Abstract:
Pregnancy is a common decompensation factor for women with post-rheumatic mitral disease. However, valvular heart diseases causing severe acute respiratory distress are rare. Use of extracorporeal membrane oxygenation (ECMO) early in the event of cardiorespiratory failure after cardiac surgery may be of benefit. Indeed, ECMO cardiopulmonary bypass (CPB) support could help pulmonary recovery if the mitral pathology is involved. A 31-year-old female patient at 30 weeks of amenorrhea was admitted to the obstetrics department with 40°C hyperthermia and New York Heart Association (NYHA) class 4 dyspnea. The patient’s medical history included a post-rheumatic mitral stenosis. Blood gases showed severe hypoxemia associated with hypocapnia. The patient needed to be rapidly intubated and was placed on ventilatory support because of acute respiratory failure. Transesophageal echocardiography showed a severe mitral stenosis, mild mitral insufficiency, and diminished left ventricular function, hypokinetic, dilated right ventricle, and a severe tricuspid regurgitation. An urgent cesarean section was performed. Because of the persistent hemodynamic instability, a mitral valvular replacement and tricuspid valve annuloplasty were performed. In view of the preoperative acute respiratory distress, we decided, at the beginning of the operation, to carry on circulatory support with oxygenation through an ECMO-type CPB at the end of the operation. This decision was totally justified by the unfeasible CPB weaning off. ECMO use led to an efficient hemodynamic state without inotropic drug support. The surgical post-operative course was uneventful. Early use of cardiorespiratory support with veno-arterial ECMO allows pulmonary and right heart recovery after cardiac surgery, thus avoiding the use of inotropic drugs and complex ventilatory support.
Keywords: respiratory failure, mitral valve, extracorporeal membrane oxygenation
Post-rheumatic mitral stenosis, despite its decreasing incidence in occidental countries, remains a major public health problem in developing countries. Pregnancy is a common decompensation factor of this valve disease because of the increased output and observed tachycardia over the last two quarters, leading to arrhythmia and cardiac failure. However, valvular heart diseases causing severe acute respiratory distress are rare (1).
Extracorporeal membrane oxygenation (ECMO) is a technique for providing life support in case the natural lungs are failing and are not able to maintain sufficient oxygenation of the body’s organ systems. The ECMO technique was an adaptation of conventional cardiopulmonary bypass (CPB) techniques and introduced into treatment of severe acute respiratory distress syndrome (ARDS) in the 1970s. Patients with ARDS respond favorably to advanced methods of intensive care, which include, but are not limited to, various forms of mechanical ventilation with positive end-expiratory pressure (PEEP) and permissive hypercapnia, positional maneuvers, sophisticated fluid regimens, and inhalational pulmonary vasodilators. There remains, however, a small number of patients with ARDS whose pulmonary gas exchange can not be improved sufficiently by the above-mentioned methods, and ECMO may be an additional therapeutic option during the acute phase. The initial reports of the use of ECMO in patients with ARDS were quite enthusiastic; however, in the following years, it became clear that ECMO was only of benefit in newborns with acute respiratory failure. In addition, the safety and efficacy of ECMO in pregnancy are unknown.
We report a clinical case of a pregnant patient who presented with an acute respiratory failure with a medical history of post-rheumatic mitral valve stenosis.
