Abstract:
The coagulation-fibrinolytic profile during cardiopulmonary bypass (CPB) has been widely documented. However, less information is available on the possible persistence of these alterations when autotransfusion is used in management of perioperative blood loss. This study was designed to explore the influence of autotransfusion management on intravascular fibrin degradation and postoperative transfusions. Thirty patients, undergoing elective primary isolated coronary bypass grafting, were randomly allocated either to a control group (group A; n = 15) or an intervention group (group B; n = 15) in which mediastinal and residual CPB blood was collected and processed by a continuous autotransfusion system before re-infusion. Intravascular fibrin degradation as indicated by D-dimer generation was measured at five specific intervals and corrected for hemodilution. In addition, chest tube drainage and need for homologous blood were monitored. D-dimer generation increased significantly during CPB in group A, from 312 to 633 vs. 291 to 356 ng/mL in group B (p = .001). The unprocessed residual blood (group A) revealed an unequivocal D-dimer elevation, 4131 ± 1063 vs. 279 ± 103 ng/mL for the processed residual in group B (p < .001). Consequently, in the first post-CPB period, the intravascular fibrin degradation was significantly elevated in group A compared with group B (p = .001). Twenty hours postoperatively, no significant difference in D-dimer levels was detected between both groups. However, a significant intra-group D-dimer elevation pre- vs. postoperative was noticed from 312 to 828 ng/mL in group A and from 291 to 588 ng/mL in group B (p < .01 for both). Postoperative chest tube drainage was higher in the patients from group A, which also had the highest postoperative D-dimer levels. Patients in group A perceived a higher need for transfusions of red cells suspensions post-operatively. These data clearly indicate that autotransfusion management during and after CPB suppresses early postoperative fibrin degradation.
Keywords: cardiopulmonary bypass, cardiotomy suction, coronary surgery, autotransfusion, fibrin degradation
During cardiopulmonary bypass (CPB), exposure of blood to large surgical wounds and conditions in the extracorporeal circuit, as well as reinfusion of shed blood containing tissue factor and other activated coagulation and fibrinolytic proteins, results in nonspecific (nonphysiologic) activation of numerous plasmatic systems (1,2).
The complexity of these responses makes effective therapeutical strategy a difficult challenge. Improvements in hemocompatibility of the extracorporeal circuit, elimination of the air-surface interface in the reservoir, infusion of antifibrinolytics, and washing and reinfusing of shed red cells intraoperatively have been shown to affect various factors of the blood coagulation system (2–7). Because activation of the clotting system during CPB is predominantly driven by the tissue factor pathway, one can expect a dysregulation of the hemostatic profile immediately after surgery with or without processing the residual CPB blood before re-transfusion (8,9).
The aim of our study was to investigate the influence of processing both shed mediastinal blood and residual CPB blood, so-called autotransfusion management, before retransfusion on the intravascular fibrin degradation, measured as plasma D-dimer levels (10–12). In addition, chest tube drainage and postoperative transfusion requirements were monitored.
MATERIALS AND METHODS
Patients
Thirty adult patients, undergoing isolated primary elective myocardial re-vascularization, were enrolled in this explorative clinical trial. The patients were randomly allocated either to a control group (group A; n = 15) or an intervention group (group B; n = 15) using sealed, opaque, sequentially numbered envelopes. The sequence of allocations was obtained from a computer-generated random number list. In the intervention group, the mediastinal and residual CPB blood was processed by a continuous autotransfusion system (CATS; Fresenius HemoCare, Bad Homburg, Germany) before reinfusion, using the quality wash protocol as described in the user manual by the manufacturer. Clinicians in the intensive care unit were blinded to the group.
Informed consent was obtained from each patient the day before the operation. The study was approved by the local ethical and research council. No patient had evidence of severe heart failure, renal or hepatic dysfunction, or preoperative coagulopathies. Moreover, no patient was treated with coumarin derivatives or nonsteroidal antiinflammatory agents within 5 days before the operation. All patients were on aspirin treatment before and after their operation. The study patients were not to receive antifibrinolytic agents during or after CPB.
