Abstract:
In this paper we describe a high-fidelity perfusion simulation system intended for use in the training and continuing education of perfusionists. The system comprises a hydraulic simulator, an electronic interface unit and a controlling computer with associated real-time computer models. It is designed for use within an actual operating theatre, or within a specialized simulation facility. The hydraulic simulator can be positioned on an operating table and physically connected to the circuit of the institutional heart-lung machine. The institutional monitoring system is used to display the arterial and central venous pressures, the ECG and the nasopharyngeal temperature using appropriate connections. The simulator is able to reproduce the full spectrum of normal and abnormal events that may present during the course of cardiopulmonary bypass. The system incorporates a sophisticated blood gas model that accurately predicts the behavior of a modern, hollow-fiber oxygenator. Output from this model is displayed in the manner of an in-line blood gas electrode and is updated every 500 msecs. The perfusionist is able to administer a wide variety of drugs during a simulation session including: vasoconstrictors (metaraminol, epinephrine and phenylephrine), a vasodilator (sodium nitroprusside), chronotropes (epinephrine and atropine), an inotrope (epinephrine) and modifiers of coagulation (heparin and protamine). Each drug has a pharmacokinetic profile based on a three-compartment model plus an effect compartment. The simulation system has potential roles in the skill training of perfusionists, the development of crisis management protocols, the certification and accreditation of perfusionists and the evaluation of new perfusion equipment and/or techniques.
Keywords: cardiopulmonary bypass, computer simulation, extracorporeal circulation
The place of simulation is now widely accepted in the training of practitioners in many fields of medicine—including both surgery and anesthesia (1,2). However, perhaps surprisingly (given the technical nature of perfusion), relatively few simulation systems are available for use in perfusion education (1–6), and none seem to enjoy widespread use.
When the use of simulation has been attempted, the most common application has been in the preliminary training of perfusionists, where a primed circuit is connected to an arterio-venous loop, and the trainee is “stepped through” the process of cardiopulmonary bypass (CPB). This facilitates initial familiarization with the heart-lung machine (HLM), ancillary equipment, and the bypass circuit.
Various computer-based applications have also been described. Riley and O’Kane (3) were the first to develop a software model of CPB in 1977 and subsequently enhanced their system to include a simulator control box in 1984 (4). More recently, Davis (5) has also described a system in which a microcomputer-based simulator is controlled by means of an electronic console that represents the hardware of a modern HLM. The most recent contribution seems to be that of Boschetti et al. (7), who have reported a “virtual” extracorporeal model of the circulation.
In this paper, we describe an “immersive” perfusion simulation system (Orpheus; Ulco Technologies, Marrickville, New South Wales, Australia), which is able to reproduce the gamut of both normal and abnormal events that may present during the course of CPB. The system consists of a hydraulic simulator interfaced to a controlling computer and associated real-time computer models. The hydraulic component of the simulator functions as a “patient” who can be connected to the majority of perfusion and hemodynamic monitoring equipment in current use in cardiac surgery. It is designed for use within an actual operating theater or within a specialized simulation facility.
The system has many possible applications that include skill training; certification and accreditation of perfusionists; development and practice of crisis and team management protocols; and evaluation of new or existing perfusion equipment and/or techniques.
DESCRIPTION OF THE SYSTEM
The system is made up of the hydraulic simulator itself, an electronic interface unit, and a computer that runs the control application and its associated real-time computer models. The configuration of these elements is shown in Figure 1.
Figure 1.
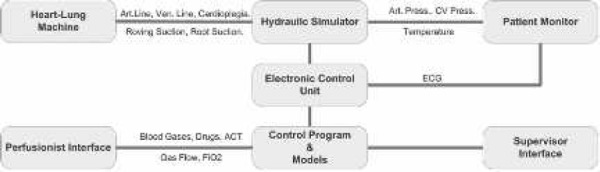
Schematic representation of the main elements of the simulation system. The institutional HLM and CPB circuit are connected to the hydraulic simulator by arterial, venous, and cardioplegia lines. Roving (handheld) and aortic root suction can also be attached. The institutional patient monitor displays arterial, central venous, and ECG waveforms and the nasopharyngeal temperature. The perfusionist’s interface displays the arterial and venous blood gases, activated clotting time, and gas flow and FiO2 to the oxygenator. The interface is also used for drug administration. All functions of the system can be controlled using the supervisory interface.