CASE REPORT
A 31-year-old-pregnant woman at 30 weeks of gestation was admitted to the obstetrics department with 40°C hyperthermia and a NYHA class 4 dyspnea associated with bilateral pulmonary rales. The patient’s medical history included a post-rheumatic mitral stenosis operated by commissurotomy at the age of 12 years and asthma crises treated with beclomethasone and salbutamol. Chest x-ray showed bilateral generalized interstitial infiltrates and pulmonary venous congestion (Figure 1). Blood gases showed severe hypoxemia (arterial oxygen pressure, Pao2: 55 mmHg) associated with hypocapnia (carbon dioxide arterial pressure, Paco2: 23 mmHg). The patient needed to be rapidly intubated and was placed on ventilatory support because of acute respiratory failure. A new chest x-ray revealed obvious worsening of radiologic images with bilateral alveolar infiltrates (Figure 2). Pao2/Fio2 (fractional inspired oxygen) was <200, and NO was administered to improve oxygenation and reduce pulmonary hypertension (PHT). Because of the hyperthermia associated with marked leukocytosis with neutrophils polynucleosis (white blood cells = 16,300/mm3), an infectious check-up was performed, and antibiotic treatment was started with erythromycin 1 g × 3 and cefotaxime 2 g × 3. Transesophageal echocardiography showed a 0.6-cm2 mitral stenosis by continuity equation, a grade 2 mitral insufficiency, subnormal left ventricular function (EF 50%), hypokinetic, dilated right ventricle, and a grade 3 tricuspid regurgitation (proximal isovelocity surface area [PISA] = 0.8, regurgitant orifice area = 0.3 cm2). Right atrio-ventricular gradient was 49 mmHg; under-ventilation (PEEP at 15) vena cava was congestive and associated with a PHT of 70 mmHg. Rapidly, the clinical picture of biological disseminated intravascular coagulation syndrome (DICS) with a prothrombin time at 46%, platelets at 99,000/mm3, V factor of 52%, and an oligo-anuric renal failure (creatinine at 184 mmol/L) took place. Cesarean urgently was performed to extract the fetus; a 1300-g newborn was extracted, intubated, and under respiratory support, was transferred to the neonatal intensive care unit.
Figure 1.
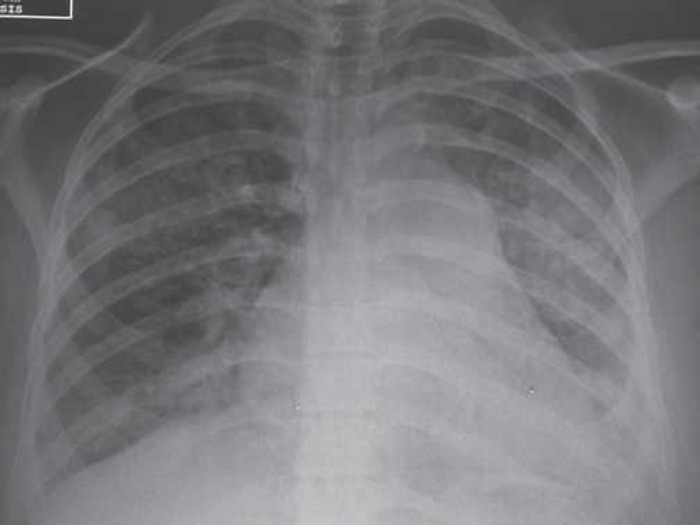
Pulmonary x-ray showed bilateral interstitial syndrome.
Figure 2.
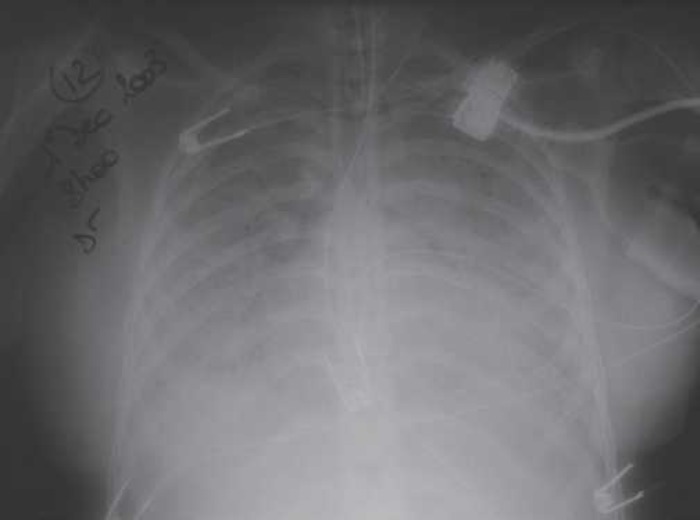
Pulmonary x-ray revealed a clear worsening of radiologic pictures with bilateral alveolar syndrome.
