Abstract:
Techniques for pediatric cardiac surgery requiring cardiopulmonary bypass (CPB) have significantly improved over the years. The use of fresh whole blood (FWB) and pre-bypass ultrafiltration (PBUF) has been suggested as means for improving perioperative and postoperative outcomes. It is the intent of this study to show that fresh whole blood along with PBUF will result in balanced CPB prime that can offer a reduction in blood product exposures and blood loss along with improving several measured postoperative outcomes. After institutional review board approval, a retrospective review was conducted on 100 patients to analyze the benefits of FWB and PBUF on outcomes in neonatal and pediatric cardiac surgery. Data analysis included preoperative and CPB data, perioperative inotrope and blood product exposure, and postoperative blood loss and blood product exposure measured for up to a 12-hour period in the intensive care unit (ICU). The three groups compared were FWB prime vs. packed red blood cell (PRBC) prime, <5 kg FWB prime vs. <5 kg PRBC prime, and 5+ kg FWB prime and 5+ kg PRBC prime. Cumulative blood product exposures for the FWB prime group found 62% received one blood exposure for the operative and postoperative period (p < .0001). The majority of patients who received a PRBC prime (64%) received three or more cumulative exposures (p < .0003). The <5 kg FWB group also received significantly less cumulative blood exposure, with 64% receiving just one exposure. Comparatively, 85% of the <5 kg PRBC patients received three or more blood product exposures perioperatively and postoperatively (p < .0001). Perioperative inotrope and postoperative blood loss did not differ among the groups. Outcomes for intraoperative death, intraoperative extubation, delayed sternal closure, and mediastinal reexploration were also not statistically different. The results of this study found that FWB leads to significantly less blood exposure, specifically in the <5-kg population. Finally, the use of PBUF is an effective method for achieving a balanced, physiologic prime. Future research would be helpful in determining which specific patient populations would receive the greatest benefit from FWB and PBUF.
Keywords: cardiopulmonary bypass, fresh whole blood, pre-bypass ultrafiltration, pediatric perfusion circuitry
Current techniques and equipment for neonatal and pediatric cardiopulmonary bypass (CPB) have significantly improved over the past 20 years. Perfusionists have been instrumental in facilitating improvements by aggressively reducing the prime volumes needed for CPB. Despite successful improvements, the CPB circuit is still a relatively large prime volume in comparison with the small blood volume of the neonatal patient. As a result, most patients still need homologous blood in the CPB prime to maintain an acceptable hematocrit for oxygen delivery. However, the transfusion of homologous blood is not without consequence. Clinicians have long recognized the detrimental effects of stored red blood cells including acid–base, glucose, and electrolyte imbalances, all of which can contribute to severe complications (1). Consequently, clinicians have looked at ways to improve outcomes associated with pediatric CPB where homologous blood transfusions are needed. Many centers have advocated the use of fresh whole blood (FWB) for CPB to help improve hemostasis, reduce exposures to cytokines and inflammatory mediators seen with stored packed red blood cells (PRBCs), and reduce overall blood exposures (2–4). Further refinements to CPB techniques have been suggested after many open heart centers discovered the impressive benefits from modified ultrafiltration (MUF). These centers suggested that the benefits of ultrafiltration could be applied to the pre-bypass stages of the cardiac operation to help reduce the deleterious effects of stored blood (5). This technique, called pre-bypass ultrafiltration (PBUF), is purported to further reduce the inflammatory, electrolyte, and metabolic disturbances by ultrafiltrating the blood primed circuit before instituting CPB (5–7). Many centers support this technique because the CPB prime is often a primary determinant in the patient’s metabolic response to the cardiac operation (8).
Using FWB in the CPB prime and practicing techniques such as PBUF may assist in improving outcomes. Although data exist for each practice, there is little information when the two techniques are combined during open heart surgery (6). Accordingly, it is the intent of this retrospective study to evaluate if there are benefits to our practice of using a fresh whole blood CPB prime and pre-bypass ultrafiltration on patient outcomes in neonatal and pediatric cardiac surgery.
MATERIALS AND METHODS
After approval by the Institutional Review Board, a retrospective chart analysis was conducted on patients 1 day to 4 years old who presented for cardiac surgery requiring CPB between January 2003 and June 2006. To meet the requirements for the study, the participants all received blood products, either fresh whole blood (<48 hours old) or packed red blood cells, for priming of the CPB circuit. The participants may or may not have received subsequent blood transfusions during the operative procedure or postoperatively. Subjects were excluded from the study if their cardiac procedure did not require the use of CPB, if they did not need a blood transfusion for priming of the CPB circuit, or if they failed to receive PBUF or aprotinin. Subjects were also excluded if they had preoperative mechanical support, a bleeding disorder, or liver disease. Additionally, any subjects who received recombinant factor VII during the perioperative or postoperative time period were excluded from the study.
Conduct of Perfusion
The CPB circuit consisted of a roller pump (Stockert Compact Perfusion System; Cobe Cardiovascular, Arvada, CO) and either a Lilliput 1 or Lilliput 2 membrane oxygenator (Cobe Cardiovascular), appropriately sized for each patient’s predicted CPB flow. The CPB circuit also consisted of an arterial line filter (Terumo Cardiovascular, Ann Arbor, MI), Sorin Vanguard for 4:1 blood cardioplegia delivery (Cobe Cardiovascular), and an appropriately sized arterial–venous loop. A Minntech HPH 400 hemoconcentrator (Minntech Corporation, Minneapolis, MN) was also used for PBUF, continuous ultrafiltration (CUF), and MUF for each case.
