Abstract:
Several surveys showed that cardiopulmonary bypass (CPB) is associated with incidents that negatively affect outcome and suggested that improved monitoring and safety could be associated with a decreased rate of incidents. In 2004, the French “Haute Autorité de Santé”(an independent French government advisory agency) and the French College of Perfusion issued recommendations concerning safety and monitoring devices for CPB. The aims of this study were to investigate the difference between the recommendations and the clinical practice of CPB shortly after publication of the recommendations and compare the 2005 situation with the results of a previous survey performed in France and to investigate the rate of perfusion incidents and their outcome. A 62-item questionnaire was sent in January 2006 to all 66 centers performing cardiac surgery and CPB in France. The survey investigated the use of safety and monitoring devices as well as perfusion incidents for 2005. Fifty-seven centers (response rate, 86%) returned the questionnaire, totaling 34,496 CPB procedures. There was a wide difference between the recommendations and the reported use of safety and monitoring devices with no clinically relevant change from the previous French survey concerning 2001. An incident was reported for every 198 CPB procedures with death at a frequency of 1:4864 and permanent sequelae of 1:11,349, respectively (a permanent injury or death in 1:3220 procedures). The three most frequent perfusion incidents were adverse effects to protamine (1:1702), dissection at the arterial cannulation site (1:1792), and coagulation of the circuit (1:4864). In conclusion, this survey showed that an important effort must be made in France to implement into clinical practice the recommendations concerning CPB monitoring and safety devices. The analysis of CPB-related incidents suggest that, with the exception of protamine adverse effects, the majority of deaths and severe permanent injuries in this survey could probably be avoided by improved use of the monitoring and safety devices.
Keywords: cardiopulmonary bypass, survey, safety, cardiac surgery
Despite the recent interest in off-pump myocardial revascularization techniques, the majority of cardiac surgery procedures require the use of cardiopulmonary bypass (CPB). Within the past decades, the evolution of CPB has been marked on one side by sophistication/complexity and on the other side by an increased requirement for safety. To meet these two goals, the medical community in several countries has issued recommendations concerning the initial training of perfusionists and the use of monitoring and safety devices for CPB (1–3). Meanwhile, several surveys, published in the late 80s and 90s, showed that CPB was associated with a relatively high number of severe complications and deaths (4–9). Although these reports suggest a decrease in CPB-related mortality from 1:1419 CPB procedures in 1972–1977 (4) to 1:4567 CPB procedures in 1996–1999 (9), the incidence of serious non-lethal complications remained relatively constant [1:1030 CPB procedures in 1972–1977 (4) and 1:1453 in 1996–1999 (9)]. Although there is no shown cause-to-effect relationship, the decrease in CPB-related mortality has partly been attributed to improved monitoring and safety procedures (10).
In late 2003, the French “Agence Nationale d’Accréditation et d’Evaluation en Santé” (National Agency for Healthcare Accreditation and Evaluation), presently included in the “Haute Autorité de Santé” (HAS), an independent French government advisory agency, asked a group of professionals (perfusionists, cardiac anesthesiologists, and surgeons) to make recommendations concerning monitoring and safety devices for both adult and pediatric CPB for cardiac surgery. The working group issued, in December 2004, recommendations available in French on www.has-sante.fr (11). There were two follow-up measures to these recommendations: (i) to investigate the difference between the everyday use of monitoring and safety devices for CPB and the recently published recommendations in France; and (ii) to estimate the types of incidents and their rates before the implementation of the recommendations. The aims of this study were first to estimate the difference between the recommendations and routine practice of CPB and second to perform a survey concerning the rate of CPB-related incidents in 2005, when professionals had become familiar with the recommendations but did not implement them.
MATERIALS AND METHODS
In January 2006, a questionnaire, prepared by the French College of Perfusion in collaboration with HAS, was mailed to the 66 centers performing cardiac surgery in France [all identified in the French Cardiac Surgery Centers Repertoire (12)]. The questionnaire and a cover letter containing detailed instructions were sent to the chief perfusionist and the head of cardiac surgery or anesthesia and intensive care departments at each center. In March 2006, the perfusionists that had not responded to the first mail were contacted again. The chief perfusionists were subsequently contacted for additional information (incomplete forms or detailed explanations in case of inconsistencies). Confidentiality was assured by allowing access to surveys only to two authors (JMC and DL). After entering the results in a computer database, the original questionnaires were destroyed.
