Abstract:
The recovery of patients undergoing total shoulder arthroplasty (TSA) can be adversely affected by a number of complications. Autologous platelet gel (APG), produced by activating platelet-rich plasma (PRP), has been shown to improve hemostasis and wound healing and reduce infections in some surgical procedures. Activated platelet-poor plasma (PPP) has also been used as a hemostatic agent. This study examines the effects of APG and PPP treatment on TSA patients postoperatively. After Institutional Review Board (IRB) approval, 40 patients undergoing TSA at our institution were prospectively enrolled in our study. They were randomized into either a control (n = 20) or study (n = 20) group, with the study group receiving APG and PPP treatment. Preoperative demographic data, pre- and postoperative laboratory data, pain scores, pain medication, complications, pre- and postoperative range of motion measurements, and postoperative lengths of stay were recorded for each group. The preoperative internal rotation index was significantly higher in the control group compared with treatment patients (4.64 ± 4.46 vs. 1.88 ± 2.44, p < .05). The percent hemoglobin retained postoperatively was higher in the treatment group at 24 (84.54 ± 5.32 vs. 79.87 ± 8.73) and 72 hours (87.46 ± 16.03 vs. 76.70 vs. 5.96), but neither difference reached statistical significance. The treatment group had significantly lower pain scores (p = .007) and total fentanyl requirements (p < .05) compared with control patients. The internal rotation index improvement factor (postoperative internal rotation index/preoperative internal rotation index) was significantly higher in the treatment group vs. the control group (p < .05). Although it did not reach statistical significance, the treatment group was discharged almost 9 hours earlier than the control group (64.44 ± 15.23 vs. 73.39 ± 15.37). APG and PPP treatment decreased pain and provided a greater increase in internal rotation measurements postoperatively.
Keywords: osteoarthritis, total shoulder arthroplasty, platelet-rich plasma, platelet-poor plasma, autologous platelet gel, growth factors
The shoulder offers the greatest range of motion of any joint in the human body. However, with osteoarthritis, the cartilage lining between the bones of the joint, the humerus, and the glenoid fossa of the scapula degenerates. Reactive new bone, called osteophytes, form at the margins and subchondral areas of the joint. Small fragments of bone or cartilage may float freely in the synovium, the glenohumeral joint space narrows, and ligaments contract. Movement becomes painful, and motion and strength in the joint may be lost. Shoulder arthroplasty surgery can be used to alleviate these symptoms when other non-surgical options have failed.
In total shoulder arthroplasty (TSA) surgery, a portion of the humeral head is osteotomized and removed. A metal stem is placed down the intermedullary canal of the humerus, and a second metal implant the shape and size of a normal humeral head is attached to the stem. In addition, the glenoid fossa is reamed down to a clean bone surface, and a plastic implant with high molecular weight polyethylene cement is secured into place to form a new functional socket. The effect is a relatively large ball articulating with a shallow socket (1,2).
In most cases, patients are discharged home 2–3 days postoperatively. However, their recovery can be hampered by a number of complications, including loosening of the glenoid or humeral implants, dislocation or instability of the joint, rotator cuff tears, periprosthetic fractures, infections, or damage to the blood vessels or nerves surrounding the shoulder. In addition, greater than expected pain, less range of motion, and a decrease in strength in the joint postoperatively can slow recovery (1,3,4).
Autologous platelet gel (APG) is produced intraoperatively by activating platelet-rich plasma (PRP) with CaCl2 and thrombin. PRP has been shown to have three to eight times the patient’s baseline platelet count and baseline or slightly above baseline levels of leukocytes (5–9). When activated, platelets also release a number of substances, such as platelet-derived growth factors and clotting factors. Those substances contribute to hemostasis and the healing process and would be readily available in APG (5,8–11).