The CPB prime consisted of Plasmalyte-A (Baxter Healthcare Corporation, Deerfield, IL), 25% mannitol (1.0 g/kg, not to exceed 12.5 g), 25% albumin (1 g/kg, not to exceed 25 g), 15–20 mL sodium bicarbonate (1 mEq/mL), and 100 units of heparin/100 mL of prime. Additionally, aprotinin (Bayer Healthcare Pharmaceuticals, West Haven, CT) was used on all patients included in this study. The pump prime dose was calculated at 240 mg/m2, with a similar infusion at the beginning of the operation and a continuous maintenance infusion of 56 mg/m2/h. CPB pump flow rates were calculated on a 2.4 cardiac index, and mild hypothermia was used (28°C) unless deep hypothermic circulatory arrest (DHCA) was warranted. Cold blood cardioplegia (4:1) was infused every 20–30 minutes, with an induction dose of 40–50 mL/kg and subsequent doses of 20 mL/kg. Hematocrit levels were maintained on CPB at 28% or greater, either by using ultrafiltration to remove excess water or by the transfusion of red cells (either FWBs or PRBCs). After weaning from CPB, arterio–venous modified ultrafiltration (A-V MUF) was performed for approximately 20 minutes.
Ultrafiltration Techniques
PBUF:
All patients had a baseline arterial blood gas drawn after insertion of an invasive arterial monitoring line. After the measurement of the patient’s baseline hematocrit levels, a CPB/dilutional hematocrit value was calculated. The target hematocrit for the patient was 28%, and to achieve this, either FWBs or PRBCs were added to the CPB prime. The circuit prime was recirculated through the Minntech 400 ultrafiltrator with aliquots of Plasmalyte added to allow for continuous removal of fluid through the ultrafiltrator. Including the initial amount of Plasmalyte-A used to prime the CPB circuit, approximately1Lof Plasmalyte-A was added and ultrafiltrated. PBUF was carried out until the minimum safe operating level for initiating CPB was reached in the venous reservoir. The volume removed by the ultrafiltrator from the CPB prime was dependent on two factors: the amount of red cells added to reach a target hematocrit of 28% and the maintenance of a safe volume in the venous reservoir for CPB initiation. During PBUF, 10–15 mEq of sodium bicarbonate was added to replace the amount removed by ultrafiltration. Once the maximum amount of volume had been removed from the CPB prime, a blood gas specimen was drawn and analyzed to adjust the prime for pH neutrality.
CUF:
During the course of the CPB run, CUF was performed to remove any excess crystalloid accumulated from the field and to remove excess volume acquired with cardioplegia administration. The goal during CUF was to maximize the hematocrit, maintain electrolyte balance, and decrease circulating levels of systemic inflammatory mediators.
MUF:
Once the patient was weaned from CPB and was hemodynamically stable, A-V MUF was performed. MUF was performed at a rate of 5–20 mL/kg/min for a 20-minute duration or until the CPB circuit was cleared of blood and chased through with Plasmalyte-A.
Transfusion Triggers
The transfusion of blood for CPB requires a dilutional hematocrit calculation after the measurement of the patient’s hematocrit from their initial blood gas in the operating room (OR). The target hematocrit for the patient was 28%, and a dilutional hematocrit value less than this required a blood primed CPB circuit. During the course of CPB, ultrafiltration was performed to maintain a hematocrit of 28%, and if necessary, further transfusions of processed cell saver blood, FWBs, or PRBCs were used.
The use of fresh frozen plasma (FFP) in the CPB prime was only warranted when fresh whole blood was not available. Once it was acknowledged that PRBCs were available, a discussion ensued between perfusion, anesthesia, and the surgeon on whether or not they believed that the transfusion of FFP would be beneficial. In these cases, the transfusion of FFP was individualized for each patient. However, factors such as preoperative laboratories, difficulty and length of the required operation/CPB run, and risk of perioperative and postoperative coagulopathy problems were all considered.
The administration of postoperative blood products is always individualized; however, the criteria listed in Table 1 was used to help define when the transfusion of blood products was necessary.
Table 1.
Postoperative transfusion guidelines.
RCB tranfusion
|
Platelet transfusion
|
Fresh frozen plasma transfusion
|
Cryoprecipitate transfusion
|
RBC, red blood cell; Hct, hematocrit; PT, prothrombin time; PTT, partial thromboplastin time.
Data Collection and Analysis
Approximately 200 data parameters were collected for each patient with the main data categories including anthropometrical data and preoperative laboratories, CPB data, perioperative inotrope use and blood product exposure, and postoperative blood loss and blood product exposure for a 12-hour duration in the intensive care unit (ICU). One hundred patients were included in this retrospective study. Comparisons were made between the following groups: FWB prime vs. PRBC prime; <5 kg FWB prime vs. <5 kg PRBC prime; and 5+ kg FWB prime vs. 5+ kg PRBC prime.
A registered statistician performed the statistical analysis. SAS software, version 9.1 (SAS Institute, Cary, NC) was used for data analysis. t Tests were used to compare continuous variables between the groups, and the Wilcoxon rank sum test was used when data were skewed. χ2 tests were used when comparing categorical variables between the groups; for small sample situations, the Fisher exact test was used. Results are presented as means and SD for each group or as frequencies and percentages. p < 0.05 was considered statistically significant.
RESULTS
A total of 100 participants met the eligibility requirements for this retrospective study. Results were analyzed according to each of the three groups that received comparisons. The results of the post-PBUF prime are shown in Table 2. These results show the effects that PBUF can have in reducing the overall metabolic load that patients receive when either FWBs or PRBCs are needed for CPB. PBUF is shown to be especially useful in decreasing the overall lactate and glucose concentrations seen with both FWB and PRBC units.