Hemofiltration was performed associated with inotropes drug induction to maintain perfusion pressure. Because of the persistent hemodynamic instability and tight mitral stenosis, a mitral valvular replacement was performed emergently. The patient was intubated and placed under NO (4 ppm) ventilatory support with a Fio2 at 100% together with permissive hypercapnia ventilation (Vt = 300 mL, rate = 30, mean pressure = 30 mmHg). Sedation was achieved using midazolam, sufentanil, and cisatracurium besilate. In addition, the patient received 1 mg/h of adrenaline and insulin therapy. In the immediate post-operative period, right pulmonary catheterization confirmed the PHT (positive end expiratory pressure, PAPs at 88 mmHg). Arterial gasometry was as follows: pH = 7.23 mmHg, Paco2 = 72.4 mmHg, Pao2 = 79.6 mmHg, and HCo3− = 31 mmol/L.
The operation consisted of mitral valve replacement with a Saint Jude 27-mm mechanical prosthesis (St. Paul, MN) and tricuspid annuloplasty with a Carpentier Edwards 32-mm ring (Irvine, CA). The procedure was carried out through a median sternotomy under moderate hypothermia (28°C) CPB with intermittent anterograde cardioplegia at 30-minute intervals. In view of the preoperative acute respiratory distress, we decided, at the beginning of the operation, to carry on circulatory support with oxygenation through ECMO-type CPB at the end of the operation. This decision was totally justified by the unfeasible CPB weaning off. There were two reasons for this unfeasibility: the impact of a right failure with severe right ventricular hypokinesia and the existence of a preoperative major pulmonary impairment with high insufflation pressures and severe hypoxemia. This partial CPB (Quadrox “D” Bioline coated circuit; Total Concept, Jostra AG. Hirrlingen Germany) was set up after a right femoro-femoral cannulation (17 Fr × 18 cm in the arterial site and 21 Fr × 50 cm in the venous one) using a Biomedicus cannulae percutaneous set (Medtronic, Minneapolis, MN) and Biomedicus pump. Weaning off the standard CPB was achieved under ECMO support, and no inotropic drug support was needed to obtain satisfactory hemodynamics and hematosis.
The surgical post-operative course was uneventful. The hemodynamic conditions remained stable, with a pulsed arterial pressure of 135–185 mmHg and a cardiac rate of 85 beats/min without any inotropic drug. The patient was ventilated for 10 days with a tidal volume of 10 mL/kg and a positive expiratory pressure of 8 cm H2O. The first two post-operative days, Fio2 was maintained at 30% because of a limited trans-pulmonary flux and later was progressively adapted according to trans-pulmonary flux and gasometry. Under ECMO, gradual clinical and radiologic improvements were observed, and the chest x-ray was normal (Figure 3). Gradually the arterial blood gases performed on blood collected from the radial artery improved, while post-ECMO blood gases remained stable and ECMO-related assistance was progressively decreased. A sputum culture performed postoperatively showed multi-resistant Pseudomonas aeruginosa P11. An adapted antibiotherapy to the infection to pseudomonas aeruginosa was prescribed: Tienam, Gentamicine. The patient was extubated 20 days after separation from ECMO support.
Figure 3.
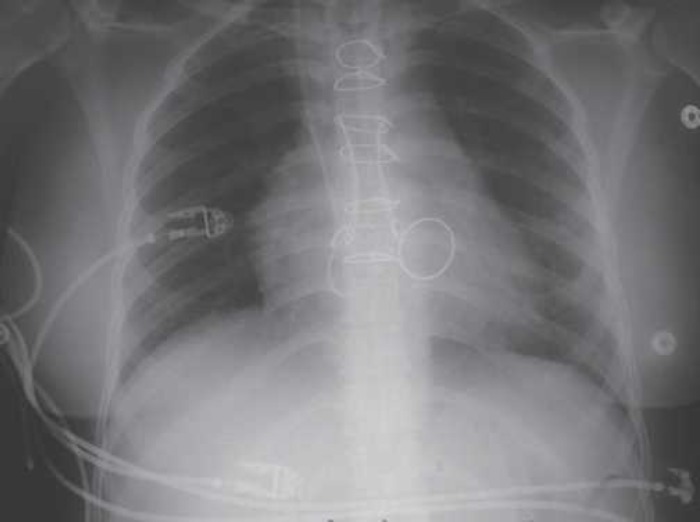
Pulmonary x-ray back to normal.