APG has been reported to decrease edema and blood loss in cosmetic surgery and to decrease blood loss, improve pain control, and recovery of range of motion, shorten hospital length of stay, and decrease the need for allogeneic blood products in TKA surgery (6,12–14). APG has increased the density of bone grafts postoperatively and increased bone and tissue in-growth into bone and other graft materials (15–17). It has decreased the incidence of sternal infections in cardiovascular surgery and improved the healing rate of chronic wounds (7,18,19). In addition, the PPP produced in the process can also be activated and used as a hemostatic agent (11,12).
The purpose for our study was to examine the effect of APG and PPP treatment on TSA patients postoperatively.
MATERIALS AND METHODS
Study Outline
After Institutional Review Board (IRB) approval and informed consent, 40 eligible patients undergoing TSA were prospectively enrolled into the study between May 2005 and June 2006. The patients were randomly assigned to either a control group or a treatment group; and their designation was blinded to the patient and all parties involved in postoperative care, with the exception of the surgeon.
Patient Eligibility Requirements
Patients were eligible for the study if they were scheduled to have TSA by the study surgeon at our institution, had a primary preoperative diagnosis of osteoarthritis, no previous surgeries on their shoulder with the exception of arthroscopy, and had a preoperative platelet count ≥100,000/μL.
Blood Draw
After the induction of anesthesia, the phlebotomy site was prepared. Using a 17-gauge hemodialysis fistula needle, 52 mL venous blood was drawn off by a staff anesthesiologist or a certified nurse anesthetist into a 60-mL syringe containing 8 mL anticoagulant (Medtronic Anticoagulant Citrate Dextrose Solution A, Medtronic Perfusion Systems, Minneapolis, MN).
PRP Preparation
The disposable kit for the Magellan Autologous Platelet Separator System (Medtronic Perfusion Systems, Minneapolis, MN) was inspected for damage and installed in a sterile fashion, and the device was programmed according to the manufacturer’s standard operating instructions to produce 9 mL PRP and ∼20 mL platelet-poor plasma (PPP). One mL of the PRP and PPP was aseptically removed for laboratory analysis, and using sterile technique, the remaining PRP and PPP and a 1:1 mixture of 5 mL 10% CaCl2/5000 units thrombin were transferred to the operative field into separate specimen cups. When the surgeon requested the APG, 8 mL PRP and 1 mL of the CaCl2/thrombin mixture were transferred to a dual syringe platelet gel applicator kit (Micromedics, Minneapolis, MN) and applied. Likewise, when the surgeon requested the activated PPP, 8 mL of it and 1 mL CaCl2/thrombin were prepared and applied in the same fashion.
Surgical Technique and PRP Application
All shoulders were implanted with Byliani-Flatau (Zimmer, Warsaw, IN) total shoulder implants. The shoulder was approached anteriorly with an incision over the clavicle running lateral to the coracoid toward the deltoid insertion. After carefully dissecting down to the joint capsule and exposing the joint, the humeral head was dislocated and osteotomized, the canal was reamed, and any circumferential osteocytes were removed. A trial prosthesis was placed down in the humerus and left in place. The glenoid was exposed, reamed, and prepared for a prosthesis. After satisfactorily seating trial components, a glenoid implant was cemented in place. The humerus was reapproached, and a trial head was placed on the trial humeral stem. After achieving a satisfactory fit, the trials were removed. Approximately 5–6 mL APG was sprayed down the intermedullary canal of the humerus, a metal humeral stem implant was placed down for a tight fit, and a humeral head implant was attached. The humeral head was reduced, and the movement and stability of the new joint was checked. Once the surgeon was satisfied, the remaining 2–3 mL APG was added extra-articularly, the joint capsule was sutured back together, and the shoulder was closed. Approximately 8 mL activated PPP was sprayed in the subcutaneous tissue while closing.
Pain Management
Patients received a preoperative interscalene nerve block, and pain management was maintained postoperatively with Fentanyl (15 μg/mL) through a patient-controlled analgesia (PCA) pump with standardized settings. Any breakthrough pain was treated with oral oxycodone (5 mg/dose). Patients were asked by a registered nurse once every 4 hours to rate their pain from 0 to 10, with 0 being “no pain” and 10 being “the worst pain possible.”