Table 2.
Lab analyses of fresh whole blood, packed red blood cells, and PBUF cardiopulmonary bypass prime.
| WB unit | PRBC unit | CPB prime | |
|---|---|---|---|
| pH | 6.9 | <6.8 | 7.44 |
| pCO2 | 67.75 | >115 | 43.22 |
| pO2 | 52.25 | 56 | 259.83 |
| BE | −14.2333 | * | 3.38 |
| Na+ | 145.75 | 120 | 147.67 |
| K+ | 4.45 | * | 4.20 |
| iCa+ | <.10 | * | 0.56 |
| Glucose | 336.25 | 343 | 43 |
| Lactate | 4.15 | >15 | 0.89 |
| Hemoglobin | 14.966 | 20 | 8.23 |
Lab values out of measurable range.
PBUF, pre-bypass ultrafiltration; FWB, whole blood; PRBC, packed red blood cells; pCO2, partial pressure carbon dioxide; pO2, partial pressure oxygen; BE, base excess; Na+, sodium; K+, potassium; iCa+, ionized calcium.
FWB Prime vs. PRBC Prime
Results showed that the FWB group had a total of 61 patients and the PRBC prime group had 39 patients. Anthropometic, preoperative, and CPB data for the FWB vs. PRBC subgroup (Table 3) found the two groups to be very similar. The two statistically significant exceptions were CPB time and percentage requiring cardioplegia for their surgical procedure.
Table 3.
FWB and PRBC preoperative and cardiopulmonary bypass data.
| FWB prime (n = 61) | PRBC prime (n = 39) | p | |
|---|---|---|---|
| BSA | 0.30 ± 0.08 | 0.33 ± 0.15 | NS |
| Redo sternotomy (%) | 23% | 33% | NS |
| Cyanotic lesion (%) | 34% | 44% | NS |
| Single ventricle (%) | 11% | 18% | NS |
| Average plyte prime (mL) | 101 ± 67 | 136 ± 125 | NS |
| Average PBUF volume (mL) | 850 ± 139 | 826 ± 163 | NS |
| Average CUF volume (mL) | 457 ± 262 | 417 ± 403 | NS |
| Average MUF volume (mL) | 593 ± 209 | 600 ± 228 | NS |
| CPB time (min) | 167.7 ± 73.8 | 126.5 ± 52.0 | 0.0015 |
| Ao Cx time (min) | 90.7 ± 46.6 | 78.0 ± 35.4 | NS |
| DHCA (%) | 7% | 10% | NS |
| CP (% receiving) | 84% | 64% | 0.026 |
| Average CP volume (mL) | 559 ± 357 | 539 ± 423 | NS |
| Lowest temp CPB (°C) | 25.5 ± 3.7 | 26.7 ± 4.6 | NS |
FWB, fresh whole blood; PRBC, packed red blood cells; BSA, body surface area; Plyte, plasmalyte-A; PBUF, pre-bypass ultrafiltration; CUF, continuous ultrafiltration; MUF, modified ultrafiltration; FFP, fresh frozen plasma; CPB, cardiopulmonary bypass; Ao Cx, aortic cross clamp; DHCA, deep hypothermic circulatory arrest; CP, cardioplegia; Temp, temperature; NS, not significant.
Comparisons of preoperative hematologic laboratory data showed that the two groups were similar in prothrombin time (PT) and partial thromboplastin time (PTT) levels but that the FWB group had a significantly greater platelet count (375.9 ± 136.7 vs. 313.9 ± 116.5/mm3, p < .024).
The CPB prime was found to be balanced and physiologic with both FWBs and PRBCs; no statistical difference was found between the two. However, there was a trend, although not statistically different, for the FWB prime to have a lower lactate level. Post-MUF laboratory data found no differences between the two groups with respect to glucose, lactate, or hemoglobin levels.
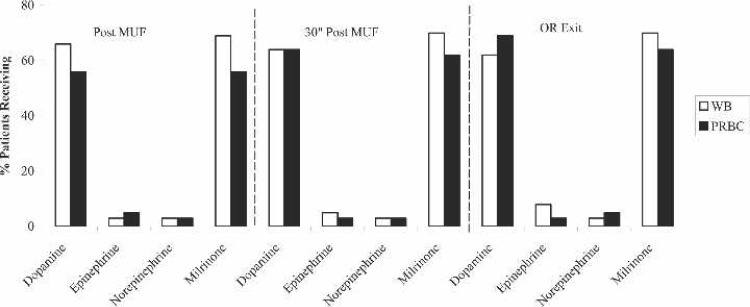
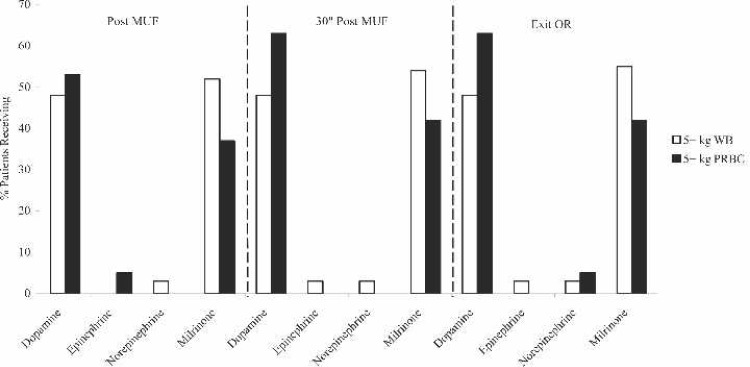
Perioperative inotropic support (Figure 1) consisted primarily of dopamine and milrinone infusions for both groups, with approximately 60% needing these inotropes post-MUF, 30 minutes post-MUF, and on exit from the OR. However, there were no statistical differences for any of the inotropes at these time points.
