME, the peak emboli diameter, and the estimated total GME volume passed during the bolus were collected and statistically compared.
Figure 3.
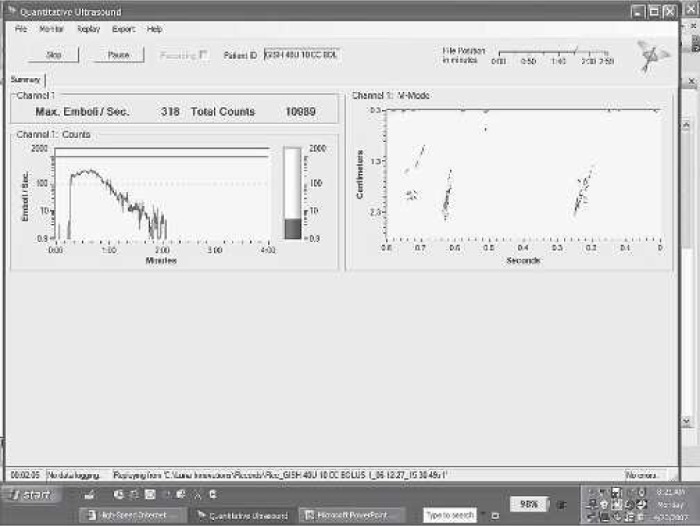
An example filter outlet GME profile measured during the room air bolus.
Blood Flow Resistance
The method and calculations described by Kopp (10) were used to evaluate ALF resistance to blood flow. The GME test circuit was modified to measure filter inlet–outlet pressure difference as described by Kopp. The filter resistance was calculated for 4.5 L/min of 20% hematocrit bovine blood flow at 32°C. Three pressure drop measurements were taken for 3 each of the 10 test filter designs, and the mean flow resistance calculations (mmHg/L/min) were statistically compared and ranked lowest to highest.
Statistical Analysis
The seven criteria used in this method to rank the 10 filters are listed in Table 2. Statistical analysis to compare the mean performance between the 10 ALFs was performed using SPSS (Version 15.0; SPSS, Chicago, IL). Multiple comparisons were made by analysis of variance with the Bonferroni procedure. Statistical significance was set at p < .05. Overall filter ranking gives equal weight to all seven comparison criteria. After ranking the filters from best to lowest performance, Bonferroni comparisons were used to discover significant statistical break points in the rank list to sort the filters into similar performance groups. Some performance criteria were compared using multiple box plot displays.
Table 2.
Seven arterial line filter ranking criteria.
| In Vitro Test | Ranking Criteria | Comment |
|---|---|---|
| Separation of flowing GME* | Percent removal at rated pore size | The percent removal of flowing GME distribution* below the reported pore size (μm) of the filter screen |
| 90% efficiency rating | Micrometer size where filter removes 90% of flowing GME distribution* | |
| Room air bolus | Largest micrometer gas emboli passed | Largest micrometer gaseous emboli measured at filter outlet after room air bolus |
| Cubic micrometers of gas passed after room air bolus | Gas volume passed in ×107 μm3 after filter inlet 10 mL room air bolus calculated from outlet GME distribution | |
| Seconds after bolus | Seconds that GME persist down to 2 GME/sec at filter outlet after room air bolus | |
| Blood flow resistance | Prime volume in cubic centimeters | The prime volume reported by the manufacturer |
| Flow resistance (mmHg/L/min) | Calculated from pressure drop measurements at 4.5 L/min for bovine 20% hematocrit blood at 32°C |
Flowing GME distribution is >104 10- to 200-μm suspended gaseous emboli flowing at 4.5 L/min, 20% hematocrit bovine blood at 32°C.
RESULTS
There were statistically significant differences in micro-and gross air-handling ability and the flow resistance between the 10 filter designs.
Separation of Flowing GMEs
Table 3 lists the statistical ranking of the GME removal efficiency of the test filters. The 10 filters ranked into two main groups, primarily by rated pore size, except for the LG-6 40-μm filter that behaved similar to the smaller pore ALFs. Note that the filters do not rank out specifically by the pore size, suggesting that there are differences in the contribution of the physical housing design to the GME separating efficiency.
Table 3.