Data Collection
Preoperative diagnosis, age, sex, date of surgery, date of discharge, preoperative platelet count, platelet count of PRP and PPP fractions, pre- and postoperative hemoglobin (at 24 and 72 hours postoperatively), preoperative leukocyte count, leukocyte count of PRP and PPP fractions, postoperative pain scores, and postoperative narcotic (Fentanyl) or other pain medication requirements were recorded. In addition, pre- and 6-week postoperative range of motion (ROM) measurements (active elevation, external rotation, and internal rotation), and any postoperative complications were recorded.
Although active elevation and external rotation are measured in degrees, internal rotation is measured by the highest vertebral anatomy that a patient can reach up their back with their thumb, starting with the iliac crest of the hip and proceeding up the spine to the fifth thoracic (T5) vertebra. Because the measurement is a landmark instead of a number, the measurements were assigned a number from 1 to 15, with 1 being the iliac and 15 being T5. That number made up the internal rotation index.
Statistical Analysis
Descriptive statistics and analyses were done with PC SAS version 9.0. Data that were normally distributed are reported as means ± SD, whereas those variables that were not normally distributed are also presented with their medians and minimum and maximum values.
Four types of statistical analyses were used. The first was the standard t test, which we applied to data that were approximately normally distributed. The second was the nonparametric Wilcoxon test, which we applied to variables that were not normally distributed. The Fisher exact test was used for data that was categorized as a “yes” or “no.” The final test used was a repeated-measures mixed model. We applied it to repeatedly measured data, such as pain medication over timed intervals. p < 0.05 was considered significant.
RESULTS
Forty patients (20 control and 20 treatment) were enrolled into the study. One control patient and one treatment patient suffered complications unrelated to APG and PPP therapy, extending their lengths of stay. They were considered outliers, and their data were not analyzed. That brought the effective study population to 19 for each group.
There was no significant difference between the control or treatment groups with respect to their age, sex, preoperative platelet count, hemoglobin, leukocyte count, active elevation, and external rotation. There was a significant difference in the preoperative internal rotation index.
Table 1 lists the demographic and preoperative data for the two groups. Table 2 characterizes the PRP and PPP fractions.
Table 1.
Demographic and preoperative data.
| Variable | Control Group | Treatment Group |
|---|---|---|
| Age (years) | 66.42 ± 8.93 | 66.42 ± 8.47 |
| Sex M/F | 9/10 | 10/9 |
| Preoperative platelet (×103/μL) | 238.28 ± 56.10 | 256.17 ± 53.84 |
| Preoperative hemoglobin (g/dL) | 14.24 ± 0.96 | 14.18 ± 1.75 |
| Preoperative leukocyte count (×103/μL) | 7.18 ± 1.76 | 6.98 ± 1.18 |
| Preoperative active elevation (deg) | 87.06 ± 35.33 | 92.37 ± 20.16 |
| Preoperative external rotation (deg) | 32.67 ± 12.23 | 27.06 ± 11.60 |
| Preoperative internal rotation index* | 4.64 ± 4.64† | 1.88 ± 2.44 |
| Median | 2.25 | 1.00 |
| Minimum | 2.0 | 1.0 |
| Maximum | 11.0 | 11.0 |
M, male; F, female.
Internal rotation measurements are the highest vertebral anatomy that can be reached with the patient’s thumb, starting with the iliac crest of the hip, the sacral vertebra, vertebra L5-L1, and T12-T5. The internal rotation index was determined by assigning the numbers 1 to 15 to the measurements, with 1 being the iliac and 15 being T5 vertebra. **p < .05.
Table 2.
Characterization of PRP and PPP fractions.
| Variable | PRP | PPP |
|---|---|---|
| Volume (mL) | 9.00 | 20.00 |
| Platelet count (×103/μL) | ||
| Mean ± SD | 1090.94 ± 388.19 | 54.97 ± 28.11 |
| Median | 1072.00 | 45.60 |
| Minimum | 526.00 | 24.70 |
| Maximum | 2100.00 | 117.00 |
| Increase over baseline* | 4.26 | 0.21 |
| Leukocyte count (×103/μL) | ||
| Mean ± SD | 16.80 ± 11.75 | 0.32 ± 0.37 |
| Median | 14.10 | 0.17 |
| Minimum | 1.96 | 0.03 |
| Maximum | 38.20 | 1.47 |
| Increase over baseline* | 2.41 | 0.05 |
PRP, platelet-rich; PPP, platelet-poor plasma.