Figure 1.
Perioperative inotrope use for FWB prime and PRBC prime patients. MUF, modified ultrafiltration; OR, operating room; WB, whole blood; PRBC, packed red blood cells.
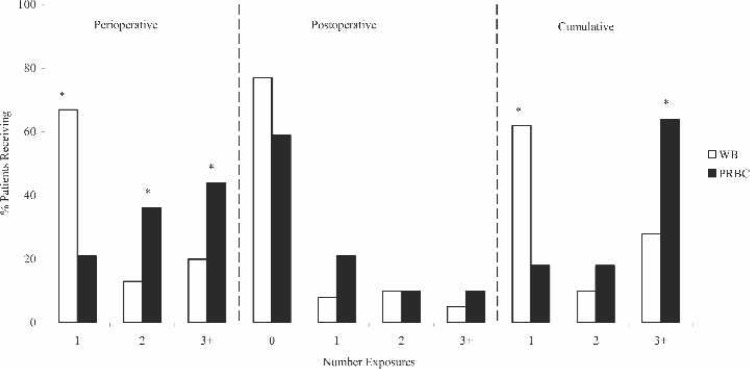
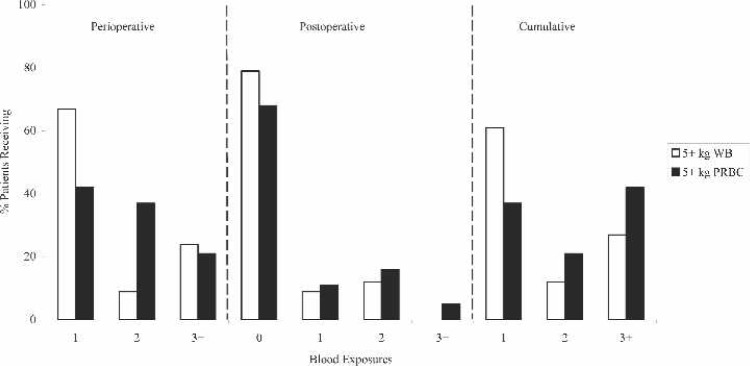
Perioperative, postoperative, and cumulative blood product exposures are shown in Figure 2. The most striking observation is that nearly 70% of the WB prime group needed only one perioperative blood exposure in comparison with 20% of the PRBC group (p < .0001). The PRBC group also received the greatest percentage of blood product exposures, with >44% receiving three or more donor exposures (p < .01). Although there was not a significant difference found in postoperative donor exposures, the data did show that the majority of both groups did not require any further blood exposures (77% WB vs. 59% PRBC). Cumulative donor exposures, totaling operative exposures and ICU exposures for up to 12 hours, showed that 62% of WB patients received only one exposure in comparison with 18% of PRBC patients (p < .0001). The data also showed that 64% of the PRBC prime group needed three or more cumulative donor exposures, which was significantly greater than the WB group (28%; p < .0003).
Figure 2.
Blood product exposures for FWB prime and PRBC prime patients. WB, whole blood; PRBC, packed red blood cells. *p < .05.
Blood loss for the ICU time periods (1, 6, and 12 hours) did not show any statistical difference between the WB or PRBC groups at any of the times. The greatest blood loss occurred at the 6-hour time period, with the loss averaging 3.3 ± 2.4 mL/kg for the WB group and 3.5 ± 2.6 mL/kg for the PRBC group (though not statistically significant). Postoperative outcomes for the WB and PRBC groups were not statistically different. In fact, the percentages were similar for each group, with 31% achieving extubation in the OR, 0% operative deaths, 8% needing delayed sternal closure, and 3% returning for mediastinal re-exploration.
WB Prime vs. PRBC Prime for <5 kg
Analysis of the preoperative and operative data for the <5 kg FWB vs. <5 kg PRBC prime groups (Table 4) found some differences between the two groups, including weight and temperature 30 minutes after CPB. Hematologic laboratory data showed that the <5 kg WB group had a greater preoperative platelet count compared with the <5 kg PRBC group (385.0 ± 170.8 vs. 282.2 ± 82.2/mm3, p < .0094). PT and PTT values were not significantly different. The CPB prime and post-MUF laboratory data did not vary significantly between the WB prime and the PRBC prime for patients <5 kg.
Table 4.
Cardiopulmonary bypass prime laboratory values.
| <5 kg FWB prime (n = 28) | <5 kg PRBC prime (n = 20) | p | |
|---|---|---|---|
| Weight (kg) | 3.84 ± 0.62 | 3.48 ± 0.61 | 0.047 |
| Redo sternotomy (%) | 11% | 5% | NS |
| Cyanotic lesion (%) | 46% | 65% | NS |
| Single ventricle (%) | 14% | 15% | NS |
| Average plyte prime (mL) | 91 ± 67 | 74 ± 81 | NS |
| CPB time (min) | 181.4 ± 77.1 | 142.4 ± 53.4 | NS |
| Ao Cx time (min) | 96.6 ± 51.1 | 85.1 ± 41.8 | NS |
| DHCA (%) | 14% | 75 | NS |
| CP (% receiving) | 89% | 64% | NS |
| Average CP volume (mL) | 416 ± 226 | 317 ± 169 | NS |
| Lowest temp CPB (°C) | 23.6 ± 3.6 | 24.4 ± 4.8 | NS |
| Temp 30″ post CPB (°C) | 36.4 ± 0.9 | 35.6 ± 0.7 | 0.0024 |
PRBC, packed red blood cells; pCO2, partial pressure carbon dioxide; pO2, partial pressure oxygen; BE, base excess; Na+, sodium; K+, potassium; iCa+, ionized calcium; NS, not significant.
