Abstract:
There is little information showing the use of microporous polypropylene hollow fiber oxygenators during extracorporeal life support (ECLS). Recent surveys have shown increasing use of these hollow fibers amongst ECLS centers in the United States. We performed a retrospective analysis comparing the Terumo BabyRx hollow fiber oxygenator to the Medtronic 800 silicone membrane oxygenator on 14 neonatal patients on extracorporeal membrane oxygenation (ECMO). The aim of this study was to investigate the similarities and differences when comparing pressure drops, prime volumes, oxygenator endurance, and gas transfer capabilities between the two groups.
Keywords: hollow fiber, silicone, oxygenator, extracorporeal membrane oxygenation
The silicone membrane oxygenator has been the workhorse for extracorporeal membrane oxygenation (ECMO) in the United States since the inception of ECMO as a medical treatment. Despite the technological advancement of hollow fiber oxygenators used for cardiopulmonary bypass, few, if any, had crossed over to the ECMO population until recently. Presently, routine hollow fiber use in the United States is 16%–19% in cardiac centers (1,2) compared with 3% in 2002 (3). However, there is little published information regarding the performance characteristics of these oxygenators for periods of extended use.
We performed a retrospective analysis comparing the BabyRx microporous polypropylene hollow fiber oxygenator (Terumo Cardiovascular, Ann Arbor, MI) to the 800 silicone membrane oxygenator (Medtronic, Minneapolis, MN) on 14 neonatal ECMO patients. The aim of this study was to investigate the similarities and differences when comparing pressure drops, prime volumes, oxygenator endurance, and gas transfer capabilities between the two groups.
MATERIALS AND METHODS
After Institutional Review Board approval, patients’ charts were retrospectively reviewed after undergoing ECMO for either respiratory or post-cardiac support at Doernbecher Children’s Hospital. The time period for the study was from May 2004 through December 2005. Two groups of patients were garnered from this time frame. The Silicone Membrane patient group (SIL) included patients placed on ECMO from May 2004 to January 2005 in which the Medtronic 800 silicone oxygenator was used. The Hollow Fiber Oxygenator patient group (HFO) included patients from March 2005 to December 2005 and used the BabyRx hollow fiber oxygenator with Xcoating. All other ECMO patients using different oxygenators were excluded from the study.
All circuits were pre-primed with Normosol-R for up to 30 days. Silicone membranes were also preprimed up to 30 days. One hundred units of heparin was added to each unit of packed red blood cells (pRBCs), and the pRBCs were added to the circuit to chase out the Normosol. Two units of pRBCs and 100 mL of fresh frozen plasma (FFP) were used to prime the SIL circuit, whereas only one unit of pRBCs and 50 mL of FFP was used to prime the HFO circuits. The SIL group circuits included the Ecmotherm (Medtronic) heat exchanger post-oxygenator, whereas the HFO contained an integrated heat exchanger. All circuits were made up of a HL20 roller pump (Jostra, Hirrlingen, Germany) with interfaced pressure transducing, a bladder, arterial CDI in-line blood gas, venous saturation, and hematocrit monitoring (Terumo Cardiovascular, Ann Arbor, MI). Nine of the 14 patients’ circuits were coated with Smartx (Sorin Cardiovascular, Arvada, CO) surface coating (2 of 7 in the SIL group; 7 of 7 in the HFO group). The hematocrit (>40%), activated clotting times (180–220 seconds), international normalized ratio (INR) (<1.5), fibrinogen levels (>150 mg/dL), and platelet counts (>100,000/pL) were all kept the same for both patient groups.
Pressure drops across oxygenators were measured by subtracting the post-oxygenator line pressure from the pre-oxygenator inlet pressure. Pressure drops were recorded hourly while on support. The two groups were compared for statistical significance hourly during the first 72 hours of support.
Oxygenator FiO2 and sweep values were recorded hourly for each patient on support. The two groups were compared for statistical significance for each hour in the first 72 hours of ECMO. The protocols for paO2 and paCO2 parameters were kept the same for all patients in the retrospective study.
Costs were compared by analyzing the three variables in the circuit that were different when the switch was made to the HFO group. These variables included donor blood costs, cost of oxygenators (including changeouts), and additional heat exchanger costs. Expenses were tabulated for each group and divided by seven to give an average cost per case. Oxygenator endurance was analyzed and is defined as the length of time the oxygenator was used before incapable of efficient gas exchange. All data were loaded onto Excel spreadsheets. Two-tailed two-sample t tests were used to determine statistical significance (p < .05).