The ECMO management was as follows: ECMO duration was 9 days at a 2.5 L/min/m2 indexed outflow, which was progressively weaned off. Gradual improvement of the right ventricle contractility was confirmed by daily transthoracic echocardiography (TTE) and complete PHT regression (27 mmHg), allowing reduction of assistance outflow. Lowering the assistance outflow permitted us to observe the improvement of pulmonary gas exchanges. TTE confirmed normal right ventricular function, the absence of PHT, and a left ventricular ejection fraction of 50%. Simultaneously, while on an Fio2 of 50%, the arterial blood gases were as follows: pH = 7.29 mmHg, Paco2 = 53 mmHg, Pao2 = 94 mmHg, and HCo3− = 27.8 mmol/L. Anti-coagulation was achieved using a 1000-UI/h dose, which maintained a 24-hour steady plasma heparinemia between 0.3 and 0.6 U/mL. It was necessary to replace the oxygenator after 7 days of ECMO support because of the occurrence of hemolysis.
The hemofiltration initially installed was sustained on a discontinuous basis until day 21, with a satisfactory recovery of renal function.
Outcomes of the various ventilatory, hemodynamic, and biological parameters are summarized in Tables 1 and 2.
Table 1.
Evolution of the various ventilatory and haemodynamic parameters.
| Preoperative | CPB Arrest | Postoperative (D1) | ECMO Arrest (D9) | Extubation (D20) | |
|---|---|---|---|---|---|
| RR (cycle/min) | 30 | 30 | 14 | 10 | — |
| FiO2 (%) | 100 | 30 | 40 | 30 | 100 |
| Vt (mL) | 300 | 300 | 450 | 410 | — |
| PEEP (cm H2O) | 6 | 8 | 8 | 5 | — |
| PaO2 (mmHg) | 79 | 282 | 119 | 105 | — |
| PAs/PAd (mmHg) | 125/69 | 94/58 | 100/60 | 130/70 | 140/70 |
| PAPs (mmHg) | 88 | 47 | 31 | 27 | — |
| HR (beat/min) | 110 | 100 | 70 | 88 | 93 |
| ECMO flow (1/min/m2) | — | — | 2,54 | — | — |
| FiO2 ECMO (%) | — | — | 70 | — | — |
| SVO2 | — | — | 76 | 72 | — |
CPB: cardiopulmonary bypass, ECMO: extracorporeal membrane oxygenation, RR: respiratory rate, Fio2: fractional inspired oxygen, Vt: tidal volume, PEEP: positive end expiratory pressure, Pao2: arterial oxygen tension, Pas/Pad: systolic/diastolic arterial pressure, PAPs: systolic pulmonary arterial pressure, HR: heart rate, SVo2: venous oxygen saturation.
Table 2.
Evolution of biological parameters.
| Preoperative | CPB Arrest | Postoperative (D1) | ECMO Arrest (D9) | Extubation (D20) | |
|---|---|---|---|---|---|
| Creatinine (mmol/L) | 448 | 316 | 396 | 159 | 88 |
| Diuresis (mL/h) | 400 | 100 | 200 | 0 | 16 |
| Hemoglobin (g/dL) | 10,2 | 7,1 | 7,8 | 9,1 | 10,2 |
| Platelets (/mm3) | 99,000 | 80,000 | 51,000 | 150,000 | 160,000 |
| Prothrombin time (%) | 46 | 37 | 23 | 48 | 62 |
| Partial thromboplastin time (patient/control) | 30/33 | >220 | 86/33 | 104/33 | 61/33 |
| Thrombin time (patient/control) | 15/17 | >60 | 31/16 | >60/18 | 32,3/18 |
| Fibrinogen (g/L) | 3,9 | 2,1 | 2,6 | 5,8 | 7,49 |
CPB: cardiopulmonary bypass, ECMO: extracorporeal membrane oxygenation.
The patient left the intensive care unit (ICU) on postoperative day 28. Unfortunately, on post-operative day 33, we observed a severe decrease in white blood cells to 1500. A myelogram revealed a neutrophil granulocytic hypoplasia, with suspected toxic origin, and all potentially hematotoxic treatments were stopped, especially imipenem/cilastatin. Neutropenia worsened, despite filgrastim introduction. The patient who was apyretic showed hyperthermia on day 36. Multiple bacteriological sampling was harvested and bacteriological testings achieved on blood, nasal, and oropharyngeal samples. Vancomycin and ciprofloxacin antibiotic treatment was subsequently administered. The patient was readmitted to the ICU on day 37 with septic shock requiring the use of norepinephrine. White blood cell count showed severe neutropenia (840 white cells/mm3; 1% of neutrophil polynuclears). The patient presented a medullar aplasia and died on day 38 in an irreversible toxi-infectious shock with clinical presentation with anuria and disseminated intravascular coagulation syndrome. Hemocultures harvested before death isolated P. aeruginosa.