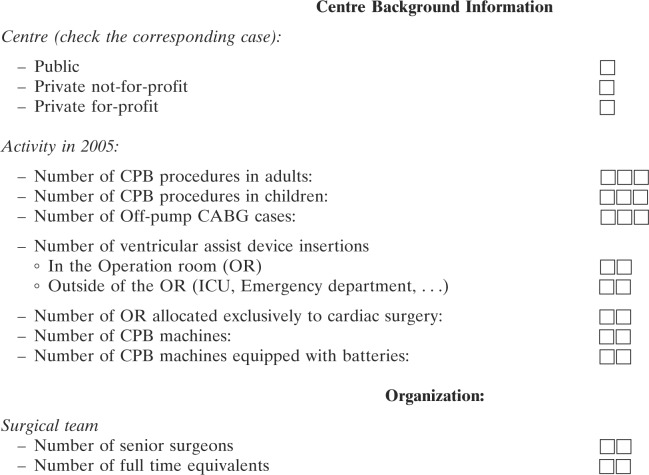
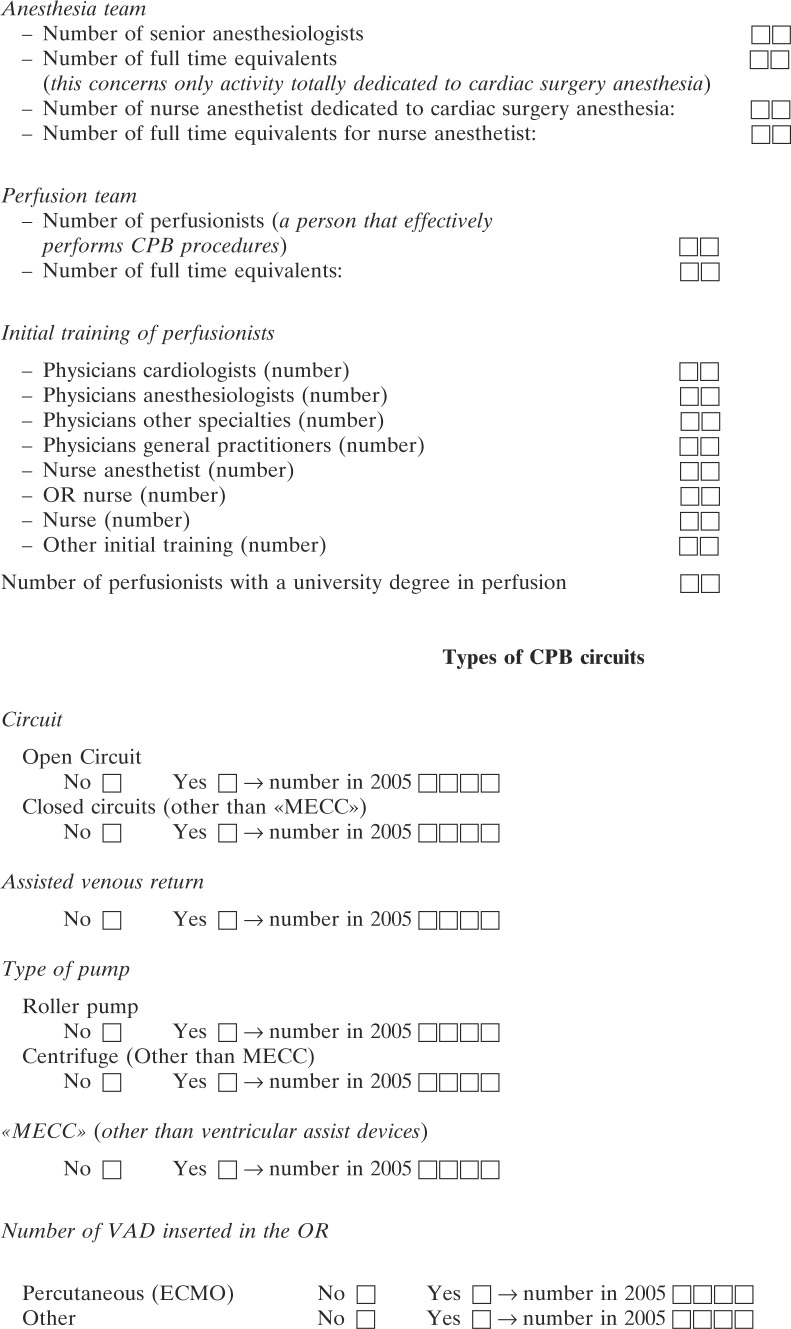
The survey concerned CPB procedures that were performed from January 1 to December 31, 2005. The questionnaire (see Appendix 1) contained 62 questions in three categories: the first category covered center background information and contained 12 items on the number and types of cardiac surgery and CPB procedures. This category also contained questions on the type of practice with regard to age group (pediatric vs. adults) of patients. The total number of hospitals that returned surveys was divided by the total number of centers identified in the French Cardiac Surgery Centers Repertoire (12) to calculate the percent of hospitals represented in the survey. The second category covered the routine use of monitoring and safety devices and procedures as recommended by the French HAS in December 2004 (11) during the period concerned by the survey. The third category concerned perfusion incidents that occurred during the CPB procedure or were considered as its direct consequences. Incidents were separated into 12 different types: coagulation or thrombosis of the CPB circuit; severe reactions to protamine; arterial wall dissection at the cannulation site; disconnection or rupture of the CPB lines; gas embolism in the main circuit; gas embolism in the cardioplegia line; oxygenator failure (either as inadequate oxygenation or increased pressures proximal of the oxygenator); electrical or mechanical pump failure; heater/cooler failure ; main electrical supply failure; and miscellaneous complications. For each type of incident, the chief perfusionist was asked to report. Centers that could not remember, did not record, or did not want to report a specific type of incident were asked to fill in “data not available.” These two issues explain why the denominator for each type of incident can be different. We classified the gravity of each type of incident into four levels: (i) no injury; (ii) injury without permanent sequelae but increased hospital length of stay; (iii) injury with permanent sequelae; and (iv) death.
Averages and percents were calculated from the returned questionnaires. Percentage of routine use of a device or a procedure was calculated from the total number of returned questionnaires. The incident rate was calculated from the number of CPB procedures divided by the number of reported incidents during the time frame of the survey. Because some centers could not or did not want to report on specific incidents, the denominator can change from one type of incident to another.