RESULTS
A total of 14 patients were included in this review. Patient demographics are summarized in Table 1. The HFO circuit prime was significantly less than the SIL group (p < .05). The HFO circuit required 236 mL to prime, whereas the SIL circuit needed 450 mL to prime.
Table 1.
Patient demographics
| Group | Medtronic Silicone | Terumo BabyRx Hollow Fiber |
|---|---|---|
| No. of patients | 7 | 7 |
| Male patients | 5/7 | 5/7 |
| Mean weight (range) | 4.0 kg (2.2–6.0 kg) | 3.4 kg (2.9–4.3 kg) |
| Mean age (range) | 53 days (14–140 days) | 35 days (3–71 days) |
| Respiratory ECMO | 1/7 | 2/7 |
| Veno-veno ECMO | 0/7 | 1/7 |
| Mean hours on ECMO (range) | 149 (15–278) | 130 (76–160) |
| Survival to discharge rate | 4/7 | 4/7 |
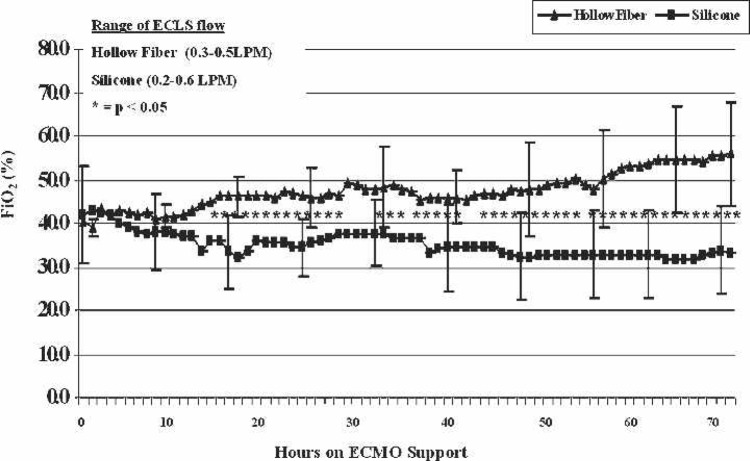
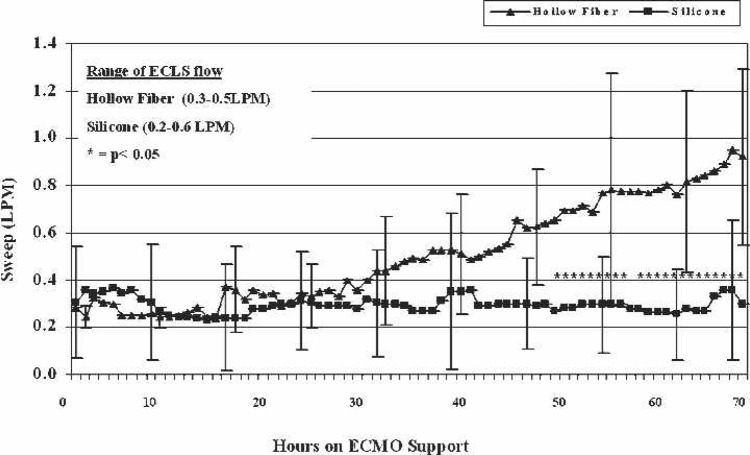
FiO2 requirements are shown by mean values of each group in Figure 1. Values that reached significance (p < .05) between the two groups are highlighted by asterisks. The SIL group favored lower FiO2 settings to achieve acceptable arterial pO2 (>90 mmHg). The SIL group also needed significantly (p < .05) lower oxygenator sweep settings to maintain adequate paCO2 (35–45 mmHg) in the majority of data points after hour 49 of ECMO (Figure 2). Significant values are highlighted by asterisks.
Figure 1.
FiO2 requirements during the first 72 hours of ECMO support. Hours in which statistical significance is shown between the two groups are noted by asterisks.
Figure 2.
Sweep requirements during the first 72 hours of ECMO support. Hours in which statistical significance is shown between the two groups are noted by asterisks.