Ranked filter percent removal of suspended flowing GMEs at 4.5 L/min, 32°C, and 20% hematocrit.
| At 40- to 45-μm GME size |
At 20- to 25-μm GME size |
|||||
|---|---|---|---|---|---|---|
| Filter | Percent Removal | Rank* | Significance† | Percent Removal | Rank* | Significance† |
| Cobe 21 | 0.995 | 1 | NS | 0.962 | 1 | NS |
| LG-6 40 | 0.988 | 1 | NS | 0.964 | 1 | NS |
| Gish 25 | 0.985 | 1 | NS | 0.964 | 1 | NS |
| Affinity 20 | 0.982 | 1 | NS | 0.899 | 1 | 0.039§ |
| Dideco 27 | 0.971 | 1 | <0.001 | 0.818 | 1 | NS¶ |
| Cobe 43 | 0.785 | 2 | NS | 0.739 | 2 | NS§ |
| Affinity 38 | 0.769 | 2 | NS | 0.691 | 2 | NS |
| Capiox 37 | 0.750 | 2 | NS | 0.606 | 2 | <0.001** |
| Capiox 40 | 0.750 | 2 | NS | 0.724 | 2 | NS |
| Gish 40 | 0.721 | 2 | <0.001‡ | 0.638 | 2 | NS |
NS, no significant difference by ANOVA at p < .05.
Filters ranked the same are not significantly different.
Compared to the filter group ranked next and lower.
Compared to Cobe 43 only and the rank 1 group.
Affinity 20 is significantly greater than Cobe 43 group.
The Dideco 27 is NS to the Cobe 43 and Affinity 38.
Capiox 37 is significantly lower than the Dideco 27 but NS to the Cobe 43.
Room Air Bolus Test
Figure 4 presents the boxplots of the peak GME measured at the filter outlet after the 10-mL room air bolus. Figure 5 presents the average times that the filters released GME after the air injection. Figure 6 ranks the estimated GME volume released at the outlet of the filters in the time period presented in Figure 5.
Figure 4.
Boxplot of filter average peak GME released during room air bolus.
Figure 5.
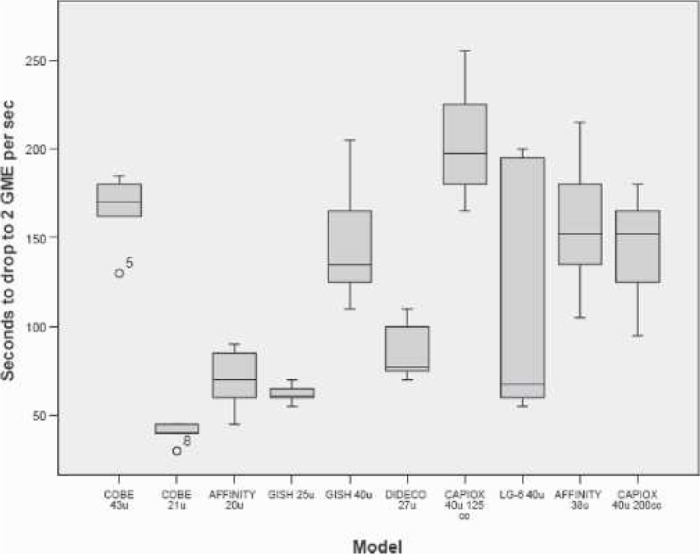
Boxplot of filter average GME release times during room air bolus.
Figure 6.
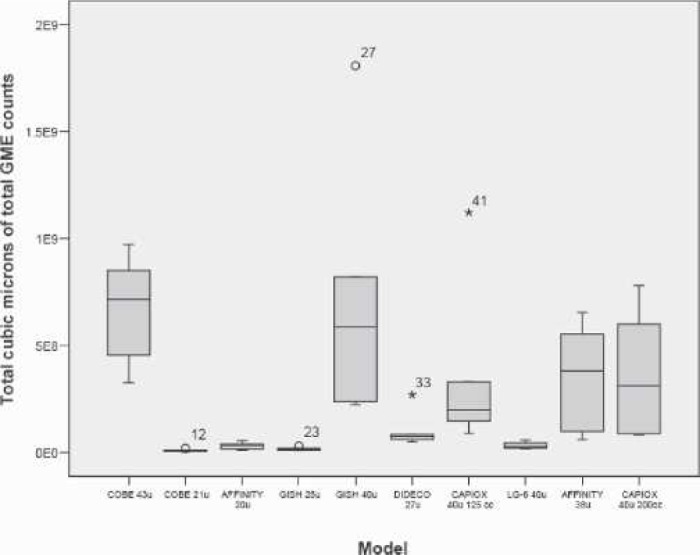
Boxplot of filter average GME volume released during room air bolus
Blood Flow Resistance
Table 4 lists the statistical comparison of the test filters’ resistance to blood flow measurements. The filter mean blood flow resistance results rank statistically into four groups.
