Abstract:
Stimulating the body’s natural healing at the cellular level can be achieved through the application of growth factors located within platelets. Once combined with a mixture of calcium and thrombin, this substance, now referred to as autologous platelet gel (APG), can be applied to surgical wound sites for patients undergoing cardiac surgery. The purpose of this study was to examine the effects of APG on surgical site infection, post-operative pain, blood loss, and bruising. After 30 mL platelet-rich plasma (PRP) was processed, 10 mL PRP was distributed on the sternum after re-approximation and 7 mL PRP before skin closure. Ten milliliters PRP was used on the endoscopic leg harvest (EVH) site. The remaining 3 mL was sent to the laboratory for hematologic testing. Both the control (CTR) and treatment (TRT) groups were well matched, with the exception of ejection fraction and pre-operative platelet count, which was significantly higher in the TRT group. Average platelet count yield was 4.2 ± 0.5 × 103/mcL, white blood count (WBC) yielded 1.9 ± 0.7 × 103/mcL, and fibrinogen yielded 1.2 ± 0.2 mg/dL above baseline. There were no deep or superficial sternal infections. However, one patient from each group did experience a leg infection at the EVH site, which occurred after hospital discharge. More patients in the TRT group experienced less pain on postoperative day (POD) 1 and at the post-operative office follow-up. Blood loss and bruising was less in the TRT group on POD 2; however, there was no statistical significance. The application of APG seems to confer beneficial effects on pain, blood loss, and bruising. However, further studies with a greater sample size are needed to power significant differences.
Keywords: growth factors, autologous platelet gel, surgical site infection, pain, nociceptors, bruising
Advancements have been made in understanding the systematic processes of wound healing. Wound healing is traditionally explained in terms of three classic phases: inflammation, proliferation, and maturation (1). A clot forms and cells of inflammation debride injured tissue during the inflammatory phase. Epithelialization, fibroplasia, and angiogenesis occur during the proliferative phase; additionally, granulation tissue forms and the wound begins to contract. Collagen forms tight cross-links to other collagen and with protein molecules, therefore increasing the tensile strength of the scar during the maturation phase.
During the inflammatory phase, the body rushes many cell types to the wound. Platelets are the first cell components to invade the wound site and initiate the wound healing process. Initially, hemostasis and cross-linked fibrin formation occur. Platelet degranulation leads to the release of growth factors (GFs) and cytokines, which play a major role in the recruitment and activation of neutrophils and macrophages (2). Platelet-derived growth factor, transforming growth factor, epidermal growth factor, and insulin-like growth factor are examples of GFs, which function to assist the body in repairing itself and stimulate new tissue regeneration (3). Major benefits of GFs include enhanced angiogenesis, improved bone regeneration, enhanced wound strength, and reduction in infection.
With multi-component pheresis and the evolution of new sequestering devices, these two elements have helped to concentrate platelets from smaller blood samples instead of the traditional one-unit phlebotomy. The ability to collect platelet-rich plasma (PRP) has allowed clinicians to use the healing properties of platelets. Specialties such as plastic and reconstructive and orthopedic have used PRP because of their ability to deliver a plethora of GFs (4,5).
The application of activator reagents such as thrombin and calcium with PRP to form autologous platelet gel (APG) provides a release of concentrated wound healing GFs. Despite anecdotal use of APG in cardiac surgery to reduce or eliminate wound infection and accelerate healing, there is little evidence in literature to support the efficacy of APG on surgical wound healing in cardiac procedures. The purpose of this study was to examine whether the application of APG on the sternum and saphenous vein harvest site was beneficial to patients undergoing coronary artery revascularization, in terms of pain, blood loss, discoloration, and surgical site infection (SSI).
MATERIALS AND METHODS
Experimental Protocol
After approval from the Siouxland Institutional Review Board, 38 patients undergoing elective coronary artery bypass grafting (CABG) with endoscopic vein harvesting (EVH) were prospectively randomized to either a control (CTR) or treatment (TRT) group. Randomization was obtained using a block method with Microsoft Excel. Because of the endpoints being measured in this research study, patients undergoing cardiac surgery, requiring either the use of the extracorporeal circuit or standby in off-pump CABG, were included. All subjects were provided information about the research study, questions were answered to the patients’ satisfaction, and informed consent was obtained. The study followed Health Insurance Portability and Accountability Act guidelines to protect patient information. Computerized spreadsheets were password protected, and patient data and consent forms were kept in a locked drawer that was only accessible by the perfusionists.
Individuals with the following criteria were excluded from the research study: hemoglobin (Hb) < 11.8 g/dL; platelet count (PLT Cnt) < 147 × 103/mcL, white blood cell count (WBC) > 9.90 × 10−3/mcL; anti-platelet medication (unless discontinued 10 days before surgery); fibrinolytic medication (unless discontinued 48 hours before surgery); anticoagulant medication (heparin, unless discontinued 4 hours before surgery, direct thrombin inhibitors). In addition, patients on intra-aortic balloon counterpulsation therapy were excluded.