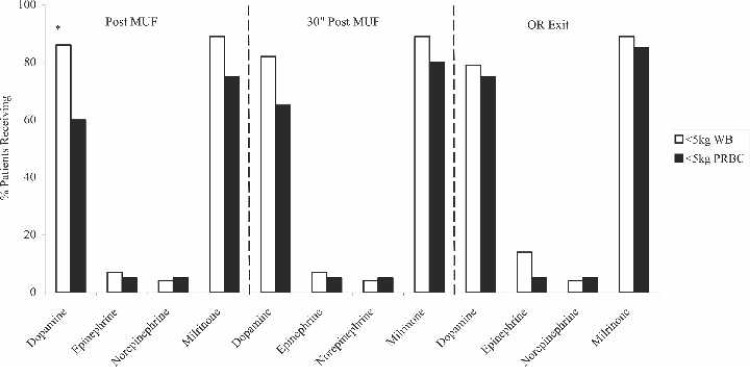
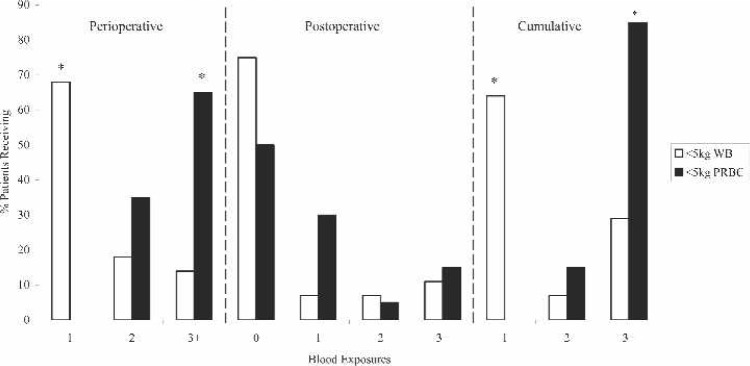
Perioperative inotropic support (Figure 3) consisted primarily of dopamine and milrinone for both groups. The <5 kg WB group trended for a greater percentage of patients to need dopamine and milrinone, although the only statistically significant point was for dopamine post MUF. Additional perioperative data showed that the <5 kg PRBC group had a statistically greater percentage needing multiple blood transfusions (Figure 4). Specifically, this data showed that 68% of the <5 kg WB group received only one blood exposure compared with 0% of the <5 kg PRBC group (p < .0001). In fact, the majority of the <5 kg PRBC group (65%) received three or more blood exposures perioperatively (p < .0003).
Figure 3.
Perioperative inotrope use for < 5 kg FWB prime and <5kg PRBC prime patients. MUF, modified ultrafiltration; OR, operating room; WB, whole blood; PRBC, packed red blood cells. *p < .05.
Figure 4.
Blood product exposures for < 5 kg FWB prime and <5kg PRBC prime patients. WB, whole blood; PRBC, packed red blood cells. *p < .05.
Postoperatively, blood loss did not differ significantly between the two groups. Subsequently, there was no difference between the <5 kg WB group and <5 kg PRBC group in postoperative transfusions, with the majority of patients, 75% of <5 kg WB and 50% of <5 kg PRBC, not requiring any further blood exposures in the ICU. Cumulative blood product exposure data showed that 64% of <5 kg WB patients needed only one exposure compared with 0% of PRBC patients (p < .0001). Furthermore, 85% of the <5 kg PRBC population needed three or more blood product exposures (p < .0001).
Results for OR extubation, intraoperative death, delayed sternal closure, and mediastinal re-exploration did not differ between the <5 kg WB group and the <5 kg PRBC group. It is of interest that 11% of the FWB patients achieved OR extubation vs. 0% of the PRBC patients, but again, this did not reach statistical significance.
WB Prime vs. PRBC Prime for 5 kg
Preoperative and operative data for the 5+ kg FWB prime and 5+ kg PRBC prime groups showed several statistical differences (Table 5). Statistical significance was reached for the percentage needing repeat sternotomy, volume of Plasmalyte prime, CPB time, lowest temperature on CPB, and percentage requiring cardioplegia for their operative procedure. Preoperative and CPB laboratory data were similar for both populations, with the exception of the glucose level in the CPB prime. The 5+ kg WB group had a significantly greater glucose level than the 5+ kg PRBC group (39.1 ± 13.9 vs. 26.0 ± 12.9 mg/dL, p < .0092). The post-MUF laboratory data did not vary significantly between the 5+ kg WB group and the 5+ kg PRBC group, with the lactate averaging 2.5 ± 1.0 vs. 2.5 ± 0.9 mmol/L (P = 0.97), the hemoglobin concentration averaging 14.0 ± 1.7 vs. 13.6 ± 2.1 g/dL (P = 0.50), and the glucose averaging 189.1 ± 50.8 vs. 190.3 ± 45.4 mg/dL (not significant).
Table 5.