Hydraulic Simulator
The primary function of the hydraulic simulator is to replicate the behavior of a patient’s circulation. It is made up of a physical analog of the venous capacitance, native heart, and arterial capacitance, as well as functional arterial and venous valves. The heart’s force of ejection is determined by filling of the venous capacitance according to the Frank-Starling relationship. In addition, the hydraulic device also incorporates a myocardial circulation through which either antegrade or retrograde cardioplegia solution can be pumped, a pleural cavity into which shed blood can be lost (or from which it can be recovered), and a thermal mass that reproduces the thermal behavior of a patient.
The simulator can be positioned on an operating table and physically connected to the circuit of the institutional HLM. It is compatible with both centrifugal and roller pump-based systems. The circuit is primed and connected to the arterial, venous, cardioplegia, and roving and aortic root suction ports on the simulator. The institutional monitoring system is used to display the arterial and central venous pressures, the ECG, and the naso-pharyngeal temperature using appropriate connections.
The flow delivered to the circulation and the myocardium from the HLM is measured by separate flow sensors and is reported continuously to the controlling application.
The thermal mass can be switched in or out of the circulation. When switched in, it can be adjusted to reproduce the behavior of a patient weighing between 20 and 100 kg. When switched out of the circulation, the system supervisor can either elect to simulate a patient with a very small thermal mass or can choose to model thermal behavior entirely in software.
Specific fault insertion devices also form part of the system. By these means, the arterial and venous lines can be kinked or air can be entrained into the venous line.
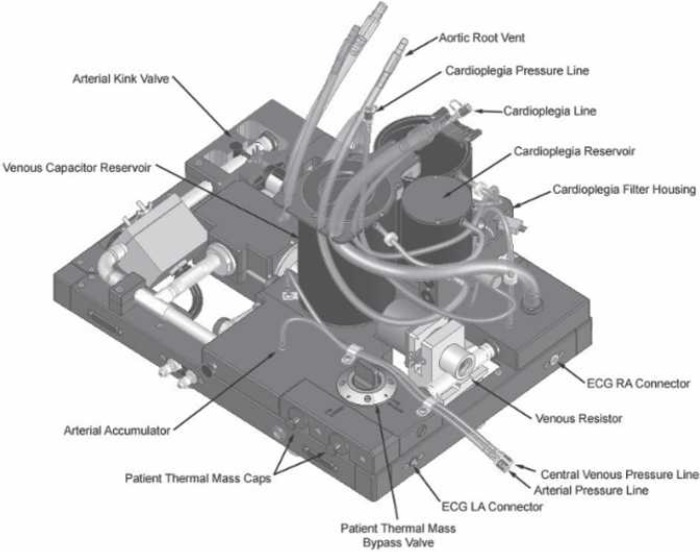
An illustration of the hydraulic simulator is shown in Figure 2.
Figure 2.
The hydraulic simulator. The device behaves as a dual-chamber univentricular circulation. The principal connections of the simulator are shown.
Electronic Interface Unit
The electronic interface unit serves two primary functions. First, it controls some of the basic behavior of the hydraulic simulator (for example, the behavior of the atrial chamber during drainage). Second, it manages the bidirectional transfer of data and control commands between the hydraulic simulator and the controlling computer.
It also generates an ECG signal that simulates a range of heart rates and rhythms and controls the fault insertion devices that enable interruption of the gas supply to the HLM or power supplies to the HLM, patient monitor, and heater/cooler.