Autologous blood was removed the morning of surgery using the central line in the operating room. First, 10 mL whole blood was collected for baseline analysis. Approximately 5.4 mL whole blood was injected equally into two blue top BD Vacutainers containing 0.109 mol/L, 3.2% sodium citrate. In addition, 3 mL was injected into a lavender top BD Vacutainer containing 5.4 mg K2EDTA. Laboratory analysis for the following hematologic and coagulation parameters were measured: Hb, hematocrit (Hct), PLT Cnt, platelet function, WBC, and fibrinogen concentration (FIB). Second, after baseline samples were procured, whole blood was removed using three 60 mL syringes. Each syringe contained 8 mL anticoagulant citrate dextrose (0.022 g/mL sodium citrate) before blood collection. Baseline laboratory analysis was not possible with samples from the 60 mL syringes because of the anticoagulant used. Because each 60 mL syringe was diluted with anticoagulant citrate dextrose, a hemodilution factor of 0.133 was used to calculate baseline Hb, Hct, FIB, PLT Cnt, and WBC. This method allowed the empirical values from the PRP samples to be used for descriptive statistics.
Designed as a fully automated, tabletop device, the Medtronic Magellan Autologous Platelet Separator (Minneapolis, MN) uses density differences and centrifugal forces to separate PRP from whole blood. The disposable kit was installed according to manufacturer’s instruction for use. Each 60 mL syringe was processed to obtain 10 mL PRP. After the PRP was collected, 1 mL of air was aspirated into the syringe, and the syringe was inverted 10 times to allow for proper mixing. A 1 mL sample from each PRP specimen was sent to the laboratory for the following analysis: Hb, Hct, Plt Cnt, WBC, and FIB. Overall, a total of 30 mL PRP was collected for each TRT patient, and 27 mL was transferred to the surgical field for use. One vial consisting of 5000 units bovine thrombin (GenTrac, Middleton, WI) was reconstituted with 5 mL 10% CaCl2 (Amphastar-IMS, So. El Monte, CA) to yield a concentration of 1000 units bovine thrombin per 100 mg CaCl2. After reconstitution, the thrombin-calcium activator mixture was passed to the surgical field. A Micromedics FibriJet Ratio Procedure Kit (St. Paul, MN) was delivered to the surgical field, and a 10:1 ratio of PRP to activator was used to form APG.
Once the sternum was re-approximated using stainless steel wires and tightened, a Micromedics dual spray tip was used along with 10 mL APG for the deep tissue. The spray applicator tip was used again to distribute 7 mL APG subcutaneously before presternal fascia closure. A total of 10 mL APG was used on the subcutaneous layer of the saphenous EVH site with a Micromedics 31.8 cm dual lumen endoscopic tip. The tip was inserted into an incision by the surgical technologist and simultaneously depressed both syringes while pulling back from the incision site. Patients in the CTR group had similar wound care without APG treatment. Dressings on the sternum and leg were applied according to surgeon protocol.
For both groups, a pain scale of 0–10 was used to assess pain starting in the morning of post-operative day (POD) 1 by the primary author. In addition, sternal and leg dressings were removed in the morning of POD 2 per surgeon protocol by the registered nurse and placed in a biohazard bag for further analysis. Estimated blood loss was ascertained by measuring the difference between the dry and wet dressings. The difference was used to calculate estimated blood loss using the conversion 1 mL = 1 g. Sternum and leg discoloration was measured in centimeters and continuously assessed throughout the patients’ hospital length of stay. In addition, the primary author assessed pain and discoloration during the post-operative office follow-up.
Post-operative wound infection was assessed starting on POD 2 until patients were discharged from the hospital and ∼3 weeks during the post-operative office follow-up. Diagnosis of superficial and or deep SSI was determined using the Guideline for Prevention of Surgical Site Infection (6). Finally, baseline pictures were taken before dressings were applied on the leg and sternum incision sites in both groups in the operating room. Photos were also taken once dressings were removed and every morning thereafter until discharged from the hospital. In addition, photos were taken when patients returned for their postoperative follow-up with the surgeon.
Statistical Analysis
SAS software Version 9.1 (SAS Institute, Cary, NC) was used for data analysis. Outcome data were compared between the treated and control groups using t tests for continuous data and Fisher exact test for categorical data. Pain scale, blood loss, and discoloration variables were compared between the groups with Fisher exact test. p < .05 were considered to be statistically significant. All data are expressed as mean ± SD or n (%) for categorical data.