Anesthesia and CPB
Anesthesia was induced with midazolam, sufentanil, and pancuronium. All patients had Swan-Ganz and arterial catheters placed. All subjects underwent standard CPB using a closed heparin-coated extracorporeal system (Bioline; Maquet Cardiopulmonary, Hirrlingen, Germany). Before connection of the extracorporeal circuit for CPB, each patient received 300 IU/kg porcine heparin to achieve a kaolin-based activated coagulation time (ACT) >480 seconds. If necessary, additional boluses of heparin were administered to maintain an ACT >400 seconds during bypass.
CPB was conducted using non-pulsatile flow. The patients were cooled to a blood temperature of 32–34°C, at which a flow rate of 2.2–2.4 L/min/m2 and an arterial blood pressure >50 mmHg was maintained. Myocardial protection was achieved using 600–1000 mL St. Thomas’ II cardioplegic solution at 4°C through the aortic root. Cardiotomy suction, blood aspirated from the pericardium and pleural space, was returned to a separate Biolinecoated cardiotomy reservoir (Maquet Cardiopulmonary) and retransfused either processed or unprocessed. On completion of the distal anastomoses during a single period of cross-clamping, the proximal anastomosis was performed using a partial occluding clamp, while the rewarming of the patient to 37°C continued. After CPB, heparin was reversed by 3 mg/kg protamine chloride. All pump blood was returned to the patient through the aortic cannula or intravenously through infusion bags with or without a cell-washing process.
Blood Sampling
During and after the operation, blood samples were collected from each patient at five specific intervals: before induction of anesthesia (baseline), after 200 mL cardiotomy suction blood was collected (onset CPB), before cessation of bypass (cessation CPB), and 2 and 20 hours after arrival at the intensive care unit (2 hrs ICU and 20 hrs ICU). Additional samples were taken from the processed or unprocessed residual CPB blood before retransfusion (infusion bag).
In addition, the volume of the chest tube drainage was noted 2 hours after arrival at the ICU, and the transfusion requirements were noted during the entire ICU period.
Assay
Blood samples were collected in 2.5-mL Vacutainer tubes containing a buffered citrate solution (0.109 mol/L), theophylline, adenosine, and dipyridamole. Plasma for determination of intravascular fibrin degradation (measured as D-dimer levels) was obtained by centrifugation at 1500g for 20 minutes and stored at −80°C until assayed. D-dimer generation was measured using an immunoturbidimetric assay (Tina-quant; Roche Diagnostics Nederland BV, Almere, The Netherlands). Reference range for D-dimer concentrations is <500 ng/mL. All samples were corrected for hemodilution.
Statistics
The results were expressed as mean ± SE, except for patient demographics, which were expressed as mean ± SD. The Wilcoxon rank sum test was used to compare groups (on fixed time parameters), whereas the Wilcoxon signed ranks test was used to compare the groups on increase of parameter over time. To study the association between intravascular fibrin degradation and postoperative chest tube drainage, Pearson test for linear correlation was used. The χ2 test was used to compare proportions. p < .05 was considered statistical significant between measured values.
RESULTS
Patient Demographics
Demographic data of patients in the experimental groups are shown in Table 1. There was no significant difference in baseline characteristics between the two groups.
Table 1.
Patients’ demographics.
| Group A (n = 15) | Group B (n = 15) | |
|---|---|---|
| Age (years) | 66 ± 8 | 62 ± 11 |
| Sex (male/female) | 11/4 | 13/2 |
| Body surface area (m2) | 1.92 ± 0.10 | 2.03 ± 0.20 |
| Bypass time (min) | 86 ± 21 | 98 ± 25 |
| Aortic cross-clamp time (min) | 55 ± 17 | 57 ± 17 |
Data are shown as mean ± SD.