RESULTS
Of the 66 cardiac surgery centers identified in the French Cardiac Surgery Centers Repertoire, 57 responded (86% response rate): 35 (of 57) from the public sector, 5 (of 57) from the private not-for-profit, and 17 (of 57) for-profit centers sectors. The nine centers that did not respond were from the public sector (one), from the private not-for-profit (one), and from the private sector (seven). Four of nine centers were in the same city, and one of the nine centers performed exclusively pediatric cardiac surgery. A second mail or a telephone contact was necessary to obtain information from 22 centers. Six centers sent multiple responses. They were concordant for the same center except for one center that required a telephone conversation. The 57 centers that returned the completed questionnaires performed 34,496 CPB procedures in 2005. The median number of CPB procedures per center was 515 (range, 38–1608). Seventeen centers performed pediatric cardiac surgery procedures with CPB totalling 2182 procedures. The median number of pediatric CPB procedures per center was 56 (range, 1–536). Of these 17 centers, 3 performed pediatric cases exclusively. Of the 54 centers that performed adult CPB procedures, 45 (83%) reported performing off-pump coronary artery bypass (OPCAB) procedures totaling 2429 cases (median number of OPCAB procedures, 32; range, 1–389). The percentage of adult cardiac surgery procedures without CPB varied among centers (median, 5%; range, 1%–55%); OPCAB procedures were not included in the analysis of CPB-related incidents.
CPB procedures were performed by 261 perfusionists, 56 physicians, and 185 non-physicians, with their specialty or initial training shown in Figure 1. The proportion of physicians as perfusionists varied according to the type of practice. Physicians represented 57% in the private for-profit centers, 11% in the private not-for-profit, and 8% in the public centers. Sixty-nine percent of perfusionists reported having followed a university level training in per-fusion. If one eliminates from the analysis one center that reported employing six perfusionists to perform 37 CPB procedures in 2005, the average number of procedures performed in 2005 by each perfusionist was 134 (range, 22–322), with 135 perfusionists (51%) reporting a mean number of procedures <150 per year. Sixty-seven percent of perfusionists followed a university degree in perfusion, and for 37% of centers; all perfusionists followed a university degree in perfusion.
Figure 1.
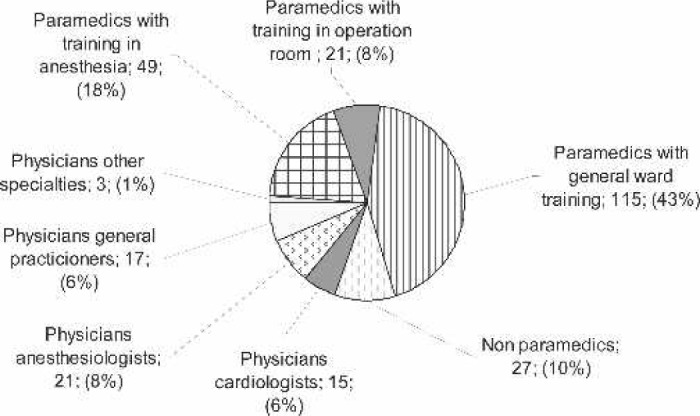
Initial training of the perfusionists.
Monitoring and Safety Devices
A centrifugal pump and no cardiotomy reservoir were used by 32% of the centers and represented 6% of all CPB procedures. The majority of CPB procedures were performed with an open circuit (78%) and roller pumps (73%) as the primary arterial pump. Closed circuits were used exclusively by 14% of the centers; open circuits were used exclusively by 68% of the centers, and 18% of the centers used both types of circuits. Centrifugal pumps as the main arterial pump were used exclusively by 21% of the centers, and 53% reported episodic use. Overall, 21% of CPB procedures used a centrifugal pump as the primary arterial pump. Assisted venous return was used by 30% of the centers but concerned only 8% of all procedures.
The section of the questionnaire concerning the use of general procedures is presented in the first part of Table 1. All centers used a dedicated chart to record vital parameters during CPB, but only 67% reported having written protocols and only 79% performed a checklist before CPB onset. A prospective registry for incidents was reported by 33% of the centers. All centers but one reported preventive maintenance of the CPB machine (33% of the centers twice a year; 67% once a year). Regular use of antiseptic solutions for disinfection of the cooler/heater was reported by 88% of the centers at intervals varying from 1 to 26 weeks (median, 4 weeks).
Table 1.
Monitoring and safety devices.
| General recommendations | |
| Use of written protocols | 67% |
| Use of a checklist before onset of CPB | 79% |
| Dedicated follow-up chart | 100% |
| Computerized chart | 37% |
| Registry of CPB-related complications | 33% |
| Preventive maintenance of CPB machines | 98% |
| Regular disinfection of the heater-cooler unit | 88% |
| Microbiologic control of water of the heater-cooler unit | 45% |
| Monitoring and safety devices for cardiovascular function | |
| ST segment monitoring | 64% |
| Measure of superior vena cava pressure during CPB | 70% |
| Arterial line pressure measurements | 21% |
| Arterial line pressure measurements with pump shutdown regulator | 18% |
| Cardioplegia line pressure measurements | 69% |
| Cardioplegia line pressure measurements with pump shutdown regulator | 14% |
| Monitoring and safety devices for respiratory function | |
| Oxygen gas analyzer | 39% |
| In-line venous hemoglobin saturation monitor | 95% |
| In-line PaO2 monitoring for adults/children | 40%/44% |
| Oxygenator exhaust capnography | 7% |
| Monitoring of temperature | |
| One site (or more) during CPB performed in normothermia | 95% |
| Two sites (or more) during CPB performed with mild hypothermia | 69% |
| Two sites (or more) during CPB performed with profound hypothermia | 87% |
| Monitoring of the temperature of the venous line | 77% |
| Monitoring of the temperature of the arterial line | 84% |
| Monitoring of the temperature of the heat exchanger | 89% |
| Biology | |
| Arterial blood gas (in line monitoring) | 100% (28%) |
| Serum Potassium | 100% |
| Serum Glucose | 72% |
| Ionized Calcium | 63%/88% |
| Delay to obtain results >10 minutes | 29% |
| Monitoring of anticoagulation | |
| Heparinisation protocol | 98% |
| Anticoagulation monitoring protocol | 93% |
| Activated Clotting Time (ACT) | 98% |
| One ACT measuring device per operation room | 91% |
| Interval between two successive ACT measurements <30 minutes | 75% |
| Anti Xa activity monitoring | 32% |
| Monitoring and safety devices to prevent gas embolism | |
| Venous reservoir level detector | 74% |
| Venous reservoir level detector with pump shutdown regulator | 61% |
| Arterial line bubble detector | 32% |
| Arterial line bubble detector with pump shutdown regulator | 28% |
| Arterial line filter | 70% |
| Electrical safety | |
| Uninterruptable power supply | 88% |
| Arterial pump battery backup* | 81% |
| Uninterruptible power supply and/or arterial pump battery backup | 96% |
| Flashlight or emergency light on pump | 70% |
| Miscellaneous | |
| Emergency oxygen tank available in the operation room | 45% |
| Left ventricular vent line one-way pressure relief valve | 41% |
| Arterial line filter with a one-way valve in a purge line† | 77% |
| Epiaortic echography to choose canulation site‡ | 7% |
The numbers in the right column represent percentage of centres that systematically use the safety devices or monitoring, except, if noticed.