RESULTS
A total of eight patients were excluded from the study because of complications associated with CABG. Of the eight, one patient expired in the operating room because of a peri-operative myocardial infarction with subsequent ventricular failure; one patient suffered a stroke on POD 1 in the coronary intensive care unit (ICU) because of atrial fibrillation; and one patient was brought back to the operating room for exploratory bleeding, which was later discovered to be from a graft anastomosis. Five of the eight patients were excluded because EVH was initiated and eventually converted to an open technique because of technical difficulty and exposure. Therefore, data from 30 patients were used for statistical analysis for the remainder of the study.
Table 1 depicts a partial complete blood count (CBC) panel for the TRT group. A dilution factor along with intra-operative baseline CBC values were used to calculate FIB, PLT Cnt, and WBC yields (Figure 1). Average PLT Cnt yield for all three PRP samples was 4.2 ± 0.5 × 103/mcL times above baseline, which was consistent with Medtronic’s data for a 60 mL blood sample. In addition, average WBC yield was 1.9 ± 0.7 × 103/mcL, and FIB yielded 1.2 ± 0.2 mg/dL times above baseline.
Table 1.
Hematology assessment for patients in the TRT group (N = 19).
| Institution Normal Ranges | Operative Baseline | Dilution Factor | |
|---|---|---|---|
| Hemoglobin (g/dL) | M (13.2–17.4) | 12.8 ± 1.0 | 11.0 ± 0.9 |
| F (11.8–15.8) | |||
| Hematocrit (%) | M (41–52) | 37.5 ± 2.6 | 32.5 ± 2.3 |
| F (37–48) | |||
| Fibrinogen concentration (mg/dL) | 160–480 | 336.5 ± 82..8 | 291.7 ± 71.7 |
| Platelet count (×103/mcL) | 147–369 | 179.9 ± 35.5 | 156.0 ± 30.8 |
| Platelet function-ADP (seconds) | 62–100 | 90.8 ± 24.3 | — |
| Platelet function-EPI (seconds) | 82–150 | 148.4 ± 59.5 | — |
| White blood count (×103/mcL) | 3.10–9.90 | 6.4 ± 1.4 | 5.6 ± 1.2 |
M, male; F, female; ADP, adenosine diphosphate; EPI, epinephrine.
Figure 1.
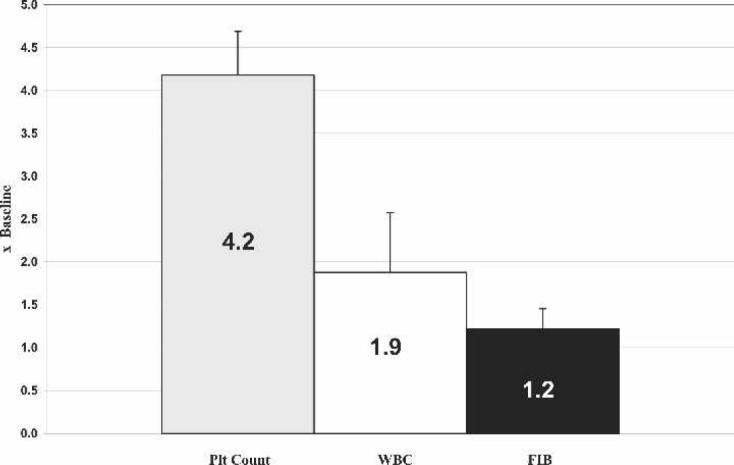
Product increase over baseline using the Medtronic Magellan Autologous Platelet Separator.
Patient demographics and pre-operative data were comparable between the groups, with the exception of ejection fraction, which was significantly lower in the CTR group than the TRT group (Table 2). Co-morbidities were also similar between groups; however, no statistical analysis was performed. There was also a significant difference in pre-operative PLT Cnt, with the CTR having fewer on average than the TRT group (p = .047). Perfusion and clamp times were similar between groups (Table 3). Three patients in the CTR group underwent off-pump CABG, and there were two patients in the TRT group. More individuals in the TRT group received less platelets intraoperatively, which was likely caused by a significantly higher pre-operative PLT Cnt. Surgeons evaluated hemostasis based on activated clotting time, PLT Cnt, coagulation tests, intra-operative bleeding, and mediastinal tube output before ordering platelets or any other blood products. During the 48-hour post-operative period, blood product use was similar between groups. The 24- and 48-hour mediastinal tube outputs were also similar between groups. Other outcome parameters, such as ventilation time, ICU, and hospital length of stay, were not statistically significant.
Table 2.