DISCUSSION
This clinical case shows the benefit of the early use of ECMO in the event of a cardiorespiratory failure after cardiac surgery. In our case, the patient’s pre-operative status led us to expect post-operative respiratory failure aggravated by unavoidable fluid overload resulting from the use of CPB. Only an ECMO-type CPB could lead to sufficient pulmonary recovery if the mitral pathology was involved; this support could also avoid both diffused tissue hypoxia induced by hypoxemia and right heart failure, allowing for the protection of other organs, such as brain, kidneys, and mesentery.
Although percutaneous mitral dilation was chosen as the treatment of choice for mitral stenosis during pregnancy (2), it was not possible to perform it in our case, because of the clinical status, associated moderate mitral insufficiency, and severe tricuspid failure (3). The results would have been unsatisfactory. We therefore decided to perform mitral valve replacement and tricuspid annuloplasty, allowing complete correction of the lesions.
The use of ECMO in case of acute respiratory failure in adult goes back to the 1970s (4). The initial technique purpose was to obtain blood oxygenation through highflow veno-arterial extracorporeal circulation (5). This technique progressed during the 1980s toward a ventilation method with reduced flows and low peak inspiratory pressures (low-frequency positive-pressure ventilation [LFPPV]) (6). This technique allows the separation of blood oxygenation physiologically produced by the patient’s lung from the Co2 cleared performed by the artificial lung (extracorporeal carbon dioxide removal [ECCo2-R]). Hence, a new low flow veno-venous extracorporeal circulation technique was perfected and described by Kolobow et al. (7).
The aim of these techniques is either to increase oxygenation or remove CO2 but never to ensure circulatory assistance. The correlation of this patient’s extreme clinical severity, radiographic major alveolar syndrome (Figure 2), and major difficulties in obtaining proper hematosis despite an optimal usual treatment associating mechanical ventilation support with high pressures and a Fio2at 100%, and moreover the use of NO, justified the use of an external respiratory support during the immediate postoperative period. However, unlike ECMOs for genuine pulmonary failures, we were facing a dual pulmonary and heart failure, which justified the use of veno-arterial circulatory assistance with oxygenation to allow right ventricular unload. In the absence of such techniques, we would have encountered refractory hypoxia together with heart failure-related hypoperfusion.
Using ECMO support may be attractive, but its success depends on several major factors.
First, an early set-up is needed to avoid irreversible tissue lesions. The set-up in the operating theatre before the operation allowed optimal hemodynamic management without inotropic drugs and led to myocardial recovery.
The literature does not provide any data assessing the respiratory impact of prompt management with ECMO-type circulatory assistance after cardiac surgery. The mechanical ventilation commonly used during ECMO-type assistance for respiratory purposes is very much like respiratory assistance used for acute respiratory distress, i.e., with small volumes (<10 mL/kg), PEEP near 10 cm H2o2, lowest possible Fio2, and respiratory rate near 10/15 cycles/min (8). We did not choose this type of ventilation because we considered that this patient’s acute respiratory failure was not related to lesion-type pulmonary edema but to cardiogenic shock. This hypothesis was confirmed by broncho-alveolar lavage performed during a preoperative bronchus fibroscopy revealing a low protein content, which led us to a surgical approach. Despite the risk of barotraumas (4), we were able to use more powerful ventilation with high running volumes and high mean pressures to start. This ventilation was possible because the myocardial response, especially from the right ventricle to such assistance, was compensated by the high output of ECMO.
Furthermore, the assistance allowed an important right side unload, thus avoiding right ventricle dilation and allowing proper function. This left ventricular activity permitted gradual venting of the pulmonary apparatus, as well as a decrease in the pulmonary edema.