Arterial pump percentage.
Centers using arterial filter.
Centers with occasional use.
The section concerning monitoring during the CPB procedure is presented in the second and subsequent parts of Table 1 as the device of interest followed by the percentage of centers using the device routinely. Of the 21% centers that reported monitoring of the arterial line pressures, only four monitored the pressure proximal and distal of the oxygenator, and one center monitored proximal and distal of the arterial line filter. Pressure monitoring on the cardioplegia line was reported by 69% of the centers, but only 14% of the centers reported the use of a cardioplegia pump shutdown regulator.
Monitoring of the respiratory function was based on monitoring of the inspired oxygen fraction (FiO2) by 39% of the centers and CO2 at the oxygenator vent (7% of the centers). Continuous in-line venous hemoglobin saturation (SvO2) monitoring was reported by 95% of the centers. Continuous arterial in-line PO2 monitoring was reported by only 40% of the centers performing adult cases and by only 44% of the centers performing pediatric cases. Temperature was monitored on the patient in at least one site by 95% of centers during normothermic CPB (>35°C); 33% of centers monitored two sites, and 7% of centers monitored three sites. During moderate hypothermia (28–35°C), 33% of the centers monitored one site, 57% monitored two sites, and 10% monitored more than two sites. For deep hypothermia (<28°C), the figures were 13%, 62%, and 25% for one, two, and three sites, respectively.
Blood gas measurements were systematic for all centers, and 28% of centers reported continuous in-line monitoring of arterial blood gas. Serum potassium was measured by all centers, but glycemia and calcemia were measured by 72% and 63% of centers, respectively, for adults. Total and ionized serum calcium concentrations were measured by 88% of centers performing pediatric CPB procedures. Delays to obtain results were >5 minutes for 46% of the centers, >10 minutes for 29% of centers, and >15 minutes for 21% of the centers.
Anticoagulation with unfractionated heparin (UFH) and its monitoring were part of a written protocol in 98% and 93% of the centers, respectively. The anticoagulant effect of UFH was monitored by all but one center using the activated clotting time (ACT) monitors. One ACT monitor per operation room was reported by 91% of the centers. Only 75% of the centers monitored ACT at intervals <30 minutes. Heparin concentration was monitored by 32% of the centers, one center using a point-of-care monitor. One center used heparin concentration as the only monitoring of the anticoagulant effect of UFH.
An alarm on the venous reservoir level detector was reported by 74% of the centers, and this alarm was associated with an arterial pump shutdown regulator in only 61% of the centers. This alarm was used episodically by 10% of the centers, and 16% reported never using it. Arterial line bubble detectors were reported as systematic by 32% of centers (28% with pump shutdown regulator), episodic by 14%, and non-existent by 54% of the centers. Arterial line filters were used systematically by 70% of the centers, and 16% of the centers never used them. When an arterial line filter was used, 77% of the centers reported the use of one-way purge lines. One-way pressure relief valves were used in left ventricular vent lines by only 41% of the centers.
Electrical safety devices and procedures concerned the use of uninterruptible power supply (UPS) by 88% of centers; 81% of the 160 identified CPB machines had arterial pump battery backup. In total, three CPB machines without arterial pump battery backup were reported, as well as two centers that did not have UPS. Emergency, independent lights were reported by 70% of the centers.
Epiaortic echocardiography to choose the cannulation site was reported to be used episodically by 7% of the centers.
Perfusion Incidents
The reported events are summarized in Table 2. Five centers (of which one that had a prospective registry of perfusion-related incidents) could not or did not want to report on CPB-related incidents occurring in 2005. These five centers performed 2298 CPB procedures, which were excluded from the analysis of incidents. The denominator used to calculate the incidence of CPB-related incidents is 32,198. One hundred sixty-three incidents were reported (1 in 198 CPB procedures). Centers that had a prospective registry of incidents did not report more incidents than those without a prospective registry: 1:168 vs. 1:207 (p = .19). These 163 incidents were followed by transient sequelae for 13 patients, persistent sequelae for 3 patients, and death for 7 patients. Taken together, morbidity was 1:1400 CPB procedures and mortality was 1:4600 procedures. Severe morbidity or mortality was calculated and resulted in 1:3220 procedures.
Table 2.