Patient pre-operative data between groups.
| CTR (N = 15) | TRT (N = 5) | p Value | |
|---|---|---|---|
| Age (years) | 64 ± 9 | 63 ± 11 | NS |
| Weight (kg) | 95.3 ± 22.7 | 86.2 ± 16.0 | NS |
| Height (cm) | 177.2 ± 5.8 | 173.9 ± 12.6 | NS |
| Body surface area | 2.11 ± 0.24 | 2.01 ± 0.24 | NS |
| Sex | |||
| Female | 2 (13%) | 1 (7%) | NS |
| Male | 13 (87%) | 14 (93%) | |
| Ejection fraction | 53 ± 12 | 63 ± 11 | .022 |
| Comorbidities | |||
| Diabetes mellitus | 7 (47%) | 6 (40%) | — |
| Chronic obstructive pulmonary disease | 1 (7%) | 2 (13%) | — |
| Hypertension | 10 (67%) | 9 (60%) | — |
| Hypercholesterolemia | 4 (27%) | 3 (20%) | — |
| Hyperlipidemia | 6 (40%) | 8 (53%) | — |
| Previous myocardial infarction | 2 (13%) | 3 (20%) | — |
| Renal insufficiency | 2 (13%) | 1 (7%) | — |
| Unstable angina | 1 (7%) | 2 (13%) | — |
| Pre-operatrive CBC | |||
| Hemoglobin (g/dL) | 13.8 ± 1.6 | 14.0 ± 0.9 | NS |
| Hematocrit (%) | 41 ± 5 | 41 ± 3 | NS |
| Platelet count (×103/mcL) | 184 ± 30 | 208 ± 33 | .047 |
| White blood count (×103/mcL) | 6.78 ± 1.16 | 7.16 ± 1.78 | NS |
NS, not significant.
Table 3.
Intra-operative data, blood product use, and outcome parameters between groups.
| CTR | TRT | p Value | |
|---|---|---|---|
| Perfusion time* | 131 ± 25 | 145 ± 28 | NS |
| Clamp time* | 87 ± 20 | 97 ± 20 | NS |
| Operative blood product use | |||
| PRBC | |||
| 0 | 12 (80%) | 10 (67%) | NS |
| 1 or more | 3 (20%) | 5 (33%) | |
| PLT | |||
| 0 | 11 (73%) | 13 (87%) | NS |
| 1 or more | 4 (27%) | 2 (13%) | |
| FFP | |||
| 0 | 15 (100%) | 14 (93%) | NS |
| 1 or more | — | 1 (7%) | |
| CRYO | |||
| 0 | 15 (100%) | 15 (100%) | NS |
| 1 or more | — | — | |
| 48-hour post-operative blood product usage | |||
| PRBC | |||
| 0 | 14 (93%) | 14 (93%) | NS |
| 1 or more | 1 (7%) | 1 (7%) | |
| PLT | |||
| 0 | 12 (80%) | 14 (93%) | NS |
| 1 or more | 3 (20%) | 1 (7%) | |
| FFP | |||
| 0 | 14 (93%) | 13 (87%) | NS |
| 1 or more | 1 (7%) | 2 (13%) | |
| CRYO | |||
| 0 | 15 (100%) | 15 (100%) | NS |
| 1 or more | — | — | |
| Outcome parameters | |||
| 24-hour MT output | 562 ± 161 | 509 ± 33 | NS |
| 48-hour MT output | 257 ± 144 | 251 ± 174 | NS |
| Ventilation time | |||
| ≤6 hours | 8 (53%) | 6 (40%) | NS |
| >6 hours | 7 (47%) | 9 (60%) | |
| ICU length of stay | |||
| ≤1 day | 3 (20%) | 5 (33%) | NS |
| 1–2 days | 7 (47%) | 6 (40%) | |
| >2 days | 5 (33%) | 4 (27%) | |
| Hospital length of stay | |||
| ≤4 days | 7 (47%) | 6 (40%) | NS |
| >4 days | 8 (53%) | 9 (60%) | |
Comparison between groups was based on 12 on-pump CABG cases in the CTR group and 13 on-pump cases in the TRT group.
NS, not significant; PRBC, packed red blood cells; PLT, platelets; FFP, fresh frozen plasma; CRYO, cryoprecipitate; MT, mediastinal tube.
Pain Scores, Blood Loss, and Discoloration
Because of the small number of patients experiencing pain, the pain scale was collapsed to compare pain vs. no pain between the groups. Table 4 shows the comparisons at various time-points. Although not statistically significant, 87% of patients in the TRT group experienced less pain on the sternum on POD 1 vs. 67% in the CTR group. On POD 2, sternum pain scores were identical between the groups. When looking at EVH pain, all patients in the TRT group experienced no pain on POD 1 and continued to do so on POD 2. Patients in the CTR group experienced similar results with EVH pain; however, only 80% had no pain on POD 1. When patients returned for their 3-week post-operative office follow-up, there were 11 TRT and 10 CTR patients who reported having no pain.
Table 4.