Controlling Computer
The controlling computer is mounted in the same chassis as the electronic interface unit and runs under the Windows XP operating system. The control application comprises a supervisor’s interface through which complete control of the simulator can be achieved, as well as real-time computer models of cardiovascular, blood gas, drug, and thermal behavior. These models execute synchronously during a simulation session and provide data and control settings to the system according to the current state of the HLM and the native circulation. An example of one of the supervisor’s interfaces is shown in Figure 3.
Figure 3.
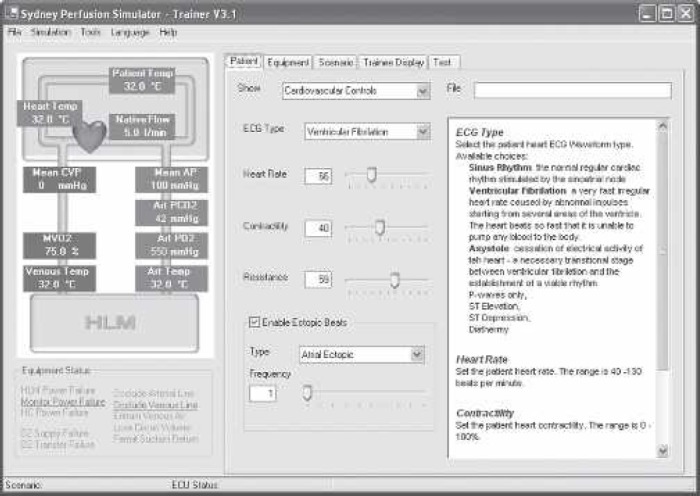
The supervisor’s user interface. The interface comprises several “tabbed” pages through which all functions of the system can be controlled.
A perfusionist’s interface is also provided in the form of an LCD touch-screen that can be separately mounted on the institutional HLM. This interface is used to display the current arterial and venous blood gas values, the gas flow and FiO2 supplied to the oxygenator, and the activated clotting time. It is also used to allow the perfusionist to administer a variety of drugs either as boluses or as infusions. An example of the perfusionist’s interface is shown in Figure 4.
Figure 4.
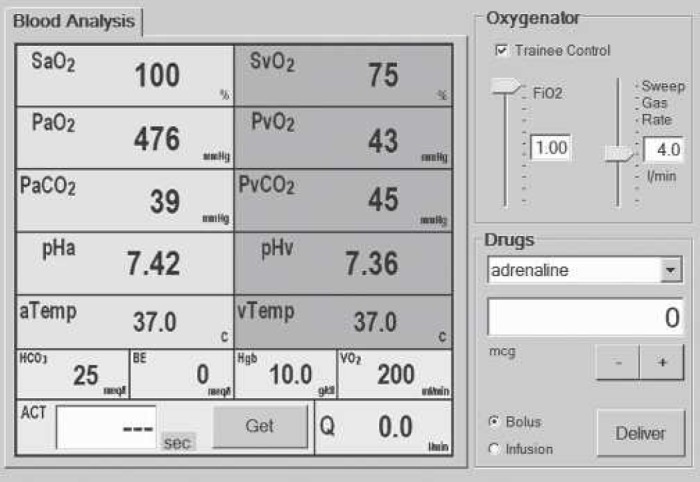
The perfusionist’s user interface. The interface is made up of three main areas. To the left of the screen, arterial and mixed venous blood gases are displayed continuously. These values are updated every 500 ms. In the upper right quadrant of the display are the controls for adjusting the inspired oxygen concentration and gas flow to the artificial lung. In the lower right quadrant are the controls for administering a variety of drugs.
COMPUTER MODELS
Cardiovascular Model
The behavior of the patient heart during initiation of and weaning from bypass is realistically simulated according to a Frank-Starling model. By this means, transfusion of fluid into the patient raises the central venous pressure and results in increasing ventricular ejection and a progressive rise in blood pressure.
Cardiac rate and rhythm can either be controlled directly by the system supervisor using the trainer’s interface or can be set to vary automatically in response to changes in temperature or the administration of drugs. When the aortic cross-clamp has been applied, the administration of cardioplegia results in appropriate suppression of myocardial activity.