Preoperative and cardiopulmonary bypass data for <5 kg FWB prime and <5 kg PRBC prime.
| 5+ kg FWB (n = 33) | 5+ kg PRBC (n = 19) | p | |
|---|---|---|---|
| Weight (kg) | 7.05 ± 2.05 | 9.88 ± 3.54 | 0.0038 |
| Redo sternotomy | 33% | 63% | 0.037 |
| Cyanotic lesion | 24% | 21% | NS |
| Single ventricle | 9% | 21% | NS |
| Average plyte prime (mL) | 109 ± 67 | 201 ± 132 | 0.0091 |
| CPB time (min) | 561.0 ± 69.9 | 109.7 ± 46.1 | 0.013 |
| Ao Cx time (min) | 84.9 ± 42.0 | 67.4 ± 20.1 | NS |
| DHCA (%) | 0 | 0 | NS |
| CP (% receiving) | 79 | 53 | 0.049 |
| Average CP volume (mL) | 696 ± 407 | 871 ± 479 | NS |
| Lowest temp CPB (°C) | 27.1 ± 3.1 | 29.0 ± 3.1 | 0.032 |
| Temp 30″ post CPB (°C) | 36.8 ± 0.6 | 36.6 ± 0.9 | NS |
FWB, fresh whole blood; PRBC, packed red blood cells; Plyte, plasmalyte-A; CPB, cardiopulmonary bypass; Ao Cx, cardioplegia; Temp, temperature; NS, not significant.
Inotropic support at three time points (Figure 5) found that the PRBC group had an increased trend for dopamine support, whereas the WB group trended for greater milrinone support, although statistical significance was not reached at any point. Additional results found that 67% of the 5+ kg WB group needed only one operative blood exposure vs. 42% of the 5+ kg PRBC group, although this also failed to reach statistical significance (Figure 6).
Figure 5.
Perioperative inotrope use for 5+ kg FWB prime and 5+ kg PRBC prime patients. MUF, modified ultrafiltration; OR, operating room; WB, whole blood; PRBC, packed red blood cells.
Figure 6.
Blood product exposures for 5+ kg FWB prime and 5+ kg PRBC prime patients. WB, whole blood; PRBC, packed red blood cells.
Results for postoperative blood product exposure were very similar for both groups, with 79% of the WB group and 68% of the PRBC group not receiving any further transfusions. Cumulative transfusion exposures favored the WB group, with 61% receiving only one exposure compared with 37% of the PRBC group; however, this did not reach statistical significance (p < .25, not significant).
Finally, there were no significant differences in outcomes for postoperative blood loss, percentage extubated in the OR (48% vs. 63%, P = 0.31), intraoperative death (0% vs. 0%, P = 1.0), delayed sternal closure (0% vs. 0%, P = 1.0), or mediastinal re-exploration (0% vs. 5%, P = 0.37).
DISCUSSION
The results of this study showed that PBUF is a simple and effective method for providing a balanced prime for the pediatric patient. PBUF allows for a physiologic prime that can help to attenuate the hemodynamic impairment often seen with the transfusion of stored blood products. Table 2 illustrates the CPB prime data, showing that PBUF is effective in reducing the overall metabolic load seen when either FWBs or PRBCs are used. Specifically, PBUF is shown to be particularly effective in reducing the glucose concentration. Although Table 2 emphasizes that stored red cells are the most non-physiologic, it must be recognized that FWB is not without derangements in pH, base excess (BE), glucose, and lactate levels. Therefore, PBUF can be an effective tool in reducing electrolyte imbalances; helping to provide a prime that avoids gross osmolal fluxes; and controling serum glucose and lactate concentrations during CPB (8).
The importance of presenting a balanced, physiologic CPB prime to the patient cannot be understated and is tremendously important for several reasons. First, the CPB prime is often the largest volume of fluid given to the patient during surgery, and it is given almost instantaneously, unlike other perioperative fluids. Additionally, the CPB prime can be a significant determinant in the patient’s metabolic response to the cardiac operation because of the great disparity between their size and the CPB circuit but also because of the deleterious effects of CPB being more pronounced due to their immature tissue and organ function (6,8,9). Furthermore, neonatal patients may not be able to handle the substrate load of blood transfusions during the perioperative period because of the massive metabolic stress response seen with surgery and CPB (2,10). Finally, a balanced, ultrafiltrated prime may be especially advantageous in single ventricle patients who have higher markers of inflammation and increased postoperative morbidity (11). Recognition of these high-risk patients may help reduce CPB-related morbidities by identifying strategies and interventions that help optimize the response of the patient to CPB (9).
The results of our study showed that FWB is beneficial in reducing overall blood product exposures. The data suggest that, when an FWB prime and PRBC prime are compared, the majority of the FWB patients (62%) receive only one cumulative blood product exposure compared with 18% of the PRBC patients (p < .0001). The reduction in donor exposures was also found in the <5 kg WB group, finding decreased exposures in the perioperative period and for the culmination of the perioperative and postoperative time periods. The importance of patient size in relation to incidence of transfusions has been recognized by previous studies, showing that smaller infants are at a greater risk for bleeding and increased transfusion rates (12–14). However, it must be acknowledged that the <5 kg PRBC group had a slightly smaller weight (p < .047) and lower preoperative platelet count (p < .0094), potentially influencing transfusion requirements. Additional blood exposure data showed no significant differences in perioperative or postoperative transfusion rates for the 5+ kg WB and 5+ kg PRBC groups, suggesting that transfusion requirements are influenced more in the smaller patient population (<5 kg). Although the makeup of FWB with plasma, platelets, and red cells has shown it to beneficial in reducing donor exposures, some authors have asserted no other beneficial advantages for the use FWB (14). Although the blood supply today is recognized to be relatively safe, we must appreciate that there are still multiple risks and morbidities associated with transfusions and that these risks should not be discounted. Specific transfusion-associated risks include immunosuppression and related adverse effects, graft-vs.-host disease, transfusion-related lung injury, allergic reaction, ABO-Rh incompatibility, and emerging viral risks (15).