Increase over baseline calculated as mean/baseline mean.
Although the 24- and 72-hour postoperative hemoglobins and the percentage of their preoperative hemoglobin retained were higher in the treatment group, neither was statistically significant compared with the control group. The hemoglobin values and their percent of preoperative values are shown in Figures 1 and 2. Eight of the control patients received autotransfused red blood cells intraoperatively vs. five treatment patients. There was no significant difference in the volume of autotransfused blood given back to patients between the groups (control group, 61.80 ± 95.51 mL; treatment group, 35.50 ± 64.18 mL).
Figure 1.
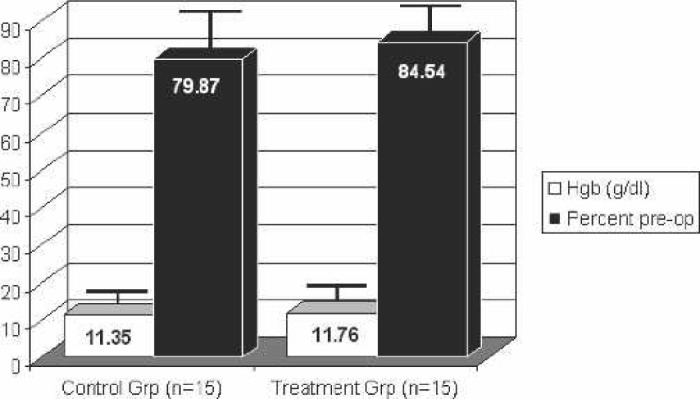
Hemoglobin 24 hours postoperatively. Hgb: hemoglobin.
Figure 2.
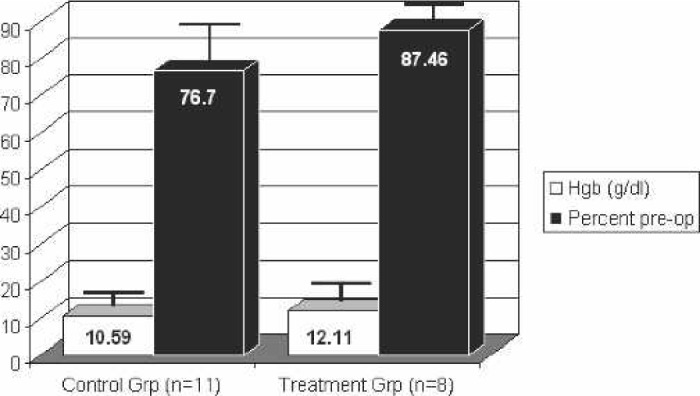
Hemoglobin 72 hours postoperatively. Hgb: hemoglobin.
The pain scores were recorded in 4-hour intervals and are graphed as such. There was a significant difference between the two groups after calculation of the area under the graphs. The graphs, which represent the degree and duration of each group’s pain, are shown in Figure 3.
Figure 3.
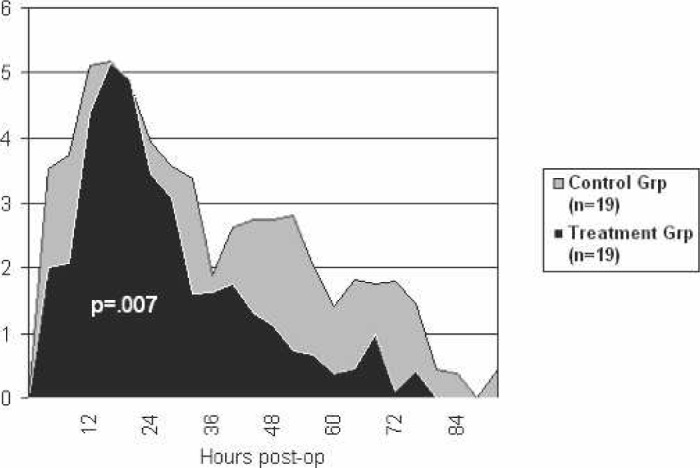
Pain scores postoperatively.
