Abstract:
Despite the advanced technologies of battery back-up for heart-lung consoles and the availability of system-wide generators, electromechanical failure is still occurring. Several heartlung machine manufacturers still provide unsafe handcranking devices to use in the case of an emergency while using a roller blood pump. A new design has been engineered to eliminate safety and quality issues for the perfusionist and the patient when the need for handcranking presents itself. A ratchet-style handcranking device was fabricated by means of a steel plate with adjustable pins. The adjustable pins allow for use with different models of the Cobe, Stockert, and Jostra heart-lung consoles, which contain roller pumps with 180° roller heads. Additional modifications such as a 1:2 transmission and fluorescent markers are also used in the design. This innovative design is an improvement in safety compared with the current handcrank provided by Cobe, Stockert, and Jostra. With this modified handcranking device, accidental reverse rotation of the roller pump head cannot occur. Fluorescent markers will improve visualization of the pump head in low-light situations. The ergonomic design improves efficiency by reducing fatigue. Most importantly, a “safe” safety device will replace the current design provided by these manufacturers, thus improving the quality of care by health care providers.
Keywords: cardiopulmonary bypass, electromechanical failure, handcranking device, heart-lung consoles, roller blood pump
Occurrences of electromechanical failure can be particularly hazardous to patients during cardiopulmonary bypass (CPB) (1). These occurrences may result in the need for safe manual manipulation of the arterial roller pump head of the extracorporeal circuit (ECC). One manufacturer of heart-lung machines, Terumo (Terumo Cardiovascular Systems, Ann Arbor, MI) (Sarns), provides users with a uni-directional hand crank made for pumps that use a central knob occluder. This design, implemented in 1974 with the Sarns 5000 heart-lung machines, is still provided with all Sarns pumps to date (2) (Figure 1). Although advanced technologies and safety mechanisms have been improved and implemented throughout the field of health care, including back-up generators and battery-powered heart-lung consoles, the current Cobe, Stockert, and Jostra heart-lung machines still allow for bidirectional handcranking to occur during a power or systems failure, which potentiates reversal of the arterial roller pump head, which in turn, may cause detrimental outcomes to the patient, including death. Therefore, a uni-directional handcranking device for Cobe, Stockert, and Jostra was fabricated (Figure 2).
Figure 1.

Terumo (Sarns) handcrank.
Figure 2.

Current handcranking device provided by Cobe, Stociert, and Jostra.
DISCUSSION
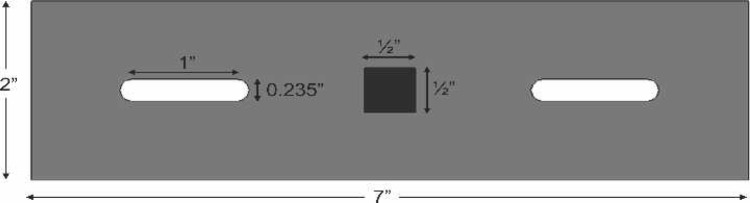
A prototype handcranking device was designed to replace the current model of handcranks provided with the Cobe, Stockert, and Jostra heart-lung machines. A ratcheting crank handle (Figure 3) was composed of black powder-coated steel and black phenolic. The switch for the ratcheting mechanism was manipulated and removed to assume a permanent uni-directional status. The two 15/64″ pins that were used as stabilizers for the handcrank were composed of a round slotted bolt with a nut made of zinc. The pins were designed so that they can be positioned in the arterial pump head for a variety of tubing diameters/occlusion settings. The spacers that were used to suspend the handcrank above the roller pump counsel are of a 1″ nylon material. A 1/2″ × 1/2″ × 1″ steel cube was welded to the top of the 2″ × 7″ steel plate (Figures 4 and 5). The device was welded together to produce one final product. Other innovative functions of the device included a 1:2 transmission and fluorescent markers for use in low-light situations. The result of the design was a uni-directional modified handcranking device for Cobe, Stockert, and Jostra heart-lung machines that eliminates safety hazards and enhances quality of patient care (Figures 6 and 7).
Figure 3.
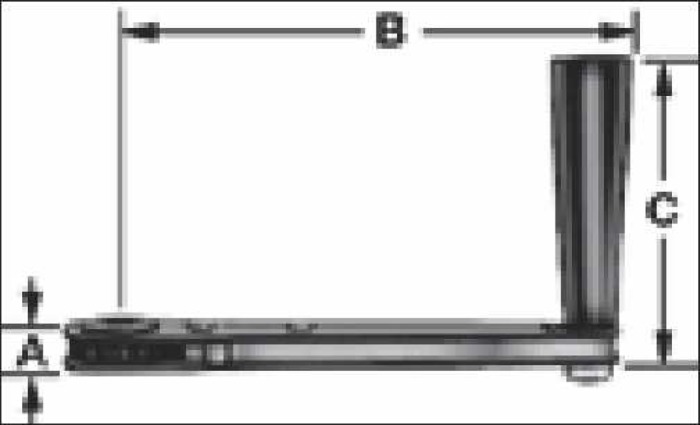
Ratcheting crank handle.
Figure 4.
Top of steel plate.
Figure 5.
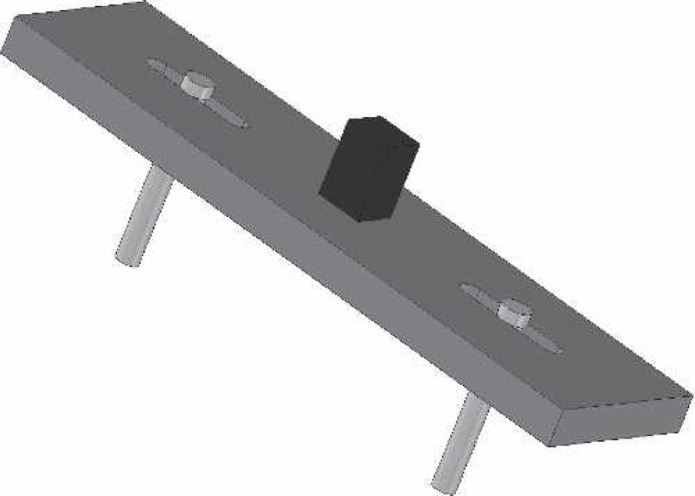
Esometric view of steel plate, pins, and square socket driver.
Figure 6.