Comparison of pain scores between groups.
| CTR [n (%)] | TRT [n (%)] | p Value | |
|---|---|---|---|
| Sternum pain | |||
| POD 1 | |||
| 0 on pain scale | 10 (67%) | 13 (87%) | NS |
| >0 on pain scale | 5 (33%) | 2 (13%) | |
| POD 2 | |||
| 0 on pain scale | 8 (53%) | 8 (53%) | NS |
| >0 on pain scale | 7 (47%) | 7 (47%) | |
| Post-operative office visit | |||
| 0 on pain scale | 11 (73%) | 12 (86%) | NS |
| >0 on pain scale | 4 (27%) | 2 (14%) | |
| EVH pain | |||
| POD 1 | |||
| 0 on pain scale | 12 (80%) | 15 (100%) | NS |
| >0 on pain scale | 3 (20%) | — | |
| POD 2 | |||
| 0 on pain scale | 15 (100%) | 15 (100%) | NS |
| >0 on pain scale | — | — | |
| Post-operative office visit | |||
| 0 on pain scale | 10 (67%) | 11 (79%) | NS |
| >0 on pain scale | 5 (33%) | 3 (21%) |
NS, not significant.
Blood loss and discoloration were also categorized, and no differences between the groups were found (Table 5). When dressings were measured, there was less sternal blood loss in the TRT group on POD 2. No differences were observed with respect to EVH blood loss on POD 2. Sternal discoloration in the form of bruising was not observed on any of the TRT patients; however, one CTR patient did have bruising, which measured between 26 and 100 cm2 (Figure 2). When these patients returned to see the surgeon in the office, there was bruising at the EVH site in both groups. Figures 3 and 4 are photographs that were taken of leg bruising from both groups. At the time of the patients’ office visits, 86% of the TRT group was noted to have no bruising compared with 73% in the CTR group with the leg.
Table 5.
Comparison of blood loss and discoloration between groups.
| CTR [n (%)] | TRT [n (%)] | p Value | |
|---|---|---|---|
| Blood loss (g) | |||
| Sternum POD 2 | |||
| 0 g | 5 (33%) | 7 (47%) | NS |
| 0.5–5 g | 10 (67%) | 8 (53%) | |
| EVH POD 2 | |||
| 1–10 g | 7 (47%) | 4 (27%) | NS |
| 10–20 g | 5 (33%) | 7 (47%) | |
| >20 g | 3 (20%) | 4 (27%) | |
| Discoloration (cm2) | |||
| Sternum POD 2 | |||
| No discoloration | 14 (93%) | 15 (100%) | NS |
| 26–100 cm2 | 1 (7%) | — | |
| Sternum office visit | |||
| No discoloration | 15 (100%) | 15 (100%) | NS |
| EVH POD 2 | |||
| No discoloration | 3 (20%) | 2 (13%) | NS |
| 1–25 cm2 | 3 (20%) | 3 (21%) | |
| 26–100 cm2 | 3 (20%) | 5 (36%) | |
| 101–225 cm2 | 2 (13%) | 4 (29%) | |
| >225 cm2 | 4 (27%) | 1 (7%) | |
| EVH office visit | |||
| No discoloration | 11 (73%) | 13 (86%) | NS |
| 1–25 cm2 | 1 (7%) | 2 (14%) | |
| 26–100 cm2 | 3 (20%) | — |
NS, not significant.
Figure 2.
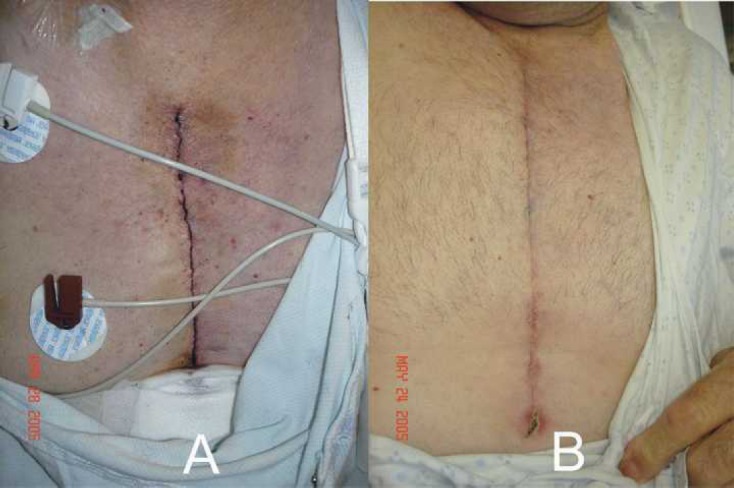
(A) Sternal bruising, which measured ∼3 × 15 cm in a CTR patient on POD 2. (B) Picture taken during post-operative office follow-up.
Figure 3.