Perfusion incidents.
| Type of complication (number of CPB)* | Total | No complication | Transient complication | Sequelae | Death | Evolution unknown |
|---|---|---|---|---|---|---|
| Coagulation of the circuit (31,303) | 7 (1/4472) | 5 (1/6261) | 2 (1/15,652) | |||
| Undesired effect to protamine (28,847) | 20 (1/1442) | 12 (1/2404) | 5 (1/5769) | 1 (1/28,847) | 1 (1/28,847) | 1 (1/28,847) |
| Dissection originating at the aortic cannulation site (24,821) | 8 (1/3103) | 3 (1/8274) | 3 (1/8274) | 1 (1/24,821) | 1 (1/24,821) | |
| Dissection originating at the arterial (non aortic) cannulation site (24,524) | 11 (1/2229) | 6 (1/4087) | 2 (1/12,262) | 1 (1/24,524) | 2 (1/12,262) | |
| Disconnection/rupture of the lines (30,512) | 7 (1/4294) | 7 (1/4294) | ||||
| Gas embolism (CPB circuit) (30,059) | 8 (1/3757) | 4 (1/7515) | 3 (1/10,020) | 1 (1/30,059) | ||
| Gas embolism (cardioplegia line) (27,859) | 14 (1/1990) | 14 (1/1990) | ||||
| Gas supply failure (27,661) | 2 (1/13,831) | 2 (1/13,831) | ||||
| Hypoxemia caused by oxygenator failure (28,803) | 4 (1/7201) | 3 (1/9601) | 1 (1/28,803) | |||
| Increased resistance in the oxygenator (9717)† | 23 (1/422) | 21 (1/463) | 2 (1/4859) | |||
| Electrical/mechanical pump failures (32,198) | 19 (1/1695) | 19 (1/1695) | ||||
| Heater/cooler failure (29,644) | 31 (1/956) | 31 (1/956) | ||||
| Main electricity supply failure (30,512) | 1 (1/30,512) | 1 (1/30,512) | ||||
| Other incidents (22,797) | 8 (1/2849) | 7 (1/3256) | 1‡ (1/22,797) |
Centers that have not transmitted data are not included in the calculations.
Only two centers with a total of 1594 CPB reporting 20 incidents have pressure monitoring proximal and distal of the oxygenator.
Coronary artery dissection upon cardioplegia injection.
The main causes of morbidity and mortality (by order of decreased rate) were related to the following.
Adverse reactions to protamine (20 patients, of which 2 died, 1 survived with sequelae, and 5 had transitory complications.
Arterial dissection at the site of cannulation. Dissection concerned the ascending aorta (8 cases, with 1 death and 3 transient sequelae) or other arterial sites (11 cases, with 2 deaths, 1 persistent sequelae, and 2 transient sequelae). It is worth mentioning a death probably caused by dissection of a coronary artery upon injection of cardioplegia.
Gas embolism originating in the CPB circuit (eight cases, of which one had sequelae and three had transitory complications).
Oxygenator thrombosis (seven cases with two deaths) required emergent oxygenator replacement for three patients.
Oxygenator failure with inadequate oxygenation was reported for four patients, was not associated with complications, and required oxygenator change-out in one case. Oxygenator dysfunction with increased resistance across the oxygenator was reported in 23 cases and was not followed by complications and required emergent change-out in 1 case. Twenty cases of increased resistance across the oxygenator were reported by four centers that monitored pressures proximal and distal of the oxygenator. The remaining three cases were reported by centers that did not monitor pressures or monitored pressure distal of the oxygenator. If the incidence of increased resistance across the oxygenator is analyzed exclusively for centers that monitored pressures proximal and distal of the oxygenator, the incidence of this event is 1:80 CPB procedures.
The other reported incidents were not associated with complications: disconnection or ruptured lines (7 cases), gas embolism originating in the cardioplegia circuit (14 cases), gas circuit failures (2 cases), electrical of mechanical failures (19 cases that required a crank for 8 patients), and heat exchanger failure (31 cases).
Among the other incidents that were not followed by sequelae, these are worth mentioning: reversed lines observed on onset of CPB, dysfunction of heat exchanger in a lot of oxygenators, increased resistance across a cardioplegia line filter, and a sequestered venous return in a double deck cardiotomy reservoir.
DISCUSSION
The percentage of centers that returned the questionnaire (85%) indicates that the results presented in this article are representative of CPB activity in 2005 in France and is higher than that of previously published surveys (4–9,13). This high percentage is probably the result of the communication policy before the survey, the involvement of the French HAS in the project, and additional mailings to hospitals that did not return the questionnaire initially. It is also probable that, in the wake of the recommendations formulated by the French HAS on monitoring and safety devices for CPB in 2004, perfusionists awarded special attention to issues concerning CPB safety. The centers that returned the questionnaire were more often from the for-profit private practice sector. In addition, centers from the public sector were geographically clustered. With these limitations, we estimate that the results of the survey are representative of CPB activity for 2005 in France.
The results of the survey showed an important difference between the recommendations formulated by the French HAS (as listed in Table 1) in December 2004 and everyday practice of CPB in 2005. This difference especially concerns the monitoring of pressure at different locations in the CPB circuit and the use of bubble detectors. Both types of devices are relatively costly, and this probably explains why implementation of the recommendations is slow. Previous surveys from France showed that implementation of routine use of a safety device is slow, as was the case for venous reservoir level detectors: 12% of centers reported their use in 1989 (14), 52% in 2001(13), and 74% in 2005 in this survey. In fact, venous reservoir level detectors represent the only safety device whose use increased recently in France (13). The use of other devices remained unchanged or decreased (monitoring of CO2 at the oxygenator vent was reported by 29% of centers in the previous French survey (13) and by only 7% in this survey; the decreased use of this monitoring devices is probably related to the absence of a Communauté Européenne (CE)-marked device).