Fentanyl requirements postoperatively were broken down into 12-hour intervals and are shown in Figure 4. Although there was not a significant difference between the two groups for any particular time interval, the control group did receive a significantly higher total Fentanyl dose compared with the treatment group. Table 3 shows the total Fentanyl dose, oxycodone dose, the number of patients receiving other pain medication, the length of time until the PCA pump could be discontinued as a result of decreased pain, and the postoperative length of stay. One treatment patient received six Darvocet N-100 doses for breakthrough pain. Two control patients also received other oral analgesics. One was given nine Darvocet N-100 (650 mg acetaminophen/100 mg propoxyphene napsylate) doses, and one received six Vicodin (500 mg acetaminophen/5mg hydrocodone bitartrate).
Figure 4.
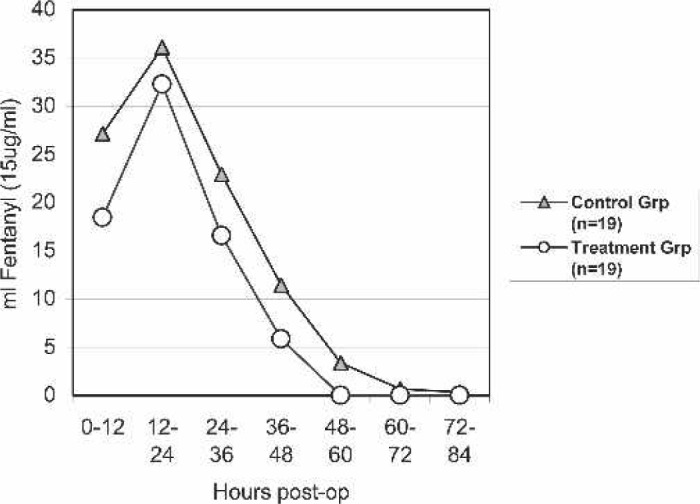
Fentanyl requirements postoperatively.
Table 3.
Postoperative data.
| Variable | Control Group | Treatment Group |
|---|---|---|
| Fentanyl dose in mL (15 μg/mL) | 101.86 ± 47.95 | 73.17 ± 39.33* |
| Oxycodone dose (5 mg/dose) | 11.84 ± 8.21 | 11.05 ± 5.76 |
| Patients receiving any other oral pain medication | 2 | 1 |
| PCA discontinued postoperatively (hours) | 46.70 ± 11.81 | 42.05 ± 9.16 |
| Length of stay postoperatively (hours) | 73.39 ± 15.37 | 64.55 ± 15.23 |
PCA, patient-controlled analgesia pump.
P < .05.
Postoperative ROM measurements for each group were also recorded at their 6-week follow-up appointment. There was no significant difference in the active elevation measurement, external rotation measurement, and internal rotation index postoperatively. There was also no significant difference with respect to the percentage that the preoperative values were improved postoperatively for active elevation and external rotation. However, there was a statistically significant difference in the internal rotation index’s improvement factor, calculated as the postoperative mean/preoperative mean. The values and percent improvement or improvement factor are shown in Figures 5–7.
Figure 5.