We focused on maintaining a sinus rhythm with amiodarone treatment to maintain an efficient atrial contractility. The absence of left ventricular failure probably permitted the maintenance of a satisfactory transmitral flow. During ECMO use, we observed a pulsed flow confirming ventricular ejection. Preservation of both atrial contraction and satisfactory transmitral flow has probably allowed prevention of prosthetic mitral valve thrombosis. ECMO use when treating post-cardiotomy cardiogenic shock seems to have a prevailing place with different results. According to different studies, mortality at 30 days varies between 58% and 73% (8–10). Nevertheless, these results concern all cardiac surgery indications: coronary artery bypass grafting, valvular surgery, and thoracic aorta surgery. When only considering results obtained with patients having undergone isolated valvular surgery, these results are disastrous. Magovern and Simpson (9) and Ko et al. (10) reported a 13% and 33% survival, respectively.
The main problem of implanting a mechanical valve in mitral position in this patient is the risk of thrombosis. We maintained high anticoagulant levels with partial thromboplastin times, three times the control, and thrombin times, three times the control, during the whole assistance period. This high level of anticoagulation did not generate excessive bleeding in the cannulae, reoperation for hemorrhage, or cardiac tamponade. Thoracic drains remained for 4 days, and total bleeding was ∼1680 mL. The patient received six red blood concentrated units.
Although the patient died later because of the occurrence of medullar aplasia and infectious shock, ECMO use allowed a favorable early recovery period.
In summary, the use of ECMO assistance is becoming more important when managing hemodynamic failures. On the contrary, in the case of severe respiratory failures in adults, this assistance seems to be less helpful. An early set-up of circulatory support with veno-arterial oxygenation allows pulmonary and right heart side recovery during post-CPB, thus avoiding the use of inotropic drugs and complex ventilator support. It requires an efficient anticoagulant therapy and a transmitral flow preservation to avoid valvular thrombosis.
REFERENCES
- 1.Hameed A, Karaalp IS, Tummala PP, et al. . The effect of valvular heart disease on maternal and fetal outcome of pregnancy. J Am Coll Cardiol. 2001;37:893–9. [DOI] [PubMed] [Google Scholar]
- 2.De Souza JA, Martinez EE Jr, Ambrose JA, et al. . Percutaneous balloon mitral valvuloplasty in comparison with open mitral valve commissurotomy for mitral stenosis during pregnancy. J Am Coll Cardiol. 2001;37:900–3. [DOI] [PubMed] [Google Scholar]
- 3.Vahanian A, Palacios IF.. Percutaneous approaches to valvular disease. Circulation. 2004;109:1572–9. [DOI] [PubMed] [Google Scholar]
- 4.Dreyfuss D, Saumon G.. Ventilator-induced lung injury: Lessons from experimental studies. Am J Respir Crit Care Med. 1998;157:294–323. [DOI] [PubMed] [Google Scholar]
- 5.Zapol WM, Snider MT, Hill JD, et al. . Extracorporeal membrane oxygenation in severe acute respiratory failure. A randomized prospective study. JAMA. 1979;242:2193–6. [DOI] [PubMed] [Google Scholar]
- 6.Gattinoni L, Pesenti A, Mascheroni D, et al. . Low-frequency positive-pressure ventilation with extracorporeal CO2 removal in severe acute respiratory failure. JAMA. 1986;256:881–6. [PubMed] [Google Scholar]
- 7.Kolobow T, Gattinoni L, Tomlinson TA, Pierce JE.. Control of breathing using an extracorporeal membrane lung. Anesthesiology. 1977;46:138–41. [DOI] [PubMed] [Google Scholar]
- 8.Doll N, Kiaii B, Borger M, et al. . Five-year results of 219 consecutive patients treated with extracorporeal membrane oxygenation for refractory postoperative cardiogenic shock. Ann Thorac Surg. 2004;77:151–7. [DOI] [PubMed] [Google Scholar]
- 9.Magovern GJ Jr, Simpson KA.. Extracorporeal membrane oxygenation for adult cardiac support: The Allegheny experience. Ann Thorac Surg. 1999;68:655–61. [DOI] [PubMed] [Google Scholar]
- 10.Ko WJ, Lin CY, Chen RJ, et al. . Extracorporeal membrane oxygenation support for adult postcardiotomy cardiogenic shock. Ann Thorac Surg. 2002;73:538–45. [DOI] [PubMed] [Google Scholar]