Blood Gas Model
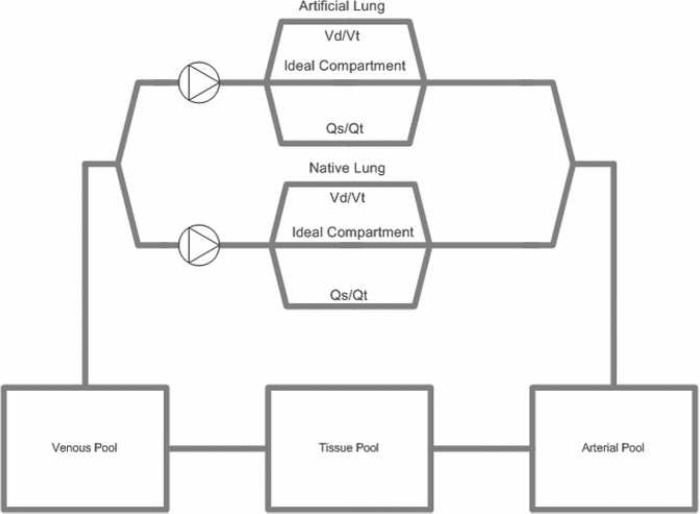
The blood gas model is loosely based on the work of Dickinson (8). It is made up of five compartments (Native Lung, Artificial Lung, Arterial Pool, Tissue Pool, and Venous Pool), which are shown in Figure 5. Oxygen and carbon dioxide are transferred between the compartments according to the prevailing conditions of blood flow within each compartment and gas flow through the native and artificial lungs.
Figure 5.
Schematic representation of the Orpheus blood gas model. The model is made up of five compartments and two pumps. In two of these compartments (Artificial Lung, Native Lung), gas exchange occurs. Two compartments (Arterial Pool, Venous Pool) largely behave as passive conduits. In the final compartment (Tissue Pool), most metabolic activity occurs. The artificial lung is assumed to be perfused at a rate equal to that of the main arterial pump. The native lung is assumed to be perfused at a rate equal to the patient’s current cardiac output.
Each lung is modeled as a traditional “Riley” three-compartment lung (9), in which both shunt fraction (Qs/Qt) and dead space can be independently varied over a wide range. Other important parameters of lung function (such as functional residual capacity) can also be adjusted. Standard equations are used to describe parameters such as the combining power of oxygen and carbon dioxide with hemoglobin, the solubility of these gases in plasma, and the relationship between PCO2 and bicarbonate. The amount and partial pressure of oxygen and carbon dioxide in each compartment are calculated repetitively at a frequency of 2 Hz.
The efficiency of oxygen transfer in an artificial lung is also known to be affected by the transit time of blood passing through the oxygenator (10). This effect is modeled by calculating the oxygenator transit time from the instantaneous flow rate and modifying oxygenator Qs/Qt accordingly. In the event of a failure of gas supply to the oxygenator, the model assumes that the artificial lung communicates freely with atmospheric air through the exhaust port.
The model is displayed as a virtual blood gas monitor and has been validated by comparing its output with that of a Terumo CDI 500 (Terumo Cardiovascular Systems, Ann Arbor, MI) blood gas analyzer during actual CPB. An example of such a comparison is shown in Figure 6.
Figure 6.