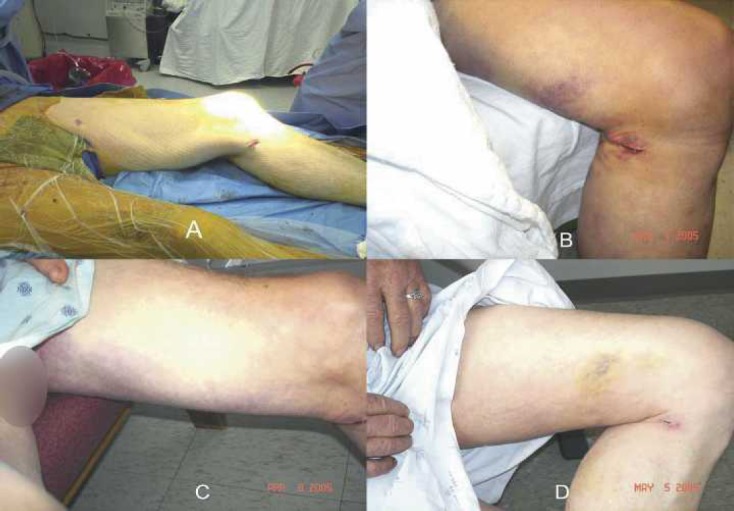
(A) Pictures of EVH incision sites in the operating room of a CTR patient. (B) Bruise noted along the medial thigh on POD 2, which measured ∼15 × 4 cm. (C) POD 3. (D) Remnant of bruise at post-operative office follow-up.
Figure 4.
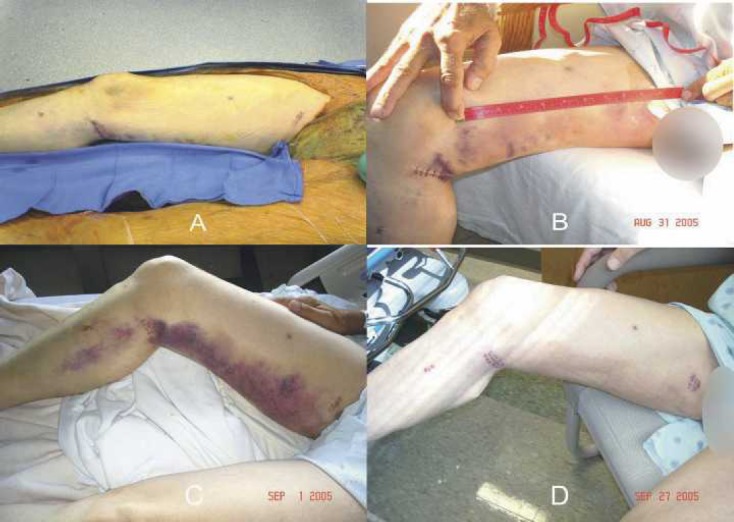
(A) Pictures of EVH incision sites in the operating room of a TRT patient with evidence of a bruise. Bruise measured ∼12 × 3 cm. (B) Evolving bruise on POD 2. (C) POD 3. (D) During post-operative office follow-up.
Surgical Site Infection
None of the patients experienced any signs of infection to either the superficial or deep sternal incision. However, when looking for signs of infection at the EVH site, one TRT patient had heat and was diagnosed as having a SSI. In addition, one CTR patient experienced redness and localized swelling at the EVH site. Both were prescribed oral antibiotics by the surgeon or primary care physician. Because of the small number of infections, no statistical tests were performed comparing both groups.
DISCUSSION
Growth factors, platelets, leukocytes, and cytokines present in APG play an important role in wound healing. The final product enhances hemostasis, provides an antibacterial component, decreases pain perception, and therefore accelerates the overall wound healing process. The antibacterial component has proven fruitful in cardiac surgery. In fact, when APG was used, patient outcomes either improved or improved significantly. Englert et al. (7) examined the effects of APG on post-operative wound infections, swelling, bruising, and blood loss. The authors observed clinically that there were improvements in the APG group in terms of decreased chest and leg pain along with reduced bruising overall. Trowbridge et al. (8) retrospectively evaluated the incidence of wound infections in patients undergoing cardiac surgery. They observed that superficial and deep sternal wound infections were significantly lower in the APG group compared with both a CTR and a historical group. In addition, there were similar leg infection rates for cases involving coronary revascularization using EVH.
The antibacterial component of APG has been efficacious in preventing SSIs. However, the mechanism of how APG alleviates pain is unclear. Does APG have analgesic properties? It has been suggested through anecdotes that APG modulates pain by sealing nerve endings or acting as a general tissue sealant. There are no scientific data to prove or disprove the interaction between APG and peripheral nerve endings; however, the latter explanation is more plausible because APG enhances hemostasis and delivers a plethora of GFs at the wound sites. The number of studies investigating APG and pain is lacking, with the exception of Gardner et al (9). These authors retrospectively analyzed patients receiving APG to a CTR group in total knee arthroplasty cases. In addition to a significantly decreased blood loss, the authors observed that there was a significant difference in pain level as indicated by less intravenous and oral narcotics in the TRT group.