Research focusing on stored blood products have noted the lack of normality in stored red blood cells, suggesting that they have an extremely high level of activated inflammatory cells and mediators, posing a risk for noscomial blood stream infections postoperatively (16,17). Additional research has documented that platelets, in particular, have high storage lesions and inflammatory mediators, with several studies suggesting an association with increased infection rates and respiratory complications (15).
Although this study did not analyze infection rates, it should be recognized that blood transfusions could be a significant risk factor for sternal wound infection (18). Confounding these problems are the shortages of safe blood and the accelerated rise in the cost because of increased testing (19). Currently, at our institution, a single unit of FWB averages approximately $500, whereas multiple exposures of PRBCs and a single FFP and platelet transfusion can average $800 to $1000+. It is also important to recognize that this only reflects the cost of the actual units of blood; it does not consider the effect of complications and transfusion-related morbidities into the overall cost. In view of the growing practice of using evidence-based medicine, it would be prudent to reduce the number of exposures and promote blood conservation, especially considering the potential risks and adverse effects from homologous blood transfusions (15,20,21).
The results of the postoperative blood loss data did not correlate with previous studies that have documented the positive effects of FWB on postoperative blood loss and coagulation (2,4,22). Although our study did support an overall decrease in blood product exposures, it did not find any differences in postoperative blood loss when either a FWB prime or PRBC prime was used. There was a slight trend, however, for the <5 kg patient population to bleed more, emphasizing previous research that smaller patients may be at a greater risk for blood loss and transfusions (12).
Although the results of our study did not show any discernible differences in postoperative blood loss among any groups, it must be recognized that this was purely a retrospective study, and although postoperative transfusion guidelines were established, actual decisions regarding transfusions may have been somewhat subjective.
Additional results for this study did not reveal any evidence in supporting a FWB or PRBC prime in reducing perioperative inotropic support. Previous work has documented that the use of FWB and PBUF is effective in reducing the overall inflammatory response to CPB by decreasing the circulating levels of bradykinin, high molecular weight kininogen, NH3, C3a, and thromboxane B2, concluding that cardiac function could be improved and inotrope use decreased (6,7). However, there are several differences in our study. Although markers of systemic inflammation were not measured in this study, it must be recognized that the use of PBUF for the blood-primed CPB circuit may have influenced the amount of inflammatory mediators that patients were exposed to and their response. Second, this was strictly a retrospective study, and therefore, no specific guidelines were established for deciding inotropic support. Rather, this decision was left up to the primary cardiac surgeon and anesthesiologist.
Additional outcomes analyzed for this study including OR extubation, intraoperative death, mediastinal reexploration, and delayed sternal closure found no differences between the FWB and PRBC group. One study suggested that FWB does not offer any benefit for improvement in postoperative outcomes (3). However, there has also been research supporting that the transfusion of older blood can be an independent predictor of multi-organ failure and in-hospital mortality, prompting many to promote the use of FWB in cardiac surgery (2,12,23–25).
It is recognized that there are several limitations to this study. First, this was not a prospective, randomized study, and perioperative decisions on inotropic support and transfusions were not unbiased. Second, the study may have been influenced by the availability of blood products. Although FWB is our preferred blood product for cardiac surgery requiring CPB, conflicts can arise and limit its use. Also, the small number of patients (n = 100) may somewhat diminish validity, specifically when the patients were broken into subgroups to be statistically compared on postoperative outcomes. Finally, although postoperative transfusion guidelines were established, decisions may have been subjective and thus influenced by the pediatric intensivist and cardiac surgeon involved.
CONCLUSIONS
The use of FWB in conjunction with PBUF allows for significantly reduced donor exposures and is particularly emphasized in the <5-kg population. The results of this study help to promote the reduction of blood product exposures and the subsequent complications that are often seen with multiple transfusions. Furthermore, this study may help aid clinicians in promoting the use of FWB for cardiac surgery and allow for further analysis into the benefits for specific patient populations, such as single ventricle patients. These high-risk patient populations may be of prime benefit in providing the most physiologic, non-detrimental CPB prime and that the reduction of donor exposures in this group may be instrumental in decreasing perioperative and postoperative complications. Finally, this study highlights that PBUF is an easy and effective method for providing a balanced, physiologic CPB prime for pediatric cardiac patients, whether FWBs or PRBCs are used.
ACKNOWLEDGMENT
The authors thank Lynette Smith at the University of Nebraska Medical Center for providing excellent statistical analysis and support on this manuscript.