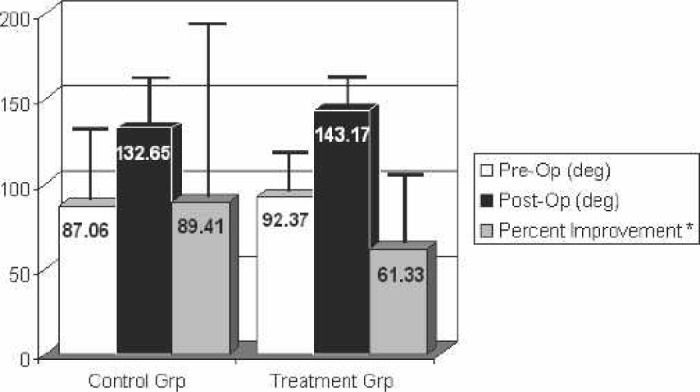
Range of motion: active elevation. Postoperative measurements taken at 6 weeks. *Percent improvement was only calculated on those patients with both pre- and postoperative measurements.
Figure 6.
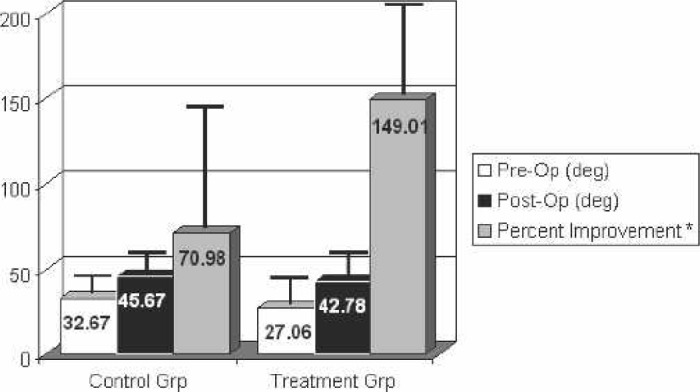
Range of motion: external rotation. Postoperative measurements taken at 6 weeks. *Percent improvement was only calculated on those patients with both pre- and postoperative measurements.
Figure 7.
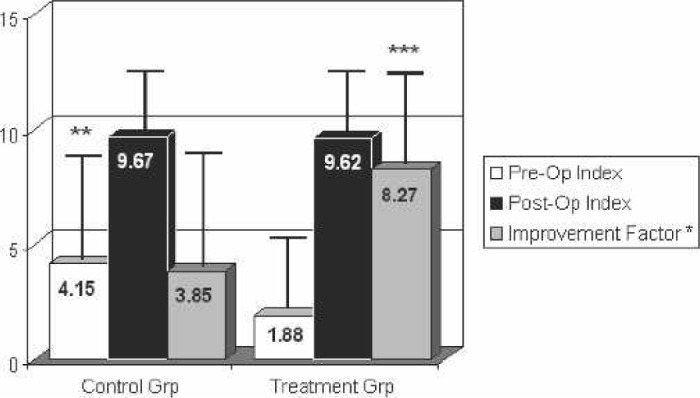
Range of motion: internal rotation index. Postoperative measurements taken at 6 weeks. Improvement factor calculated as postoperative internal rotation index/preoperative internal rotation index. *Improvement factor was only calculated on those patients with both pre- and postoperative measurements. **p < .05. ***p < .05.
No direct complications were observed from the use of APG therapy.
DISCUSSION
APG has been shown to have a positive effect on pain control, hemostasis, density, and ingrowth of bone into graft materials, wound healing, the rate of infection, and hospital length of stay with various surgical procedures (6,13–19). In our study on TSA patients, APG and PPP significantly decreased their pain (p = .007) and their pain medication requirement (p < .05) and provided a greater increase in their internal rotation measurements (p < 0.05) postoperatively.
Postoperative pain control is critical to a speedy recovery. Patients with less pain are more active and may recovery their ROM quicker. They also need fewer narcotics, reducing their chances of suffering adverse reactions associated with the drugs, such as sedation, seizures, respiratory depression, hypotension, arrhythmias, nausea, or constipation (20).
The increased internal rotation improvement factor seen in treatment patients postoperatively (Figure 7) would be consistent with their decreased pain. However, the difference in internal rotation improvement factor values may be exaggerated by the fact that control patients started out with better internal rotation measurements preoperatively. Because patient group designations were random, we are not sure why we saw a difference in one of their preoperative ROM measurements.