Modified handcrank prototype.
Figure 7.

Modified handcrank prototype.
As the amount of technology that is used in medical procedures continues to increase, so to does the demand for higher electrical capabilities. Although battery backups and system-wide generators are currently implemented at most institutions, depending solely on these “safety” devices does not benefit patients 100% of the time in a state of emergency. The consequences of electromechanical failure can be devastating. The literature is still representative of statistics that show the frequency of incidences that may occur requiring handcranking. A recent international survey that identified occurrences at facilities using CPB showed that, during a 2-year period, there were 267 of 4882 cases that needed handcranking (3). Several other incidences have been documented internationally within this survey over the past 10 years, including brief power interruption, complete power failure, mechanical failure, and battery back-up/generator failure resulting in the need to handcrank.
A question addressing the possession of uni-directional vs. bidirectional handcranking devices contained with heart-lung machines and any current information on use by perfusionists was initiated on the Perflist/Perfmail forum in the spring of 2005. Four responses from perfusionists in the United States were received. In each of the responses, it was stated that at least once in their career they had a power interruption with the indication to hand crank. One response stated that a 49-minute system-wide power failure led to 49 minutes of hand cranking.
It has been shown by Dr. Christopher Troianos that a perfusionist can adequately handcrank an average pump for ∼15 minutes before beginning to fatigue (1). The proposed modified design not only accommodates the perfusionist by presenting an ergonomic device that reduces fatigue and allows for improved energy efficiency while handcranking but also contains an option to implement a 1:2 transmission, furthering the ease of manual pump rotation in the need of an emergency. Other adaptations include fluorescence outlining the major portion of the handcrank and the perimeter of the pump console. This will allow for adequate placement in low-light situations without the need to reach for any extra pieces of equipment (i.e., a flashlight).
A protocol outlining an intraoperative plan of action for complete electrical or mechanical failure should be implemented in every health care system (1). This plan should include a handcranking protocol. While a handcranking device may seem obsolete, there is a need to provide a “safe” safety device for heart-lung/ECMO consoles to not only protect perfusionists, but the quality of care delivered to patients in a critical situation. By continually improving any device involved in patient safety, the integrity of the profession and the care provided is protected.
REFERENCES
- 1.Troianos CA.. Complete electrical failure during cardiopulmonary bypass. Anesthesiology. 1995;82:298–302. [DOI] [PubMed] [Google Scholar]
- 2.Mandl JP.. Air embolism from a reversed pump head: A case report. Perfusion. 1976;2:83–4. [Google Scholar]
- 3.Marshall C, Hargrove M, O’Donnell A, Aherne T.. Variations in battery life of a heart-lung machine using different pump speeds, pressure loads, boot material, centrifugal pump head, multiple pump usage, and battery age. J Extra Corpor Technol. 2005;37:278–81. [PMC free article] [PubMed] [Google Scholar]