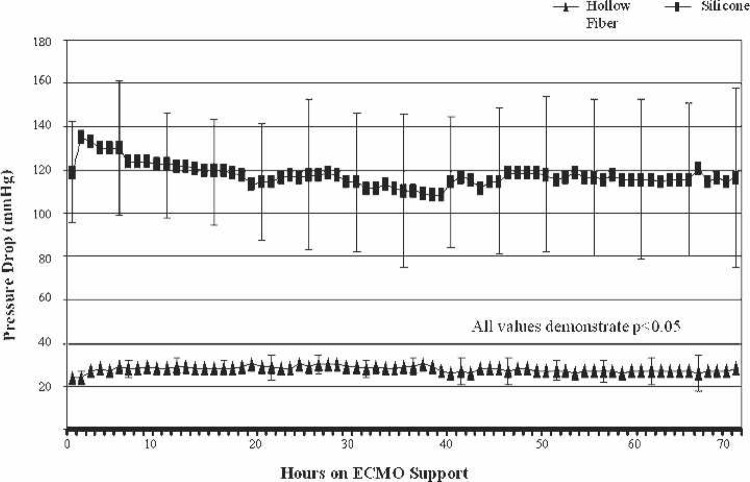
The HFO group showed significantly less transmembrane pressures (p < .001) during the first 72 hours of support at flow rates of 0.27–0.55 liters per minute (LPM) (Figure 3). Oxygenator endurance was less in the patients in the HFO group (four of seven were changed out) compared with the SIL patients (two of seven were changed out). The BabyRx hollow fiber membrane was changed out at an average of 87.7 hours (range, 78–102 hours) during the four instances it failed. The remaining three ECMO runs had uncomplicated hollow fiber performance (range, 76–160 hours). One silicone membrane was changed out after 190 hours and one clotted off after 30 minutes of support and had to be replaced. The hollow fiber group saw an average cost savings of $822.00 per patient.
Figure 3.
Transmembrane pressure comparison during the first 72 hours of ECMO support. All values show statistical significance (p < .05).
DISCUSSION
There are several benefits to using the hollow fiber for ECMO. First, the BabyRx hollow fiber takes less than a minute to prime. The primer inserts the dry oxygenator into a pre-primed circuit and keeps the outlet of the oxygenator clamped. The roller pump is slowly turned on, deairing the oxygenator through open purge lines at the top of the BabyRx. The decreased priming time has caused a change in protocols when using this oxygenator vs. the silicone lung. We no longer maintain a primed oxygenator at all times and therefore do not have to dispose of primed oxygenators if not used within a certain time frame. Also, pre-primed oxygenators averaged lower oxygen transfer values than freshly prepared oxygenators (4). The HFO group also showed significantly less membrane pressure drops under similar flow conditions than the silicone group. Less pressure drop translates into a reduction in shear forces and stresses resulting in less hemolysis and leukocyte activation (5).
Oxygenator endurance proved to be an issue when comparing HFOs with SILs. Figures 1 and 2 show the contrasting FiO2 and sweep rates between the two groups. Generally speaking, gas exchange capabilities were equal until later in the runs. All four oxygenator changeouts in the HFO group were caused by poor CO2 removal at high sweep rates. For example, one patient had an arterial CDI 500 pCO2 value in the mid-50s mmHg at a sweep of 3 L/min. The sweep rate had increased from 3 to 4 L/min over the previous 6 hours. It was apparent that we would have to continue to increase the sweep rate to higher levels to maintain acceptable arterial pCO2, so the decision to change the oxygenator was made. One reason could be the effects of plasma leakage through the hollow fibers not seen in the silicone membranes. We frequently observed a clear water-like fluid dripping from the exhaust port of the hollow fiber oxygenator after the first 24 hours of support. The condensation was evacuated by increasing the sweep rate by 1 L/min for 1 minute once an hour. The amount of fluid expelled out the exhaust was not measurable, nor did it affect the hemodynamic status or electrolyte balance of the patient.
Another advantage of the HFO group is the presence of a surface coating on the gas exchange fibers, whereas the silicone membranes cannot be coated. Although debate still exists whether coated circuits are cost effective (6) or truly evidence based, biocompatible surface coatings have shown reductions in cytokine levels (7), thrombin formations (8), chest tube output, and improved biocompatibility (9,10). Since switching to coated oxygenators and circuits, we have experienced zero changeouts caused by clot formation on circuit surfaces.
One more benefit to using the hollow fiber oxygenator was the reduced amount of blood used to prime our ECMO circuit. By decreasing the quantity of donor blood exposure, febrile reactions, incompatibility reactions, and infection transmissions may be greatly reduced (11). Although we have not eliminated blood use, transfusion dose, or the amount of RBCs transfused, is one of the most significant predictors of infection, length of hospital stay, days of fever, and antibiotic treatment (12). The complete avoidance of RBC donation will be difficult because of the variety of ECMO patients, but using less donor blood will certainly prove beneficial. In addition, costs were greatly reduced. We estimated our hospital costs to administer a unit of blood to be at $300–400, although some may place blood donation costs as high as $1500 per unit after adjusting for risks, acquisition and fixed overhead (13). Comparing total blood use throughout the ECMO run could be of value with case-matched patients. However, we could not draw a fair conclusion in this regard because of our diverse ECMO patient population and the low number enrolled in the study.