Although APG and PPP treatment seemed to increase the percent of the patient’s preoperative hemoglobin seen at 24 and 72 hours postoperatively (p = .09), shorten the time that they required a PCA pump (p = .13), and shorten their postoperative length of stay (p = .12), none of these differences reached statistical significance.
Treatment patients in our study were discharged almost 9 hours earlier postoperatively than control patients (64.44 ± 15.23 vs. 73.39 ± 15.37 hours, respectively). A shorter length of stay could decrease the patient’s chance of infection and the cost of their hospitalization.
It is impossible to separate hemostasis, wound healing, and infection, and APG has the potential to affect each process in various ways. Platelets help achieve primary hemostasis, whereas leukocytes help jump start inflammation and the healing process and fight infection at the wound site (21). Both platelets and leukocytes also contribute various growth factors to the wound that can mediate a number of processes including hemostasis, inflammation, the formation of granulation tissue, epithelialization, tissue remodeling, angiogenesis, and in some cases, osteogenesis (22,23). There is a dose-response relationship between platelet concentrations and levels of growth factors. By delivering a substance with a high concentration of activated platelets and a baseline or slightly above baseline level of leukocytes to a wound, such as APG, you may be able to further enhance the healing process (24).
Because of their autologous nature, PG and PPP offer very little risk to the patient. However, the majority of clinicians today use commercially prepared thrombin as a platelet-activating agent. Although thrombin is a very effective activator, commercially available preparations are derived from bovine plasma, which has been linked to coagulopathies. Recently, some groups have described procedures to produce autologous thrombin from whole blood or PPP. The activity of the autologous thrombin is dependent on the individual patient and the disposables used to procure it, and it is consistently lower than that seen with bovine thrombin. However, because of the decreased risk that would be seen with autologous thrombin, future studies should include looking at its efficacy with APG treatment (10,18,25).
ACKNOWLEDGMENTS
The authors thank Medtronic Perfusion Systems, Minneapolis, MN, who donated disposables for the study, and the Clinical Laboratory at North Kansas City Hospital for donating tests on PRP and PPP samples.
REFERENCES
- 1.Craig EV.. Master Techniques in Orthopaedic Surgery: The Shoulder. 2nd ed. Philadelphia: Lippincott, Williams & Wilkins; 2004. [Google Scholar]
- 2.Schilling McCann JA.. Professional Guide to Pathophysiology. Philadelphia: Lippincott, Williams & Wilkins; 2003;409–12. [Google Scholar]
- 3.Matsen FA III, Rockwood CA Jr, Wirth MA, et al. Glenohumeral arthritis and its management. In: Rockwood CA Jr, Matsen FA III, Wirth MA, et al. eds. The Shoulder. Philadelphia: Saunders; 2004; 879–1008. [Google Scholar]
- 4.Cofield RH, Chang W, Sperling JW.. Complications of shoulder arthroplasty. In: Iannotti JR, Williams GR, eds. Disorders of the Shoulder: Diagnosis and Management. Philadelphia: Lippincott, Williams & Wilkins; 1999; 571–93. [Google Scholar]
- 5.Brady C, Vang S, Christensen K, et al. Use of autologous platelet gel in bariatric surgery. J Extra Corpor Technol. 2006;38:161–4. [PMC free article] [PubMed] [Google Scholar]
- 6.Berghoff WJ, Pietrzak WS, Rhodes RD.. Platelet-rich plasma application during closure following total knee arthroplasty. Orthopedics. 2006;29:590–8. [DOI] [PubMed] [Google Scholar]