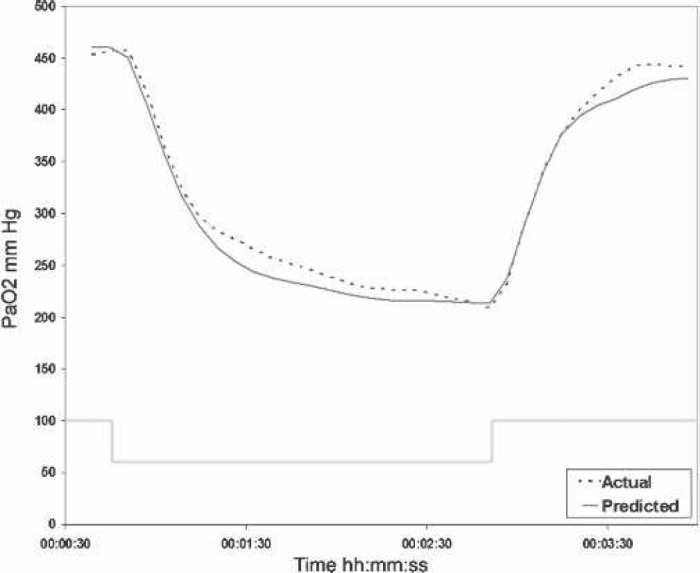
PaO2 after a change in FiO2 during cardiopulmonary bypass. The predicted PaO2 of the Orpheus model compared with the actual output of a Terumo CDI 500 inline blood gas analyzer after a step change in FiO2 from 1.0 to 0.6 in a patient on bypass.
These data were obtained from a patient on stable cardiopulmonary bypass at 32°C who was oxygenated using a Terumo Capiox RX 25 artificial lung. The effect of a step change in FiO2 from 1.0 to 0.6 for a period of 5 minutes on PaO2 is shown. The actual blood gas measurements are represented by an interrupted line, whereas the predictions of the Orpheus model are shown as a solid gray line.
Pharmacodynamic Model
The drugs modeled include vasoconstrictors (metaraminol, epinephrine, and phenylephrine), a vasodilator (sodium nitroprusside), chronotropes (epinephrine and atropine), an inotrope (epinephrine), and modifiers of coagulation (heparin and protamine). Each drug has a pharmacokinetic profile based on a three-compartment model plus an effect compartment.
Thermal Model
If a heater/cooler is used, measurements are taken from sensors in the hydraulic circuit; otherwise, temperature can be set by the system supervisor. The patient’s temperature affects the cardiac rhythm and the rate of metabolism of drugs. It also forms an input to the blood gas model by affecting metabolic rate, oxyhemoglobin binding, and gas solubilities.
Applications of the System
The simulator is able to create a wide range of events and situations, which are summarized in Table 1. These include the routine practice of bypass, problems with the perfusion system, and changes in the patient’s physiology and pharmacology.
Table 1.
Conditions that can be simulated by the system.
| Routine Bypass | Patient Emergencies |
|---|---|
| Initiation of bypass | Blood loss |
| Weaning from bypass | Left ventricular dysfunction |
| Cooling and rewarming | Cardiac arrhythmias |
| Use of centrifugal and roller pumps | Air embolism |
| Variations in vascular resistance | Anaphylaxis |
| Use of vasoactive drugs | Malignant hyperpyrexia |
| Variations in blood coagulation | Protamine reaction |
| Changes in oxygen consumption | Transfusion reaction |
| Administration of cardioplegia | Drug errors |
| CO2 insufflation of the operative site | Failure to wean |
| Equipment Malfunctions | |
| Aortic cannula obstruction | Heat exchanger failure |
| Aortic cannula displacement | Patient monitor failure |
| Venous air entrainment | Circuit leaks |
| Venous cannula obstruction | Oxygenator failure |
| Oxygen supply failure | HLM power supply failure |
DISCUSSION
CPB is a challenging clinical practice that requires considerable initial training and the ongoing maintenance of skills to remain proficient. It is characterized by a relatively low frequency of rapidly evolving but potentially serious critical incidents. Stammers and Mejak (11) surveyed the results of > 650,000 perfusions carried out at nearly 800 institutions and reported that serious injury resulting from perfusion-related incidents occurred in ∼1 in 2000 cases. Similarly, in an earlier Australasian study by Jenkins et al. (12), the overall rate of death or serious injury complicating perfusion-related incidents was estimated at 1 in 2500 cases.
The response of the perfusionist to these challenges requires a detailed understanding of the mechanics of the system and the patient’s physiology. It also requires the ability to put this understanding into practice in a timely manner.