D-Dimer Levels
The impact of re-infusion of processed shed mediastinal blood and residual CPB blood on fibrin degradation is shown in Figure 1. Plasma D-dimer levels increased from 312 to 633 ng/mL during CPB in the control group compared with a moderate increase from 291 to 356 ng/mL in the intervention group (p = .001). D-dimer levels in unprocessed residual CPB blood (group A) were 15-fold (p = .001) higher than the levels in processed residual blood (group B). During the early postoperative period (2 hrs ICU), a profound intravascular fibrin breakdown was observed in group A (D-dimer level, 1849 vs. 580 ng/mL in group B, p = .001). Later (20 hrs ICU), no significant difference in D-dimer levels was detected between both groups (p = .05). However, compared with baseline values, a significant intra-group D-dimer elevation was noticed in the postoperative period (from 312 to 828 ng/mL in group A and from 291 to 588 ng/mL in group B, p < .01 for both).
Figure 1.
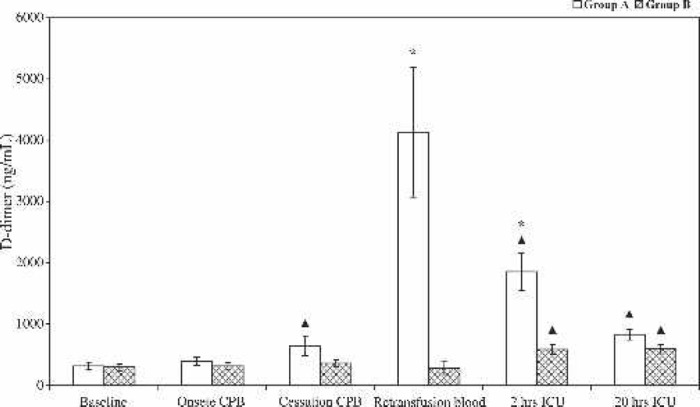
Plasma concentration (mean ± SE) of cross-linked fibrin degradation products (D-dimer) during and after CPB in group A (bars) and group B (cross-hatched bars). *Statistically significant difference between the two groups (p < .001). ΔIntra-group statistically significant difference compared with baseline values (p < .01).
A positive, however statistically not significant, association (r = 0.24, p = .22) was observed between intravascular fibrin degradation and the volume of postoperative chest tube drainage.
Chest Tube Drainage and Blood Transfusion Requirements
The volume of fluid from chest tube drainage was highest in patients from group A, who also had the highest D-dimer level (Figure 2).
Figure 2.
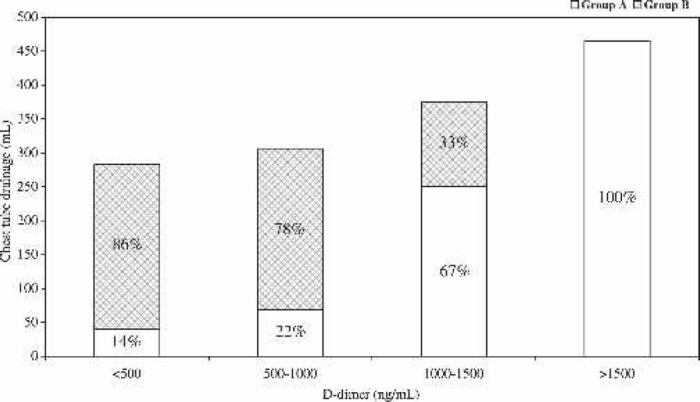
Relationship between plasma D-dimer levels and postoperative chest tube drainage. Plasma D-dimer levels were arbitrary subdivided into four groups. Each bar represents the amount of patients (in percentage) from group A and group B (cross-hatched).