Table 4.
Statistical rank of filter average resistance to blood flow measurements.
| Filter Model | Resistance Mean | Resistance SD | Rank | Significance Compared With Next ALF | |
|---|---|---|---|---|---|
| Cobe 43 μm | 2.94 | 0.98 | 1 | NS* | |
| Cobe 21 μm | 3.33 | 0.61 | 1 | NS† | |
| Capiox 37 μm | 3.47 | 0.19 | 1 | NS‡ | |
| Affinity 38 μm | 4.10 | 0.21 | 2 | NS§ | |
| Capiox 40 μm | |||||
| (125 mL) | 4.13 | 0.39 | 2 | NS¶ | |
| Gish 40 μm | 4.14 | 0.33 | 2 | NS | |
| Affinity 20 μm | 4.27 | 0.24 | 2 | NS** | |
| Gish 25 μm | 4.28 | 0.23 | 2 | NS | |
| LG-6 40 μm | 4.66 | 0.25 | 3 | NS | |
| Dideco 27 μm | 5.16 | 0.22 | 4 |
The test filters separate into four statistically significant different groups.
p < .001 compared with Affinity 38 μm.
p = .018 compared with Affinity 38 μm.
p < .001 compared with LG-6 40 μm; NS compared with Capiox 40 μm 125 mL.
p < .001 compared with Dideco 27 μm; NS compared with LG-6 40 μm.
p < .001 compared with Dideco 27 μm; NS compared with LG-6 40 μm.
p = .002 compared with Dideco 27)m; NS compared with LG-6 40 μm NS, not significant.
Table 5 lists the summary results for the filter comparison criteria. The filters are ranked in descending order of performance weighting each of the test parameters equally.
Table 5.
Arterial line filter criteria values ranked in overall net descending order of air-separating performance at 4.5 L/min, 32°C, and 20% hematocrit.
| Filter (Screen μm) | Percent Rated (μm)* | 90% (μm)* | Prime Volume (mL) | Flow Resistance† | Cubic Micrometers After Bolus‡ | Peak Micrometers After Bolus§ | Seconds After Bolus¶ |
|---|---|---|---|---|---|---|---|
| Cobe 21 | 93 | 15 | 178 | 3.33 | 0.85 | 190 | 40 |
| Gish 25 | 97 | 5 | 168 | 4.28 | 1.65 | 225 | 62 |
| Pall LG-6 40 | 99 | 5 | 220 | 4.66 | 3.06 | 215 | 108 |
| Medtronic 20 | 94 | 5 | 212 | 4.27 | 3.10 | 275 | 70 |
| Dideco 27 | 90 | 27 | 195 | 5.16 | 10.01 | 300 | 85 |
| Cobe 43 | 85 | 70 | 178 | 2.94 | 67.09 | 325 | 166 |
| Terumo 40 | 80 | 82 | 200 | 3.47 | 36.16 | 300 | 145 |
| Medtronic 38 | 77 | 75 | 212 | 4.10 | 35.42 | 290 | 157 |
| Terumo 37 | 78 | 75 | 125 | 4.13 | 34.71 | 330 | 203 |
| Gish 40 | 80 | 75 | 168 | 4.14 | 70.92 | 345 | 146 |
Percent removal at rated pore size when challenged with a high-count, 10-to 200-μm flowing distribution of GME.
Flow resistance in mmHg/L/min with 100 mmHg afterload.
Gas volume passed in ×107 μm3 after filter inlet 10-mL room air bolus.
The largest micrometer GME at filter outlet after filter inlet 10-mL room air bolus.
Seconds that filter outlet GME persist after filter inlet 10-mL room air bolus.
DISCUSSION
With our method and the EDAC Quantifier™, we were able to successfully construct a circuit and rank the GME separating performance and flow resistance of 10 ALFs (Figure 7). The in vitro test circuit and the emboli detection system provided consistent measurements with low coefficients of variation for the test criteria.
Figure 7.