Ginther et al. (13) recently surveyed > 300 American perfusionists, working in 59 centers, on the need for perfusion crisis management drills. They found that “while 97% of the perfusion departments believed that regular practice and performance of crisis management drills would improve individual proficiency, only 17% of the programs mandated that their perfusionists perform crisis management drills as a matter of departmental policy.”
In those centers where the practice of crisis management drills was not mandatory, 39% left it up to individuals to maintain their proficiency, 22% were “not motivated,” 19% were confident of their proficiency, and 17% did not have the time to conduct such drills.
The impact of the practice of crisis management on perfusion outcomes has been the subject of very little formal study. However, it is interesting to note that Ginther et al. found that a small group of students in their own program improved their performance at emergency oxygenator change-out by ∼20% if they were allowed to mentally review the process before attempting it and improved their performance by ∼300% when they were permitted to repeatedly practice the procedure.
Similarly, Anderson et al. (14) showed a significant improvement in the handling of extracorporeal membrane oxygenation (ECMO) crises when a group of nine nurse specialists were trained on a high-fidelity ECMO simulator.
Thus, there are grounds for believing that the practice of perfusion crisis management drills using high-fidelity simulation may markedly improve performance and, according to Ginther et al. (13), there are also indications that the safety culture in the profession is moving in this direction.
In a recent report, Da Broi et al. (15) analyzed data in the MAUDE database (www.fda.gov/search/databases.html) and reported that the average time required to change an oxygenator in a group of 125 patients on CPB was 8.4 minutes. This is in stark contrast to our experience, which is that a perfusionist who practices oxygenator change-out regularly can perform the maneuver successfully in < 3 minutes.
Previously, simple circuits or animal models have been used in perfusion simulation. However, both of these ap-proaches have significant limitations, and Davis (5) has outlined the advantages of the use of more sophisticated systems. These include the absence of patient risk; the nonuse of animals; flexibility; control of the pace and sequence of training; low cost per use; the ability to experiment outside lethal limits; and the ability to incorporate a variety of perfusion components.
Although screen-based simulators have a useful place in the development of mental models of various tasks, fully immersive simulation permits the practical application of this knowledge. Such an environment offers significant opportunities to enhance the practice of perfusion.
In the area of skill training of new trainees, it might be used for the repetitive execution of a basic bypass sequence. If one defines such a sequence as the initiation of bypass, administration of a vasopressor, application of a cross-clamp, administration of cardioplegia, release of a cross-clamp, and weaning from bypass, this minimal sequence can be performed in < 5 minutes using the simulator, and furthermore, it can be repeatedly practiced. In contrast, a real coronary artery graft usually occupies a perfusionist for 3–4 hours, during which time only a single bypass sequence is undertaken.
Crisis management protocols can also be developed and refined using the simulator. Typically, the development cycle of a protocol is one of repeated editing, evaluation, and refinement. In this context, the simulator can be used to validate any protocol by precipitating the crisis and observing the perfusionist while he or she responds according to the protocol under test. The response can be evaluated using a combination of qualitative measures, such as the technical and behavioral ratings described by Gaba et al. (16), together with some form of quantitative scoring, such as the achievement of key temporal or physiologic endpoints. The protocol is further refined and the cycle is repeated.
The certification and accreditation of perfusionists might be improved by examining the performance of candidates during the conduct of standard scenarios, such as changing out an oxygenator. The analysis of the performance of groups of perfusionists during the execution of standardized scenarios could also provide a tool for the establishment of acceptable clinical norms. In this context, performance can be evaluated using metrics derived from automatically collected technical and physiologic endpoints, as well as behavioral and procedural scoring systems.
The evaluation of new perfusion equipment and/or techniques can be facilitated using the simulator. For example, when new equipment is being considered for purchase, the testing of the competing alternatives under a wide range of conditions can be easily undertaken. Similarly, novel techniques (such as the use of small bore cannulae and assisted venous drainage) can be tried and evaluated before use on patients.
