Abstract:
The detection and prevention of gaseous microemboli (GMEs) during cardiopulmonary bypass has generated considerable interest within the cardiac surgical community. There have been several landmark papers that have used transcranial Doppler devices during cardiopulmonary bypass to detect gaseous microemboli activity in the patients’ middle cerebral artery during perfusionist interventions. To determine if this source of emboli could be prevented, a shunt was developed between the oxygenator’s sampling manifold and the oxygenator’s venous line. This shunt bypassed the venous line and emptied into the oxygenator’s integral cardiotomy. An in vitro experiment was performed using three open system oxygenators (Sorin Synthesis, Sorin PrimeOx2, and Terumo Capiox SX25) to compare post-arterial filter emboli detection using the Hatteland CMD20 Microbubble Detector under tightly controlled conditions. After injection of air through the sampling manifold, the PrimeOx2 and the Synthesis oxygenators had statistically significant fewer GMEs with the shunt used than when the shunt was not used. Using a shunt in the sampling manifold during perfusionist interventions will dramatically reduce or eliminate gaseous microemboli transmission to the patient during bypass with both the PrimeOx2 and Synthesis oxygenators. However, results indicate that further study of GME handling with all oxygenator’s integral cardiotomies is warranted.
Keywords: gaseous microemboli, oxygenator, perfusionist, cardiopulmonary bypass
An intervention by perfusionists has been defined as those periods during cardiopulmonary bypass (CPB) when the perfusionist withdraws blood samples for testing from the oxygenator’s manifold sampling system or injects medication into the same manifold for therapeutic results (1).
There are many procedures associated with the release of gaseous microemboli (GMEs) during the course of CPB, such as aortic cannulation, removal of aortic cross-clamp, initiation of bypass, and valvular surgery (2–4). One of the more common sources of GMEs entering the arterial circulation during CPB is when large amounts of gaseous emboli are introduced into the venous line (5,6). Attempts to avoid the latter are done through meticulous air removal from the venous line before going on bypass and attempting to avoid entraining air into the venous line during bypass. The use of arterial filters has shown an ability to reduce microemboli going to the patient; however, these devices are not capable of removing all GMEs (7,8).
Unfortunately, up to 50% of detected cerebral emboli may not be directly associated with surgical manipulation, and their actual sources are still uncertain (9). This makes it imperative that clinicians study all of the potential sources of these emboli during the management of extra-corporeal circulation.
In 1999, Taylor et al. (1) discovered a connection between perfusionists conduct during CPB and the detection of GMEs in their patient’s middle cerebral artery. In this prospective clinical study using membrane oxygenators and soft shell venous reservoirs during coronary artery bypass grafts (CABG), the authors used transcranial Doppler (TCD) to continuously monitor perfusionist interventions during CPB. During these interventions through the sampling manifold, the authors found a sevenfold increase in the cerebral embolic rate compared with any other time periods during the study.
They concluded that perfusionist interventions could account for a large proportion of the previously unexplained cerebral microemboli during CPB, which was consistent with all perfusionists who participated in the study. They also concluded that the emboli detected likely represented GMEs that were not eliminated by the membrane oxygenator or the arterial filter.
In 2001, Borger et al. (10) attempted to establish a link between GMEs created during increased perfusionist interventions and an increase in postoperative cognitive impairment. Their prospective study group involved 83 CABG patients who were divided into two groups. One group (n = 42) had <10 perfusionist interventions, whereas the other group (n = 41) had >10 perfusionist interventions. Once again they used soft shell venous reservoirs with membrane oxygenators. Patients had undergone numerous pre-bypass neuropsychological tests, which were repeated after surgery, and they were followed for 3 months after surgery. The authors found that those patients who were subjected to increased perfusionist interventions had significantly worse results in neuropsychological testing after bypass, which tended to persist three months postoperatively.
Rodriguez et al. (11) used TCD to conduct a prospective randomized study to determine if purging the manifold, drug injections through the manifold, and blood level influenced the amount of GMEs detected in the middle cerebral artery when using open systems during bypass. They concluded that 38% of all GMEs detected in 90 CABG patients, with eight different perfusionists, were the result of perfusionist interventions. However, this was minimized when the perfusionist maintained a blood level >800 mL in the reservoir, did not purge the manifold to clear syringes, and used continuous infusions of medications rather than bolus injections.
An in vitro study conducted by Rudolph et al. (12) examined high-intensity transient signals (HITS) generated in the arterial line, distal to the arterial filter. After injection of a 10-mL bolus of normal saline, using TCD, they found increased HITS activity that lasted up to 2 minutes after the injections were completed. This was a clear indication that microemboli were even being created by bolus injections of fluid, as evidenced by the HITS postarterial filter.
The hypothesis was simple. If we could create a shunt that would bypass the venous inlet during CPB and divert that blood to the oxygenators integral cardiotomy filter, we could eliminate most or all of the all GMEs being generated by perfusionist interventions.