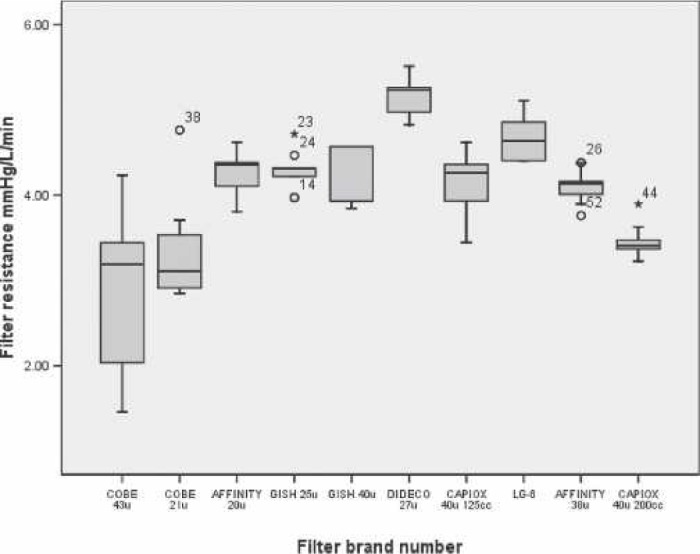
Pressure drop boxplots for 10 models of ECC arterial line filters.
Especially for the 40-μm LG-6, the filter rated pore size did not exactly predict the overall performance rank revealed by this method. Unlike the other filters, the LG-6 design is a three-stage system with automatic venting that was activated during the study according to the manufacturer’s instructions. Presumably, the LG-6 also removed smaller GME than its pore size because of the presence of its proprietary coating applied to the filter media to attract activated leukocytes. The increased LG-6 GME removal capability seems to translate into significantly fewer cerebral embolic episodes and better performance on neurocognitive tests in the clinic compared with a similar filter without the leuko-depleting filter media (12). The LG-6 had the second highest flow resistance measurement among the 10 study filters.
An important finding is that the filter-rated pore size did not predict the rank of the filters’ resistance to blood flow. The lack of correlation between filter pore size and flow resistance suggests that fluid rheology, filter housing geometry, filter media surface area, and, perhaps, surface modification are the greater determinants of filter blood path resistance.
There may be concern that smaller pore media ALFs cause higher shear rates and potentially more cellular element damage. A search of the literature yielded little evidence of attempts to measure cellular damage by ALFs except for trials with the leuko-depleting filter (13). Arterial line filters seem to be well designed to slow the velocity of flow through the housing and the large surface area media to avoid hemolytic shear stress and kinetic energy loss. Despite the 20-to 40-)m openings in the filter media, the peak velocity and apparent resistance as measured by this protocol are remarkably low, avoiding hemolytic shear through even 20-)m pores. The potential activation of leukocytes and platelets as observed when blood is flowed through much smaller radii or screens has not been observed in ALFs. Adequate anticoagulation and moderate hemodilution help to avoid cellular damage at the temperatures and flow velocities in which ALFs are clinically used.
There has been increased attention to monitoring the ECC delivery of GME to the middle cerebral artery by transcranial Doppler monitoring; therefore, the use of an ALF that limits the embolic load most effectively is warranted to protect the brain during CPB (1,4). Evidence-based guidelines have been written to guide surgical practice to reduce the chance of undesired neurologic outcomes with CPB (14). Arterial line screen filters are extremely effective at removing foreign particulate and plastic or silicon spalls greater in size than the filter media pores but probably allow smaller particulates to pass, suggesting that prebypass circuit prime ultra-pore filtration is indicated (15,16). All filters allowed the passage of some GME many times larger than the rated pore size in both the flowing GME separation and the room air bolus tests.
The 10 study filters are moderately effective in removing GME and gross air. The volume of air and the number of GME passed by the filter is small, and the embolic load, count, or volume per unit time to cause neurologic impairment after CPB has not been completely quantified. However, the goal should be complete removal of GME caused by the negative physiologic and neurologic consequences of micro-air infusion (17).
A test ECC and a method with statistical procedures were successfully used to rank ALFs. The ALF is the last line of defense in the CPB circuit, and basing ALF selection on pore size alone does not guarantee exceptional performance.
ACKNOWLEDGMENTS
The author wishes to thank Timothy Dickinson from Hospital Clinical Services Group (Nashville, TN) for his expertise and support in the laboratory. Jeffrey Crowley, also from Hospital Clinical Services Group, and Mr. Dickinson provided valuable feedback to the history and preparation of this report.