- 7.Trowbridge CC, Stammers AH, Woods E, et al. Use of platelet gel and its effects on infection in cardiac surgery. J Extra Corpor Technol. 2005;37:381–6. [PMC free article] [PubMed] [Google Scholar]
- 8.Marx RE, Carlson ER, Eichstaedt RM, et al. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–46. [DOI] [PubMed] [Google Scholar]
- 9.Altmeppen J, Hansen E, Bonnlander GL, et al. Composition and characteristics of an autologous thrombocyte gel. J Surg Res. 2004; 117:202–7. [DOI] [PubMed] [Google Scholar]
- 10.Everts PAM, Knape JTA, Weibrich G, et al. Platelet-rich plasma and platelet gel: A review. J Extra Corpor Technol. 2006;38:174–87. [PMC free article] [PubMed] [Google Scholar]
- 11.Englert SJ, Estep TH, Ellis-Stoll CC.. Autologous platelet gel applications during cardiovascular surgery: Effect on wound healing. J Extra Corpor Technol. 2005;37:148–52. [PMC free article] [PubMed] [Google Scholar]
- 12.Man D, Plosker H, Winland-Brown JE.. The use of autologous platelet-rich plasma (platelet gel) and autologous platelet-poor plasma (fibrin glue) in cosmetic surgery. Plast Reconstr Surg. 2001;107:229–37. [DOI] [PubMed] [Google Scholar]
- 13.Mooar PA, Gardner MJ, Klepchick PR, Sherk HH.. The efficacy of autologous platelet gel in total knee arthroplasty: An analysis of hemoglobin, narcotic requirements and range of motion. American Academy of Orthopedic Surgeons, 67th Annual Meeting. Annual Meeting, Orlando, FL, March 2000 American Academy of Orthopedic Surgeons; 2000. [Google Scholar]
- 14.Everts PAM, Devilee RJJ, Brown Mahoney C, et al. Platelet gel and fibrin sealant reduce allogeneic blood transfusions in total knee arthroplasty. Acta Anaesthesiol Scand. 2006;50:593–9. [DOI] [PubMed] [Google Scholar]
- 15.Zhang GC, Yuan T, Zen GB.. Experimental study of the effect of platelet rich plasma on osteogenesis in rabbit. Chin Med J (Engl). 2004;117:1853–5. [PubMed] [Google Scholar]
- 16.Lowery GL, Kulkarni S, Pennisi AE.. Use of autologous growth factors in lumbar spinal fusion. Bone. 1999;25(2 Suppl):47S–50S. [DOI] [PubMed] [Google Scholar]
- 17.Siebrecht MA, De Rooij PP, Arm DM, et al. Platelet concentrate increases bone ingrowth into porous hydroxyapatite. Orthopedics. 2002;25:169–72. [DOI] [PubMed] [Google Scholar]
- 18.Mazzucco L, Medici D, Serra M, et al. The use of autologous platelet gel to treat difficult-to-heal wounds: A pilot study. Transfusion. 2004; 44:1013–8. [DOI] [PubMed] [Google Scholar]
- 19.Driver VR, Hanft J, Fylling P, Beriou J.. A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manage. 2006;52:68–87. [PubMed] [Google Scholar]
- 20.Way WL, Way EL, Fields HL.. Opioid analgesics and antagonists. In: Katzung BG, ed. Basic and Clinical Pharmacology. 6th ed. Norwalk, CT: Appleton & Lange; 1995; 460–76. [Google Scholar]
- 21.Hunt TK, Williams Hopf H.. Wound healing and wound infection: What surgeons and anesthesiologists can do. Surg Clin North Am. 1997;77:587–606. [DOI] [PubMed] [Google Scholar]
- 22.Slater M, Patava J, Kingham K, Mason RS.. Involvement of platelets in stimulating osteogenic activity. J Orthop Res. 1995;13:655–63. [DOI] [PubMed] [Google Scholar]
- 23.Eppley BL, Pietrzak WS, Blanton M.. Platelet-rich plasma: A review of biology and applications in plastic surgery. Plast Reconstr Surg. 2006;118:147–59. [DOI] [PubMed] [Google Scholar]
- 24.Pietrzak WS, Eppley BL.. Platelet-rich plasma: Biology and new technology. J Craniofac Surg. 2005;16:1043–54. [DOI] [PubMed] [Google Scholar]
- 25.Kumar V, Chapman JR.. Whole blood thrombin: Development of a process for intra-operative production of human thrombin. J Extra Corpor Technol. 2007;39:18–23. [PMC free article] [PubMed] [Google Scholar]