Failure to use a checklist by 21% of the respondents is surprising given the fact that this security measure has no additional cost. It is expected that implementation of the French HAS recommendations will result in generalized use of checklists.
The rate of incidents without injury is highly variable in the different published surveys depending on the methodology of the survey and the number of items that compose the questionnaire, and this parameter is an imperfect estimator of the true rate of CPB-related incidents (10). When comparing, in a first approach, the rate of incidents with injury (persistent or transient) and mortality between our survey and that published by Mejak et al. (9), it is striking that at a several-year interval and on two different continents, the rates of incidents with injury (1:1400 vs. 1:1453) and mortality (1:4600 vs. 1:4567) are similar. There are nevertheless methodologic differences between the two surveys. Mejak et al. (9) included coagulation problems after CPB in their analysis, which is probably valid in that excessive hemodilution during CPB can trigger hemodilution-induced coagulopathy and excessive bleeding after CPB and surgery. This was the most frequent complication in their study, but it could be caused by factors other than CPB or could be the combined results of CPB and other factors. For this reason, we did not include this complication in our questionnaire. If this type of complication is not included in the analysis in the rate of incidents in the survey of Mejak et al. (9), the incidence of incidents with injury is not 1:1453 but is 1:2450, and the rate of mortality is not 1:4567 but is 1:6850. Adverse events to protamine are the most frequent incidents in our survey. Although classically cataloged as CPB-related incidents, the adverse reactions to protamine are nearly inevitable complications. The one death that followed protamine injection in our survey was caused by anaphylactic shock. Whatever the complexity of monitoring or safety devices during CPB, it will not be possible to prevent adverse reactions to protamine, and therapy will be the only option.
In addition to protamine-induced adverse events, three types of incidents were associated with injury and death.
Incidents related to arterial cannulation seem to be more frequent compared with previous surveys. In our survey, this type of incident occurred nine times, followed by persistent sequelae in one patient and death for three patients. This type of incident was not reported by older surveys (4–7) but represented the second cause of mortality in the surveys of Mejak et al. (9) (the first cause being incidents related to coagulation) and Jenkins et al. (8) (the first cause being emergent reinstitution of CPB, which is not exactly a CPB-related incident but a result of cardiac dysfunction). Whereas the majority of arterial cannulations for CPB concern the ascending aorta, in our survey, the incidence of complications related to other arterial sites (less frequently used) are just as frequent, and this suggests that cannulation of arteries other than aorta are associated with a much higher risk of complications. Whether or not, in case of alternative cannulation site from the conventional ascending aorta, arterial echography could be helpful to avoid such complications requires prospective studies. Similarly, it is not known whether epiaortic echography could help prevent complications related to cannulation of the ascending aorta (15). In this survey, the use of epiaortic echography was infrequent. The use of centrifugal pumps and arterial line pressure monitoring (ALPM) distal of the oxygenator with arterial pump shutdown regulator could eventually avoid (or at least attenuate the consequences of) such complications. The reported use of centrifugal pumps and ALPM with arterial pump shutdown regulator was 49% and 35%, respectively, in the survey of Mejak et al. (9) and 21% and 18%, respectively, in our survey. It is possible that the less frequent use of these two devices in our survey explains the fact that incidents related to arterial cannulation are the first cause of mortality (1:11,349 CPB procedures). In the context of incidents related to arterial cannulation, we report one death that occurred in a patient that developed coronary artery dissection diagnosed on increased pressure in the cardioplegia line. The center that reported this complication did not use a cardioplegia pump shutdown regulator, and it is possible that this contributed to the bad outcome in this case. Only 14% of the French cardiac surgery centers reported the use of cardioplegia line pressure monitoring (CLPM) with a cardioplegia pump shutdown regulator.
Clot or thrombus present in the circuit during CPB. Under this definition, two different types of problems were reported; in two cases, occlusion of the circuit was related to the aspiration of surgical debris or biological glue. These two incidents were without consequences for the patients, although in one case, the oxygenator had to be changed during the CPB procedure. These two cases are arguments in favor of controlling aspirations from the surgical field. For the five other cases, there were real thromboses of the circuit during the CPB that required oxygenator change-out in three cases and resulted in death in two patients. The five cases were reported by five centers. For four of the centers, the anticoagulation protocol stipulated that intervals between two successive ACT measurements could be >30 minutes. For the fifth center, the protocol recommended an interval <30 minutes, but the actual interval for that patient had been longer. These results suggest that the interval between two successive ACT measurements during CPB should be <30 minutes.