Pain transduction and perception is extensive and complex, involving biological events at multiple levels of the nervous system (10,11). This discussion was not intended to be a comprehensive review of pain mechanisms. Instead, the focus is on a brief overview of what occurs at the site of injury from an inflammatory response and nociceptor activation perspective and how APG may modulate pain. Noxious stimuli to tissue initiate a cellular and vascular response that sets the stage for tissue healing and regeneration. Immediately after a blood vessel is damaged, ruptured cell membranes release inflammatory factors like thromboxanes and prostaglandins that cause vasoconstriction to prevent blood loss. This vasoconstriction lasts 5–10 minutes and is followed by vasodilation, which peaks at ∼20 minutes after injury (12). Vasodilation is the result of factors released by platelets and other cells. The main chemical mediator involved in causing vasodilation is histamine. In addition, histamine causes vascular permeability changes, which increases extravascular osmolarity and edema to transpire. Increased vascular permeability also facilitates the entry of inflammatory cells like leukocytes in response to specific cytokines generated in the wound.
In addition to histamine, thromboxanes, and prostaglandins, tissue damage releases other chemicals, including substance P, serotonin, and bradykinin, which sensitize nociceptors. These substances can decrease nociceptor depolarization thresholds and may activate uninvolved contiguous nociceptors that result in hyperalgesia (11). Activation of nociceptors leads to the release of substance P and other peptides from primary afferent neurons. Subsequently, substance P acts to degranulate mast cells in the proximity of sensory endings to release histamine, which directly excites nociceptors. In addition, substance P produces peripheral blood vessels to dilate, and the consequential edema causes a further liberation of bradykinin. This kinin causes contraction of non-vascular smooth muscle, increases vascular permeability, and also is involved in the mechanism of pain. The peripheral nociceptor sensitization decreases the firing threshold and increases the responsiveness of A delta and C nerve fibers, which conduct signals at different rates (10). Once the signals reach the spinal cord, they terminate in the dorsal horn where other chemical mediators lead to sensitization of spinal neurons and the transmission of the pain signals onward to the brain.
The inflammatory phase of wound healing is clinically manifested as signs of redness, heat, swelling, and pain. The physiologic processes underlying this inflammation begin immediately on tissue injury that optimally leads to restoration of tissue integrity and function. Simultaneously, the coagulation cascade, the arachidonic acid pathways, and the creation of growth factors and cytokines work together to initiate and maintain the inflammatory phase (13). The benefits of APG in modulating pain are intuitively because of its ability to provide immediate hemostasis. Second, if vasoactive substances are released from the vascular endothelium and other cellular constituents and influenced by nociceptor sensitization, immediate hemostasis with APG would ameliorate the adverse effects of increased vascular permeability. Finally, pain would be modulated by the accelerated wound healing process by platelet GFs.
Limitations of the study should be addressed. First, the issue of patients caring for their wound sites is a potential concern once they are discharged from the hospital. We observed that all leg wound complications occurred after patients were discharged from the hospital. Therefore, relying on patients to care for their wounds according to surgeon instructions are important to preventing leg complications. Second, improvements with the TRT group with respect to pain, blood loss, and discoloration was likely because of the significantly higher baseline Plt Cnt. A higher Plt Cnt suggests an increase in GFs, which may explain the improved study parameters. Finally, although a prospective randomized trial is ideal, this study lacks the power necessary to observe a significant reduction in the study parameters. Using a statistical and power analysis software (NCSS and PASS; Number Cruncher Statistical Systems, Kaysville, UT), we were able to determine the number of patients needed in each group to obtain a power equivalent to at least 80% in parameters such as pain, blood loss, and discoloration. Table 6 depicts the power calculations and sample size needed in each group to detect observed differences, given the current sample size.
Table 6.
Power analysis of study parameters.
| CTR [n (%)] | TRT [n (%)] | Power* | Sample Size Needed Per Group | |
|---|---|---|---|---|
| Sternum pain | ||||
| POD 1 | ||||
| 0 on pain scale | 10 (67%) | 13 (87%) | 26% | 67 |
| >0 on pain scale | 5 (33%) | 2 (13%) | ||
| Post-operative office visit | ||||
| 0 on pain scale | 11 (73%) | 12 (86%) | 14% | 151 |
| >30 on pain scale | 4 (27%) | 2 (14%) | ||
| EVH pain | ||||
| POD 1 | ||||
| >0 on pain scale | 12 (80%) | 15 (100%) | 31% | 27 |
| >0 on pain scale | 3 (20%) | — | ||
| Post-operative office visit | ||||
| 0 on pain scale | 10 (67%) | 11 (79%) | 12% | 214 |
| >0 on pain scale | 5 (33%) | 3 (21%) | ||
| Blood loss (g) | ||||
| Sternum POD 1 | ||||
| 0 g | 5 (33%) | 7 (47%) | 12% | 192 |
| 0.5–5 g | 10 (67%) | 8 (53%) | ||
| EVH POD 2 | ||||
| 1–10 g | 7 (47%) | 4 (27%) | 20% | 89 |
| 10–20 g | 5 (33%) | 7 (47%) | ||
| >20 g | 3 (20%) | 4 (27%) | ||
| Discoloration (cm2) | ||||
| EVH POD 2 | ||||
| No discoloration | 3 (20%) | 2 (13%) | 30% | 50 |
| 1–25 cm2 | 3 (20%) | 3 (20%) | ||
| 26–100 cm2 | 3 (20%) | 5 (33%) | ||
| 101–225 cm2 | 2 (13%) | 4 (27%) | ||
| >225 cm2 | 4 (27%) | 1 (7%) | ||
| EVH office visit | ||||
| No discoloration | 11 (73%) | 13 (87%) | 16% | 127 |
| 1–25 cm2 | 1 (7%) | 2 (13%) | ||
| 26–100 cm2 | 3 (20%) | — | ||
Power to detect the observed difference, given the sample size.