A cross-tabulation for the postoperative transfusion requirements in both groups is shown in Table 2. Although statistically not significant, more patients in group A (67% vs. 54% in group B, p = .05) received at least one packed red cells concentrate postoperatively. In addition, up to 47% of patients in group A needed transfusion with two or more red cell suspensions compared with only 13% of patients in group B (p = .04).
Table 2.
Postoperative transfusion requirements in the control (A) and intervention (B) group.
| Group A (n = 15) | Group B (n = 15) | |
|---|---|---|
| No transfusion | 5 (33%) | 7 (47%) |
| At least one PRC | 10 (67%) | 8 (54%) |
| At least two PRC | 7 (47%) | 2 (13%)* |
p < .05.
DISCUSSION
During the past decade, increased attention was paid to the so-called material-independent coagulation pathway activation with inherent enhanced breakdown of fibrin during and after CPB. In this study, we showed that autotransfusion management during and after CPB effectively suppresses the early postoperative D-dimer elevation in patients undergoing primary myocardial revascularization.
Fibrin is a temporary and localized matrix that not only covers a wound but also provides a structure that allows easy access of invading cells during the complex events that accompany healing. As part of the healing process, most fibrin is removed by cooperative efforts of the fibrinolytic system, leukocytes, fibroblasts, and other inflammatory cells (13). Thus, fibrin formation and resolution are fundamental tissue repair processes in the human body. In our study, D-dimer was chosen as a plasma marker for determining fibrinolytic activity. D-dimer is a cross-linked fibrin degradation product that is released from the clot on action of the endopeptidase plasmin and constitutes a vital part of the wound healing process. This remodeling process requires a balanced interaction between the cells involved (14). Retransfusion of unprocessed shed mediastinal and residual CPB blood accelerates the activation of fibrin breakdown and might therefore influence the risk of bleeding. In contrast, washing steps and concentration of blood cells before retransfusion resulted in a better-balanced fibrin formation, tuning the fibrinolytic regulation postoperatively back toward normal.
During CPB, the coagulation system is predominantly triggered through the tissue factor pathway by retransfusion of highly thrombogenic blood aspirated from the thoracic cavities (2,5,7). Consequently, mitigation of the degree of activation and fibrin degradation can be expected when the aspirated blood from the thoracic cavities is washed and concentrated before re-transfusion. In our study, fibrin degradation was significantly lower in group B at the moment of cessation of bypass compared with group A. The increase in fibrinolytic activity during CPB in group A can therefore be explained by thrombinmediated endothelial activation or injury rather than by blood-material interaction or surgery per se. Previously, it has been shown that increased levels of thrombin as found during CPB stimulate the release of tissue-type plasminogen activator (t-PA) from endothelial cells (15). This observation has been corroborated recently by Oliver et al. (16), who showed that active t-PA levels in blood represent endothelial dysfunction, thereby promoting endogenous fibrinolysis. After re-transfusion of processed residual CPB blood, a significant decrease in fibrin degradation was noticed during the early postoperative period (2 hrs ICU) compared with the control group. In contrast, Daane et al. (8), using an uncoated extracorporeal circuit, did not find such a decrease in fibrin degradation when only the residual CPB blood was processed before retransfusion. This discrepancy can be explained by the potential source of activated hemostatic components from blood aspirated from the thoracic cavities, when only the residual CPB blood is processed before re-transfusion. Moreover, it has been shown that thrombin generation during CPB is likely to be an important stimulus for activation of the fibrinolytic pathway in the early postoperative period. A statistically significant correlation between thrombin-antithrombin levels at the end of the operation and plasmin-α2-antiplasmin levels 1 hour postoperatively were observed (10). This observation suggest that hyperfibrinolysis after CPB is directly or at least indirectly mediated by activation of coagulation during CPB. Therefore, we note that, to achieve clinical benefit from autotransfusion techniques during cardiac surgery, both the shed mediastinal blood and residual CPB blood needs to be cell washed before reinfusion.