Gas embolism. This type of incident did not result in any deaths in our survey. Nevertheless, the rate of gas embolism was higher than that published by Mejak et al. (9), who reported the occurrence of gas embolism in 1:13,426 procedures (with morbidity in 1:39,487 procedures), whereas in our survey, the rate of gas embolism was 1:3757 (with morbidity in 1:7515 procedures). The higher rate of gas embolism in our survey is probably not related to the use of venous reservoir level detector [70.4% reported by Mejak et al. (9) and 74% in our survey] or the use of arterial pump shutdown regulator [30.4% of centers reported by Mejak et al. (9) and 61% in our survey]. It is probable that the higher rate of gas embolism in our survey is related to the less frequent use of other safety devices such as arterial line bubble detector [87.8% of centers reported by Mejak et al. (9) with arterial pump shutdown regulators for 63% centers compared with 32% and 28%, respectively, in our survey]. Similarly, arterial line filters were reported by 98.5% of centers by Mejak et al. (9) and by 70% of centers in our survey; a similar difference exists for one-way pressure relief valves for the left ventricular vent line (83% in the United States and 41% in our survey) and one-way relief valves on the filter purge line (91.8% in the United States and 53% in our survey). It is important to mention that the most severe incident that followed gas embolism in our survey occurred in a patient where gas originated from a left ventricle vent line that did not have a one-way pressure relief valve. Similarly, it is noticeable that our survey contains a high rate of gas embolism in the cardioplegia line (1:1990 procedures). This must be compared with the results reported by Jenkins et al. (8) (1:2459) and Mejak et al. (1:1716) (9). The use of a cardioplegia delivery line bubble detector was not part of our questionnaire or the questionnaire of Jenkins et al. (8). Such a device was reported by 9.4% of centers by Mejak et al. (9). Gas embolisms on the cardioplegia line occurred in 14 patients and were not associated with reported complications. Whether or not these gas embolism episodes in the cardioplegia line resulted in transient or prolonged myocardial dysfunction is not known because this item was not part of the questionnaire. It is important to underline the fact that it is easier to prevent gas embolism than to treat its consequences.
Other types of incidents concerned device failures (oxygenator failure, gas circuit failure, electrical or mechanical pump failures). These incidents are frequently reported, and despite their potential danger for the patients, did not result in any severe complication in our survey or the above-cited surveys. Devices failures result in hypoperfusion or hypoxemia that can probably be corrected rapidly or, if short-lived, tolerated by the patients. Therefore, although such incidents must be prevented, they can also be corrected if diagnosed and treated rapidly. This could explain the discordance between the high incidence of such failures and the lack of reported clinical consequences. One separate comment should be made for emergent oxygenator change-out during the CPB procedure. The reported morbidity of this situation is very low both in our survey and in that of Jenkins et al. (8), who did not report any morbidity, or in that of Mejak et al. (9), who reported two severe injuries. Comparisons among the different surveys should nevertheless be done cautiously because under the category “oxygenator failure” there are probably several mechanisms (problems caused by manufacturing, installation, coagulation problems, etc.). The rate of oxygenator failure was 1:458 (that required change-out was 1:773) for Jenkins et al. (8) and 1:2459 and 1:4662, respectively, for Mejak et al. (9). The differences between these two surveys are probably related to the fact that failures detected before the onset of CPB procedure were taken or not into consideration. In our survey, we counted as oxygenator failures only those that occurred after onset of the CPB procedure diagnosed by increased pressure proximal of the oxygenator and/or altered oxygenation. If only the increased pressure proximal of the oxygenator is taken into consideration, this complication occurred in 1:7201 procedures that required oxygenator change-out in 1: 28,803 procedures. This low rate should be interpreted by taking into account that only four centers measured the pressure proximal of the oxygenator and only two centers reported data. The reported incidence of increased pressure proximal of the oxygenator for these two centers (1:80 procedures) is comparable to that reported by Myers et al. (16), which was 1:87 procedures. The severity of such incidents must also be taken into account. The classification into three types proposed by Fisher et al. (17) could be helpful to further study this type of incident. This last example clearly shows that many incidents are probably underreported because the tools required to diagnose them are not used or do not exist. These undiagnosed incidents could contribute to the morbidity and mortality of CPB procedures under less spectacular ways than arterial dissection or oxygenator/circuit thrombosis.
Surveys are subjected to two major sources of error: incomplete sampling and misinterpretation of the survey questions by respondents (18). Given the high response rate to our survey (86%), we believe that the sampling is sufficiently complete to make inferences about perfusion practice in France. The limitations of this survey are those generally associated with retrospective reporting of incidents, resulting in underreporting, although a shorter time period (1 year) compared with other surveys (4–9) could result in fewer “forgotten” incidents. The comparison among several surveys is also a limitation of this study because definitions were not standardized among the different published studies, and therefore, the rates of a type of incident may not be comparable.
It seems from our results that the majority of severe complications (including deaths) related to CPB could be prevented by using several safety devices and monitoring techniques that are underused as shown in this survey. Compared with previous surveys, our survey suggests that, at least for some complications, the more frequent use of safety/monitoring devices is associated with a lower incidence of complications. Given the average number of CPB procedures performed by perfusionists in France (<150/year), the low rate of the reported incidents (<1: 200) could provide a false sense that CPB is a safe procedure. Although much progress has been made concerning the safety of CPB procedures, much work remains to be done to prevent unacceptable deaths and permanent sequelae. For France, application of the recently published recommendations and incident reporting should be part of this process.
ACKNOWLEDGMENTS
The authors thank the following for contributions to the survey: Prof. B. Albat, Mr. M. Aveline, Mr. F. Bartolomé, Mme. M. Beaufils, Mme. Y. Bendahmane, Dr. F. Bertin, Dr. N. Bischoff, Prof. Y. Blanloeil, Prof. D. Blin, Dr. G. Boccara, Dr. D. Bompard, Mr. Y. Boucher, Dr. P. Brunel, Prof. J.L. de Brux, Mr. J.L. Cadusseau, Mr. D. Chassaing, Dr. O. Corre, Dr. L. Couche, Dr. F. Crepin, Mr. M. Cruet, Dr. D. Denjean-Jouannou, Dr. J.P. Depoix, Dr. D. Duterque, Mr. J.Y. Ely, Mr. G. Faur, Mr. D. Fouré, Dr. J.F. Fournier, Dr. Y. Fromes, Mme. S. Gambaudo, Dr. D. Garbi, Mr. J.M. Horras, Dr. C. Isetta, Prof. G. Janvier, Mr. J.C. Kempf, Mme. B. Kindelberger, Dr. J.P. Lançon, Dr. D. Le Houerou, Dr. E. Lemoine, Dr. P. de Lentdecker, Prof. D. Loisance, Dr. M. Maccario, Mr. S. Martinez, Dr. A. Maudiere, Dr. M. Mauduit, Dr. P. Menestret, Mr. J. Mogier, Dr. F. Montiglio, Mme. S. Mottet-Reymond, Mme. M.C. Pintard, Prof. A. Pol, Dr. A. Poncet, Dr. O. Ponzio, Mlle. F. Regneri, Mr. A. Rioux, Dr. A. Ruffenach, Dr. J.C. Sargentini, Mr. R. Sigonney, Mme. D. Vallée Lucas, and Mr. F. Werbicki.