In conclusion, APG has gained popularity as a clinical treatment in various surgical procedures. Further studies with larger sample sizes and long-term follow-up are needed to assess healing. Furthermore, results of this study would suggest a need for the continuing use of APG at the EVH site.
ACKNOWLEDGMENTS
The authors thank Medtronic (Minneapolis, MN) and Mercy Medical Center (Sioux City, IA) for financial support in funding the laboratory and medication costs of this research study. The primary author thanks Susan J. Englert, RN, BSN, CNOR, CCP (Wichita, KS), for insights into shaping the research protocol.
REFERENCES
- 1.Cohen KI, Diegelmann RF, Yager DR, Wornum IL, Graham MF, Crossland MC.. Wound care and wound healing. In: Schwartz S, ed., Principles of Surgery. New York: McGraw-Hill; 1999;263–295. [Google Scholar]
- 2.Pietrzak WS, Eppley BL.. Platelet rich plasma: Biology and new technology. J Craniofac Surg. 2005;16:1043–54. [DOI] [PubMed] [Google Scholar]
- 3.Marx RE.. Platelet-rich plasma: Evidence to support its use. J Oral Maxillofac Surg. 2004;62:489–96. [DOI] [PubMed] [Google Scholar]
- 4.Bhanot S, Alex JC.. Current applications of platelet gels in facial plastic surgery. Facial Plast Surg. 2002;18:27–33. [DOI] [PubMed] [Google Scholar]
- 5.Franchini M, Dupplicato P, Ferro I, De Gironcoli M, Aldegheri R.. Efficacy of platelet gel in reconstructive bone surgery. Orthopedics. 2005;28:161–3. [DOI] [PubMed] [Google Scholar]
- 6.Mangram AJ, Horan TC, Pearson ML.. Guideline for prevention of surgical site infection, 1999. Infect Control Hosp Epidemiol. 1999;20:247–78. [DOI] [PubMed] [Google Scholar]
- 7.Englert SJ, Estep TH, Ellis-Stoll CC.. Autologous platelet gel applications during cardiovascular surgery: Effect on wound healing. J Extra Corpor Technol. 2005;37:148–52. [PMC free article] [PubMed] [Google Scholar]
- 8.Trowbridge CC, Stammers AH, Woods E, Yen BR, Klayman M, Gilbert C.. Use of platelet gel and its effects on infection in cardiac surgery. J Extra Corpor Technol. 2005;37:381–86. [PMC free article] [PubMed] [Google Scholar]
- 9.Gardner MJ, Demetrakopoulos D, Klepchick PR, Mooar PA.. The efficacy of autologous platelet gel in pain control and blood loss in total knee arthroplasty: An analysis of the haemoglobin, narcotic requirement and range of motion. Available at http://www.springer.com/west/home/generic/search/results?SGWID_4-40109-70-1038368-0 Accessed January 10, 2007. [DOI] [PMC free article] [PubMed]
- 10.Heavner JE, Willis WD.. Pain pathways: Anatomy and physiology. In: Raj PP, ed. Practical Management of Pain. St. Louis: Mosby; 2000;107–114. [Google Scholar]
- 11.Johnson BW.. Pain mechanism: Anatomy, physiology, and neurochemistry. In: Raj PP., ed. Practical Management of Pain. St. Louis: Mosby; 2000;117–143. [Google Scholar]
- 12.Stadelmann WK, Digenis AG, Tobin GR.. Physiology and healing dynamics of chronic cutaneous wounds. Am J Surg. 1998;176:26S–38S. [DOI] [PubMed] [Google Scholar]
- 13.Lin E, Lowry SF, Calvano SE.. The systemic response to injury. In: Schwartz S, ed. Principles of Surgery. New York: McGraw-Hill; 1999;263–295. [Google Scholar]