REFERENCES
- 1.Keidan I, Amir G, Mandel M, Mishali D.. The metabolic effects of fresh versus old stored blood in the priming of cardiopulmonary bypass solution for pediatric patients. J Thorac Cardiovasc Surg. 2004;127:949–52. [DOI] [PubMed] [Google Scholar]
- 2.Manno CS, Hedberg KW, Kim HC, Bunin GR, Nicolson S, Jobes D.. Comparison of the hemostatic effects of fresh whole blood, stored whole blood, and components after open heart surgery in children. Blood. 1991;77:930–6. [PubMed] [Google Scholar]
- 3.Mou SS, Giroir BP, Molitor-Kirsch EA, et al. Fresh whole blood versus reconstituted blood for pump priming in heart surgery in infants. N Engl J Med. 2004;351:1635–44. [DOI] [PubMed] [Google Scholar]
- 4.Friesen RH, Perryman KM, Weigers KR, Mitchell MB, Friesen RM.. A trial of fresh autologous whole blood to treat dilutional coagulopathy following cardiopulmonary bypass in infants. Pediatr Anesth. 2006;16:429–35. [DOI] [PubMed] [Google Scholar]
- 5.Shimpo H, Shimamoto A, Miyake Y, et al. Effect of ultrafiltration on priming solution with preserved blood for extracorporeal circulation in infants. ASAIO J. 1996;42:M792–4. [DOI] [PubMed] [Google Scholar]
- 6.Nagashima M, Imai Y, Seo K, et al. Effect of hemofiltered whole blood pump priming on hemodynamics and respiratory function after the arterial switch operation in neonates. Ann Thorac Surg. 2000;70:1901–6. [DOI] [PubMed] [Google Scholar]
- 7.Shimpo H, Shimamoto A, Sawamura Y, et al. Ultrafiltration of the priming blood before cardiopulmonary bypass attenuates inflammatory response and improves postoperative clinical course in pediatric patients. Shock. 2001;16(Supp1):51–4. [DOI] [PubMed] [Google Scholar]
- 8.Ridley PD, Ratcliffe JM, Alberti KGMM, Elliot MJ.. The metabolic consequence of a “washed” cardiopulmonary bypass pump-priming fluid in children undergoing cardiac operations. J Thorac Cardiovasc Surg. 1990;100:528–37. [PubMed] [Google Scholar]
- 9.Shen I, Giacomuzzi C, Ungerleider RM.. Current strategies for optimizing the use of cardiopulmonary bypass in neonates and infants. Ann Thorac Surg. 2003;75:S729–34. [DOI] [PubMed] [Google Scholar]
- 10.Ratcliffe JM, Elliot MJ, Wyse RK, Alberti KG.. The metabolic load of stored blood. Implications for major transfusions in infants. Arch Dis Child. 1986;61:1208–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Madhok AB, Ojamaa K, Haridas V, Parnell VA, Pahwa S, Chowdhury D.. Cytokine response in children undergoing surgery for congenital heart disease. Pediatr Cardiol. 2006;27:408–13. [DOI] [PubMed] [Google Scholar]
- 12.Guay J, Moerloose P, Lasne D.. Minimizing perioperative blood loss and transfusions in children. Can J Anesth. 2006;53:S59–67. [DOI] [PubMed] [Google Scholar]
- 13.Williams GD, Bratton SL, Ramamoorthy C.. Factors associated with blood loss and blood product transfusions: a multivariate analysis in children after open-heart surgery. Anesth Analg. 1999;89:57–64. [DOI] [PubMed] [Google Scholar]
- 14.Lithmathe J, Boeken U, Feindt P, Gams E.. Predictors of homologous blood transfusions for patients undergoing open heart surgery. Thorac Cardiovasc Surg. 2003;51:17–21. [DOI] [PubMed] [Google Scholar]
- 15.Spiess BD.. Blood transfusion: the silent epidemic. Ann Thorac Surg. 2001;72:S1832–7. [DOI] [PubMed] [Google Scholar]
- 16.Spiess BD.. Transfusion of blood products affects outcome in cardiac surgery. Semin Cardiothrorac Vasc Anesth. 2004;8:267–81. [DOI] [PubMed] [Google Scholar]
- 17.Elward AM, Fraser VJ.. Risk factors for noscomial primary bloodstream infection in pediatric intensive care unit patients: a 2-year prospective cohort study. Infect Control Hosp Epidemiol. 2006;27:553–60. [DOI] [PubMed] [Google Scholar]
- 18.Ottino G, De Paulis R, Pansini S, et al. Major sternal wound infection after open-heart surgery: a multivariate analysis of risk factors in 2,579 consecutive operative procedures. Ann Thorac Surg. 1987;44:173–9. [DOI] [PubMed] [Google Scholar]
- 19.Shander A.. Emerging risks and outcomes of blood transfusion in surgery. Semin Hematol. 2004;41:117–24. [DOI] [PubMed] [Google Scholar]
- 20.Kwiatkowski JL, Manno CS.. Blood transfusion support in pediatric cardiovascular surgery. Transfus Sci. 1999;21:63–72. [DOI] [PubMed] [Google Scholar]
- 21.Mohr R, Martinowitz U, Lavee J, Amroch D, Ramot B, Goor DA.. The hemostatic effect of transfusing fresh whole blood versus platelet concentrates after cardiac operations. J Thorac Cardiovasc Surg. 1988;96:530–4. [PubMed] [Google Scholar]
- 22.Mohr R, Goor DA, Yellin A, Moshkovitz Y, Shinfeld A, Martinowitz U.. Fresh blood units contain large potent platelets that improve hemostasis after open heart operations. Ann Thorac Surg. 1992;53: 650–4. [DOI] [PubMed] [Google Scholar]
- 23.Zallen G, Offner PJ, Moore EE, et al. Age of transfused blood is an independent risk factor for postinjury multiple organ failure. Am J Surg. 1999;178:570–2. [DOI] [PubMed] [Google Scholar]
- 24.Basran S, Frumento RJ, Cohen A, et al. The association between duration of storage of transfused red blood cells and morbidity and mortality after reoperative cardiac surgery. Anesth Analg. 2006;103:15–20. [DOI] [PubMed] [Google Scholar]
- 25.Guay J, Rivard GE.. Mediastinal bleeding after cardiopulmonary bypass in pediatric patients. Ann Thorac Surg. 1996;62:1955–60. [DOI] [PubMed] [Google Scholar]