Appendix 1 Questionnaire that was sent to the chief perfusionist of each centre
Centre Number:
Centre identification:
City:
Perfusionist that filled-in the questionnaire:
Last Name:
First Name:
Telephone:
Email address:
Centre Number:
Please answer all questions and fill in Not available or Not applicable if you cannot answer. This will document the fact that the item was not forgotten.
REFERENCES
- 1.Society of Clinical Perfusion Scientists of Great Britain and Ireland. Recommendations for Standards of Monitoring and Alarms During Cardiopulmonary Bypass. Society of Clinical Perfusion Scientists of Great Britain and Ireland; 1988. Available at: http://www.sopgbi.org/perfusionists.html. Accessed July 9, 2007.
- 2.American Society of Extra-Corporeal Technology. AmSECT Guidelines for Perfusion Practice. American Society of Extra-Corporeal Technology; 1998. Available at: http://www.amsect.org/index.html. Accessed July 9, 2007.
- 3.Australasian Society of Cardio-Vascular Perfusionists. Standards of Clinical Practice of Perfusion for Cardiopulmonary Bypass. Australasian Society of Cardio-Vascular Perfusionists; 2000. Available at: http://www.anzcp.org/Documents/ANZCP%20Regulations.pdf. Accessed July 9, 2007.
- 4.Stoney W, Alford WCJ, Burrus G, Glassford DJ, Thomas CJ.. Air embolism and other accidents using pump oxygenators. Ann Thorac Surg. 1980;29:336–40. [DOI] [PubMed] [Google Scholar]
- 5.Wheeldon D.. Can cardiopulmonary bypass be a safe procedure? In: Longmore DB, ed. Towards Safer Cardiac Surgery. Lancaster, UK: MTP; 1981;427–46. [Google Scholar]
- 6.Wheeldon D.. Proceedings of the First World Congress on Open-Heart Technology. London, UK: Franklin Scientific Projects; 1982. [Google Scholar]
- 7.Kurusz M, Conti V, Arens J, Brown J, Faulkner S, Manning J.. Perfusion accident survey. Proc Am Acad Cardiovasc Perfusion. 1986;7:57–65. [Google Scholar]
- 8.Jenkins O, Morris R, Simpson J.. Australasian perfusion incident survey. Perfusion. 1997;12:279–88. [DOI] [PubMed] [Google Scholar]
- 9.Mejak B, Stammers A, Rauch E, Vang S, Viessman T.. A retrospective study on perfusion incidents and safety devices. Perfusion. 2000;15:51–61. [DOI] [PubMed] [Google Scholar]
- 10.Palanzo D.. Perfusion safety: Defining the problem. Perfusion. 2005;20:195–203. [DOI] [PubMed] [Google Scholar]
- 11.ANAES, ed. Recommandations Concernant le Monitorage et les Dispositifs de Sécurité Pour la Circulation Extracorporelle en Chirurgie Cardiaque. ANAES; 2004. Available at: http://www.has-sante.fr (http://www.has-sante.fr/portail/upload/docs/application/pdf/circulation-extracorporelle_chirurgie_cardiaque_rap.pdf). Accessed July 9, 2007. [PubMed]
- 12.Annuaire des centres de chirurgie cardiaque et thoracique. Saint Max, France: Editions Axis; 2005. [Google Scholar]
- 13.Charriere J, Durand C, Mandon N, Le Guen A, Jayle C, Debaene B.. Enquête française sur les dispositifs de surveillance de la CEC en 2001. Ann Fr Anesth Reanim. 2003;22:414–20. [DOI] [PubMed] [Google Scholar]
- 14.Meilhan E, Merian L, Guespin A, et al. Pratique de la CEC dans l’Ouest de la France: résultats d’une enquête pour l’année 1989. Les Cahiers du CECEC. 1990;33:55–62. [Google Scholar]
- 15.Ruchat P, Hurni M, Stumpe F, Fischer A, von Segesser L.. Acute ascending aortic dissection complicating open heart surgery: cerebral perfusion defines the outcome. Eur J Cardiothorac Surg. 1998;14:449–52. [DOI] [PubMed] [Google Scholar]
- 16.Myers G, Weighell P, McCloskey B, Holt A, McTeer S, Maxwell S.. A multicenter investigation into the occurrence of high-pressure excursions. J Extra Corpor Technol. 2003;35:127–32. [PubMed] [Google Scholar]
- 17.Fisher A, Baker M, Buffin M, et al. Normal and abnormal trans-oxygenator pressure gradients during cardiopulmonary bypass. Perfusion. 2003;18:25–30. [DOI] [PubMed] [Google Scholar]
- 18.Groom RC, Froebe S, Martin J, et al. Update on pediatric perfusion practice in North America: 2005 Survey. J Extra Corpor Technol. 2005;37:343–54. [PMC free article] [PubMed] [Google Scholar]