The elevated systemic levels of D-dimers in our study most likely represent a normal physiologic response rather than a pathologic response such as disseminated intravascular coagulation. In fact, as suggested by others, a mild (500–1000 ng/mL) to moderate (1000–2000 ng/mL) increase of D-dimer levels may result from clot remodeling at the operative site (11,17). In addition, it should be noted that significant alterations in dynamics of clot formation and rate of remodeling (secondary fibrinolysis), as the primary cause of elevated D-dimers in patients undergoing CPB, depends on the clinical setting. Returning shed mediastinal blood, inadequate systemic heparinization, and hemodilution are factors that can aggravate thrombin generation driving secondary fibrinolysis (18).
Interestingly, patients in the control group with the highest postoperative D-dimer levels also had the highest volumes of chest tube drainage. Similar results were also reported by Teufelsbauer et al. (10). This suggests that early postoperative excess bleeding predisposes to increase clot formation and subsequent clot remodeling causing elevated D-dimer concentrations. However, to further substantiate a causal relationship between the increase in D-dimer and the risk of bleeding, it would be of importance to administer an anti-fibrinolytic agent before heparin reversal to see if such a treatment would correct both the elevated D-dimer and decrease the bleeding.
Beside the enhanced fibrinolytic state, blood preservation during cardiac surgery is critically important because of the inherent risks of homologous blood transfusions (19,20). In open heart surgery, ∼70% of the patients undergoing CPB receive at least one transfusion (20). In our study, similar results were seen in group A (67%). In group B, the need for blood transfusion tended to be lower. It is tempting to speculate that the reduction in blood transfusion requirements is caused by the higher hemoglobin content of the processed blood. However, systemically, no differences in hemoglobin levels were noticed between the experimental groups (data not shown). Whether our finding is related to the washout of bioactive components and consequently reduction of blood loss altering the transfusion trigger needs to be further elucidated. Nevertheless, this explorative clinical study showed that autotransfusion management during and after CPB reduces the perceived need for transfusion of allogeneic red blood cell suspensions in patients undergoing primary myocardial re-vascularization. The ability to reduce blood transfusions may, in part, favorably affect patient outcomes and overall health care costs.
In conclusion, processing shed mediastinal blood and residual CPB blood suppresses the early postoperative fibrin degradation and may therefore move hemostatic regulation back toward normal.
ACKNOWLEDGMENTS
The authors thank Paul Lap for skillful assistance in performing the immunoturbidimetric assay. This research was conducted at the Radboud University Nijmegen Medical Centre, Nijmegen, The Netherlands.
REFERENCES
- 1.Paparella D, Brister SJ, Buchanan MR.. Coagulation disorders of cardiopulmonary bypass: A review. Intensive Care Med. 2004;30:1873–81. [DOI] [PubMed] [Google Scholar]
- 2.Weerwind PW, Lindhout T, Caberg NEH, De Jong DS.. Thrombin generation during cardiopulmonary bypass: The possible role of retransfusion of blood aspirated from the surgical field. Thromb J. 2003;1:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gunaydin S.. Clinical significance of coated extracorporeal circuits: A review of novel technologies. Perfusion. 2004;19:S33–41. [DOI] [PubMed] [Google Scholar]