REFERENCES
- 1.Pugsley W, Klinger L, Paschalis C, et al. The impact of microemboli during cardiopulmonary bypass on neuropsychological functioning. Stroke. 1994;25:1393–9. [DOI] [PubMed] [Google Scholar]
- 2.Whitaker DC, Stuygall JA, Newman S.. The use of leukocyte-depleting and conventional arterial line filters in cardiac surgery: A systematic review of clinical studies. Perfusion. 2001;15:433–46. [DOI] [PubMed] [Google Scholar]
- 3.Taggaft DP, Bhattacharya K, Meston N, et al. Serum S-100 protein concentration after cardiac surgery: A randomized trial of arterial line filtration. Eur J Cardiothorac Surg. 1997;11:645–9. [DOI] [PubMed] [Google Scholar]
- 4.Jones TJ, Deal DD, Vernon JC, Blackburn N, Stump DA.. How effective are cardiopulmonary bypass circuits at removing gaseous microemboli? J Extra Corpor Technol. 2002;34:34–9. [PubMed] [Google Scholar]
- 5.Borger MA, Peniston CM, Weisel RD, Vasiliou M, Green REA, Feindel CM.. Neuropsychologic impairment after coronary bypass surgery: effect of gaseous microemboli during perfusionist interventions. J Thorac Cardiovasc Surg. 2001;121:743–9. [DOI] [PubMed] [Google Scholar]
- 6.Wilcox TW, Mitchell SJ, Gorman DF.. Venous air in the bypass circuit: a source of arterial line emboli exacerbated by vacuum-assisted drainage. Ann Thorac Surg. 1999;68:1285–9. [DOI] [PubMed] [Google Scholar]
- 7.Norman MJ, Sistino JJ, Acsell JR.. The effectiveness of low-prime cardiopulmonary bypass circuits at removing gaseous emboli. J Extra Corpor Technol. 2004;36:336–42. [PubMed] [Google Scholar]
- 8.Cruz S, Ewing M, Riley JB, Dickinson TA.. Gaseous microemboli separation, management and elimination by the arterial line filter. J Extra Corpor Technol. 2006;28:A84. [Google Scholar]
- 9.Dickinson TA, Riley JB, Crowley JC, Zabetakis PM.. In vitro evaluation of the air separation ability of four cardiovascular manufacturer extracorporeal circuit designs. J Extra Corpor Technol. 2006;38:206–13. [PMC free article] [PubMed] [Google Scholar]
- 10.Kopp ME.. The effect of temperature on the pressure drops across 25 and 40 micron arterial line filters. J Extra Corpor Technol. 1983;15:171–4. [Google Scholar]
- 11.Kurusz M, Butler B.. Bubbles and bypass: an update. Perfusion. 2004;19(Suppl 1):S49–55. [DOI] [PubMed] [Google Scholar]
- 12.Thurlow PJ, Doolan L, Sharp R, Sullivan M, Smith B, Andersen LW.. Laboratory studies of the effect of Pall extracorporeal leukocyte filters LG6 and AV6 on patients undergoing coronary bypass grafts. Perfusion. 1996;11:29–37. [DOI] [PubMed] [Google Scholar]
- 13.Whitaker DC, Newman SP, Stygall J, Hope-Wynne C, Harrison MJG, Walesby RK.. The effect of leukocyte-depleting arterial line filters on cerebral microemboli and neuropsychological outcome following coronary artery bypass surgery. Eur J Cardiothorac Surg. 2004;25:267–74. [DOI] [PubMed] [Google Scholar]
- 14.Shann KG, Murkin JM, Baker RA, et al. An evidence-based review of the practice of cardiopulmonary bypass in adults: a focus on neurologic injury, glycemic control, hemodilution, and the inflammatory response. J Thorac Cardiovasc Surg. 2006;132:283–90. [DOI] [PubMed] [Google Scholar]
- 15.Kim WG, Kim KB, Yoon CJ.. Scanning electron microscopic analysis of arterial line filters used in cardiopulmonary bypass. Artif Organs. 2000;24:874–8. [DOI] [PubMed] [Google Scholar]
- 16.Briceño JC, Runge TM.. Tubing spallation in extracorporeal circuits. An in vitro study using an electronic particle counter. Int J Artif Organs. 1992;15:222–8. [PubMed] [Google Scholar]
- 17.Barak M, Katz Y.. Microbubbles: pathophysiology and clinical implications. Chest. 2005;128:2918–32. [DOI] [PubMed] [Google Scholar]