Outside the United States, ECMO centers are using the polymethylpentene (PMP) fiber oxygenators exclusively for neonatal ECLS support. These PMP oxygenators show all of the similar attributes (14) stated in our study, with the added benefit of longer endurance. The PMP fibers have recently been introduced for adult ECMO but still remain unavailable for neonatal oxygenators. Therefore, until the PMP fibers are available for neonatal oxygenators in the United States, we feel the advantages of decreased blood use, lower pressure drops, greater cost savings, and coated fibers all outweigh the lone drawback of oxygenator endurance.
ACKNOWLEDGMENTS
The authors thank the ECMO specialists at Doernbecher Children’s Hospital for tireless hours behind the pump and ability to adapt to changes in the ECMO circuits and equipment.
REFERENCES
- 1.Searles B, Gunst G, Terry B, et al. 2004 Survey of ECMO in the neonate after open heart surgery: Circuitry and team roles. J Extra Corpor Technol. 2005;37:351–4. [PMC free article] [PubMed] [Google Scholar]
- 2.Groom RC, Froebe S, Martin J, et al. Update on pediatric perfusion practice in North America: 2005 survey. J Extra Corpor Technol. 2005;37:343–50. [PMC free article] [PubMed] [Google Scholar]
- 3.Lawson DS, Walczak R, Lawson A, et al. North American neonatal extracorporeal membrane oxygenation (ECMO) devices: 2002 survey results. J Extra Corpor Technol. 2004;36:16–21. [PubMed] [Google Scholar]
- 4.Walczak R, Lawson DS, Kaemmer D, et al. Evaluation of a preprimed microporous hollow-fiber membrane for rapid response neonatal extracorporeal membrane oxygenation. Perfusion. 2005;20:269–75. [DOI] [PubMed] [Google Scholar]
- 5.Gu YJ, Boonstra PW, Graaff R, et al. Pressure drop, shear stress, and activation of leukocytes during cardiopulmonary bypass: A comparison between hollow fiber and flat sheet membrane oxygenators. Artif Organs. 2000;24:43–8. [DOI] [PubMed] [Google Scholar]
- 6.Stammers AH, Christensen KA, Lynch J, et al. Quantitative evaluation of heparin-coated versus non-heparin coated bypass circuits during cardiopulmonary bypass. J Extra Corpor Technol. 1999;31:135–41. [PubMed] [Google Scholar]
- 7.Ozawa T, Yoshihara K, Koyama N, et al. Clinical efficacy of heparinbonded bypass circuits related to cytokine responses in children. Ann Thorac Surg. 2000;69:584–90. [DOI] [PubMed] [Google Scholar]
- 8.Pappalardo F, Valle PD, Crescenzi G, et al. Phosphorylcholine coating may limit thrombin formation during high-risk cardiac surgery: A randomized controlled trial. Ann Thorac Surg. 2006;81:886–91. [DOI] [PubMed] [Google Scholar]
- 9.De Somer F, Francois K, van Oeveren W, et al. Phosphorylcholine coating of extracorporeal circuits provides natural protection against blood activation by the material surface. Eur J Cardiothorac Surg. 2000;18:602–6. [DOI] [PubMed] [Google Scholar]
- 10.De Somer F, Van Belleghem Y, Caes F, et al. Phosphorylcholine coating offers natural platelet preservation during cardiopulmonary bypass. Perfusion. 2002;17:39–44. [DOI] [PubMed] [Google Scholar]
- 11.Blumberg N, Triulzi D, Heal J.. Transfusion-induced immunomodulation and its clinical consequences. Transfus Med Rev. 1990;4(Suppl 1):24–35. [DOI] [PubMed] [Google Scholar]
- 12.Murphy PJ, Connery C, Hicks GL Jr, et al. Homologous blood transfusion as a risk factor for postoperative infection after coronary artery bypass graft operations. J Thorac Cardiovasc Surg. 1992;104:1092–9. [PubMed] [Google Scholar]
- 13.Spiess BD.. Blood conservation: Why bother? J Cardiothorac Vasc Anesth. 2004;18(4 Suppl):1S–5S. [DOI] [PubMed] [Google Scholar]
- 14.Khoshbin E, Westrope C, Pooboni S, et al. Performance of polymethyl pentene oxygenators for neonatal extracorporeal membrane oxygenation: A comparison with silicone membrane oxygenators. Perfusion. 2005;20:129–34. [DOI] [PubMed] [Google Scholar]