In summary, the simulator that we describe here is an attempt to provide a comprehensive solution to the challenges outlined above. It is designed to allow perfusionists to practice handling a wide variety of clinical problems, with their own set-ups, in a familiar environment, and it is our belief that the elements contained within the system provide a realistic clinical environment, which will hopefully lead to safer perfusion practice.
ACKNOWLEDGMENTS
The authors thank Craig Herbert, Stephen Fuller, and Vlad Ilic of Ulco Technologies and John Begg for ongoing contributions to the development of the Orpheus simulator.
REFERENCES
- 1.Dutta S, Krummel TM.. Simulation: a new frontier in surgical education. Adv Surg. 2006;40:249–63. [DOI] [PubMed] [Google Scholar]
- 2.Cumin D, Merry AF.. Simulators for use in anaesthesia. Anaesthesia. 2007;62:151–62. [DOI] [PubMed] [Google Scholar]
- 3.Riley JB, O’Kane KC.. A computer simulation of maintaining total heart lung bypass for basic education. J Extra Corpor Technol. Proceedings 1977. p 42–49 [Google Scholar]
- 4.Riley JB, Winn BA, Hurdle MB.. A computer simulation of cardio-pulmonary bypass: Version two. J Extra Corpor Technol. 1984;16:130–6. [Google Scholar]
- 5.Davis RB.. The heart-lung pump/human interface: A real time microcomputer-based simulation. J Extra Corpor Technol. 1990;22:96–100. [Google Scholar]
- 6.Leonard RJ.. A total heart/lung bypass simulator. ASAIO Trans. 1988;34:739–42. [PubMed] [Google Scholar]
- 7.Boschetti F, Montevecchi FM, Fumero R.. Virtual extracorporeal circulation process. Int J Artif Organs. 1997;20:341–51. [PubMed] [Google Scholar]
- 8.Dickinson CJ.. A digital computer model to teach and study gas transport and exchange between lungs, blood and tissues (‘MacPuf’). J Physiol. 1972;224:7P–9P. [PubMed] [Google Scholar]
- 9.Riley RL, Lilienthal JL, Proemmel DD, Franke RE.. On the determination of the physiologically effective pressures of oxygen and carbon dioxide in the alveolar air. Am J Physiol. 1946;147:191. [DOI] [PubMed] [Google Scholar]
- 10.Pybus DA, Lyon M, Hamilton J, Henderson M.. Measuring the efficiency of an artificial lung: 1. Carbon dioxide transfer. Anaesth Intensive Care. 1991;19:421–5. [DOI] [PubMed] [Google Scholar]
- 11.Stammers AH, Mejak BL.. An update on perfusion safety: does the type of perfusion practice affect the rate of incidents related to cardiopulmonary bypass? Perfusion. 2001;16:189–98. [DOI] [PubMed] [Google Scholar]
- 12.Jenkins OF, Morris RW, Simpson JM.. Australasian perfusion incident survey. Perfusion. 1997;12:279–88. [DOI] [PubMed] [Google Scholar]
- 13.Ginther R Jr, Fillingham R, Searles B, Darling E.. Departmental use of perfusion crisis management drills: 2002 survey results. Perfusion. 2003;18:299–302. [DOI] [PubMed] [Google Scholar]
- 14.Anderson JM, Murphy AA, Boyle KB, Yaeger KA, Halamek LP.. Simulating extracorporeal membrane oxygenation emergencies to improve human performance. Simulation in Healthcare. 2006;1:228–32. [DOI] [PubMed] [Google Scholar]
- 15.Da Broi U, Adami V, Falasca E, Malangone W, Crini S, Degrassi A.. A new oxygenator change-out system and procedure. Perfusion. 2006;21:297–303. [DOI] [PubMed] [Google Scholar]
- 16.Gaba DM, Howard SK, Flanagan B, Smith BE, Fish KJ, Botney R.. Assessment of clinical performance during simulated crises using both technical and behavioral ratings. Anesthesiology. 1998;89:8–18. [DOI] [PubMed] [Google Scholar]