- 4.Wan S, LeClerc J-L, Vincent J-L.. Inflammatory response to cardiopulmonary bypass: Mechanisms involved and possible therapeutic strategies. Chest. 1997;112:676–92. [DOI] [PubMed] [Google Scholar]
- 5.Aldea GS, Soltow LO, Chandler WL, et al. Limitation of thrombin generation, platelet activation, and inflammation by elimination of cardiotomy suction in patients undergoing coronary artery bypass grafting treated with heparin-bonded circuits. J Thorac Cardiovasc Surg. 2002;123:742–55. [DOI] [PubMed] [Google Scholar]
- 6.Eisses MJ, Seidel K, Aldea GS, Chandler WL.. Reducing hemostatic activation during cardiopulmonary bypass: A combined approach. Anesth Analg. 2004;98:1208–16. [DOI] [PubMed] [Google Scholar]
- 7.De Somer F, Van Belleghem Y, Caes F, et al. Tissue factor as the main activator of the coagulation system during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2002;123:951–8. [DOI] [PubMed] [Google Scholar]
- 8.Daane CR, Golab HD, Meeder JH, Wijers MJ, Bogers AJ.. Processing and transfusion of residual cardiopulmonary bypass volume: effects on haemostasis, complement activation, postoperative blood loss and transfusion volume. Perfusion. 2003;18:115–21. [DOI] [PubMed] [Google Scholar]
- 9.Sirvinskas E, Lenkutis T, Raliene L, Veikutiene A, Vaskelyte J, Marchertiene I.. Influence of residual blood autotransfused from cardiopulmonary bypass circuit on clinical outcome after cardiac surgery. Perfusion. 2005;20:71–5. [DOI] [PubMed] [Google Scholar]
- 10.Teufelsbauer H, Proidl S, Havel M, Vukovish T.. Early activation of hemostasis during cardiopulmonary bypass. Evidence for thrombinmediated hyperfibrinolysis. Thromb Haemost. 1992;68:250–2. [PubMed] [Google Scholar]
- 11.Comunale ME, Carr JM, Moorman RM, Robertson LK.. Significance of D-dimer concentrations during and after cardiopulmonary bypass. J Cardiothorac Vasc Anesth. 1996;10:477–81. [DOI] [PubMed] [Google Scholar]
- 12.Chandler WL, Velan T.. Plasmin generation and D-dimer formation during cardiopulmonary bypass. Blood Coagul Fibrinolysis. 2004;15:583–91. [DOI] [PubMed] [Google Scholar]
- 13.Sidelmann JJ, Gram J, Jespersen J, Kluft C.. Fibrin clot formation and lysis: basic mechanisms. Semin Thromb Hemost. 2000;26:605–18. [DOI] [PubMed] [Google Scholar]
- 14.Collen A, Koolwijk P, Kroon M, van Hinsbergh V.. Influence of fibrin structure on the formation an maintenance of capillary-like tubules by human microvascular endothelial cells. Angiogenesis. 1998;2:153–65. [DOI] [PubMed] [Google Scholar]
- 15.Emeis JJ.. Mechanisms involved in short-term changes in blood levels of t-PA. In: Kluft C, ed. Tissue-Type Plasminogen Activator (t-PA): Physiological and Clinical Aspects, Vol 2 Boca Raton, FL: CRC Press;1988:22–31. [Google Scholar]
- 16.Oliver JJ, Webb DJ, Newby DE.. Stimulated tissue plasminogen activator release as a marker of endothelial function in humans. Arterioscler Thromb Vasc Biol. 2005;25:2470–9. [DOI] [PubMed] [Google Scholar]
- 17.Parolari A, Colli S, Mussoni L, et al. Coagulation and fibrinolytic markers in a two-month follow-up of coronary bypass surgery. J Thorac Cardiovasc Surg. 2003;125:336–43. [DOI] [PubMed] [Google Scholar]
- 18.Whitten CW, Greilich PE, Ivy R, Burkhardt D, Allison PM.. D-dimer formation during cardiac and noncardiac thoracic surgery. Anesth Analg. 1999;88:1226–31. [DOI] [PubMed] [Google Scholar]
- 19.Fransen E, Maessen J, Dentener M, Senden N, Buurman W.. Impact of blood transfusions on inflammatory mediator release in patients undergoing cardiac surgery. Chest. 1999;116:1233–9. [DOI] [PubMed] [Google Scholar]
- 20.Cross MH.. Autotransfusion in cardiac surgery. Perfusion. 2001;16:391–400. [DOI] [PubMed] [Google Scholar]