MATERIALS AND METHODS
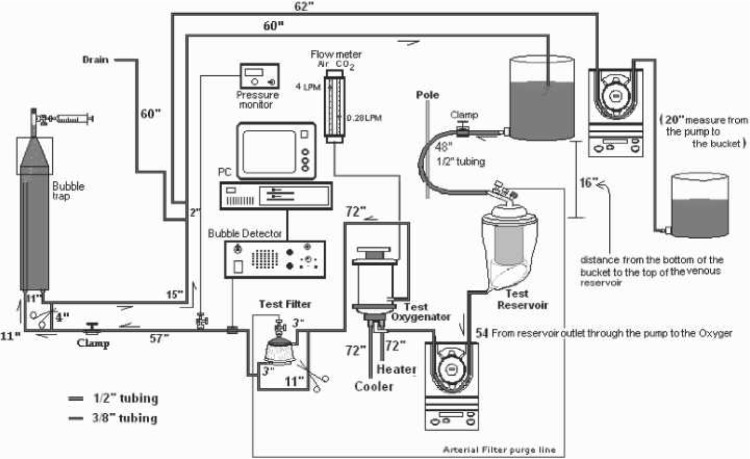
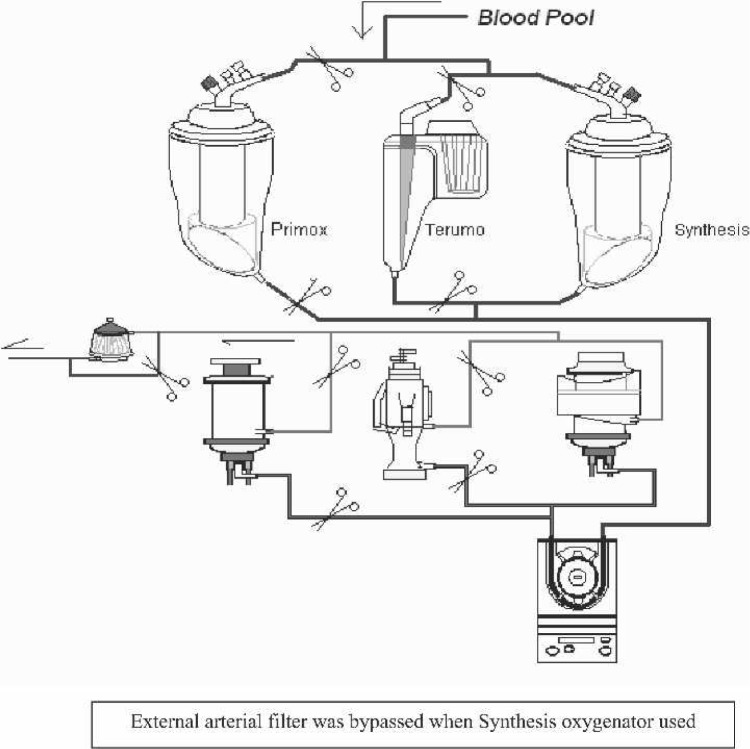
Using a standardized method to establish an in vitro model of an adult cardiopulmonary bypass circuit (Figure 1), three routinely used oxygenators were set up in series (Figure 2) and tested for the presence of GMEs after perfusionist interventions through the sampling manifold. Results obtained were repeated with the shunt in place. The perfusionist was blinded to ongoing GME detection results when this shunt was in use or not in use.
Figure 1.
Air challenge (10 mL) from the sample manifold entering the venous inlet of the oxygenator.
Figure 2.
Air challenge (10 mL) from the sample manifold entering the integral cardiotomy of the oxygenator.
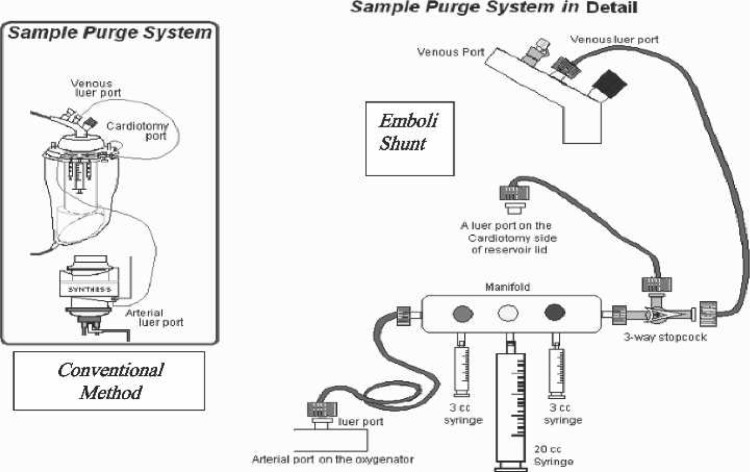
The shunt (called an emboli shunt) was a simple arrangement of a stopcock and a pressure line put in place at the venous end of the sampling manifold. The opposite end of the pressure line was connected to the oxygenator’s integral cardiotomy filter. To engage the shunt, the stopcock was turned off in the direction of the venous line and subsequently opened to the oxygenator’s integral cardiotomy (Figure 3). The oxygenator’s integral cardiotomy was chosen as the site to divert sample blood and drug injections for three reasons. One is the fact that, during CPB, the perfusionist routinely allows fluid additions through the quick prime line in this filter area with little regard for air. The second is that this is the site most perfusionists place the shunt from their arterial line filters during CPB. However, the third and most important reason is that the integral cardiotomy is where the perfusionist allows vent return and/or suction blood to enter the oxygenator during all CPB cases.
Figure 3.
Emboli shunt stopcock and line on sampling manifold.
The three open system coated oxygenators used for this in vitro experiment were the Sorin Synthesis (Mirandola, Italy), Sorin PrimeOX2 (Arvada, CO), and the Terumo Capiox SX25 (Terumo Medical Corporation, Somerset, NJ). The circuit consisted of a 3-ft bubble trap, phosphorylcholine (PC)-coated tubing and a Dideco 40 micron arterial filter used for both the PrimeOX2 and Capiox oxygenators (Synthesis had its own integrated 40 μm filter built in). All membrane oxygenators were new and had the open hard shell venous reservoir.
Microbubble detection was recorded with the Hatteland CMD20 Microbubble Detector (Royken, Norway) using the Excel compatible BUBMON software and the Gateway 2000 P5–66 computer with Excel Data Pack Software.
The total prime was 24 L, which consisted of 16 L normal saline with 35 g sodium bicarbonate to prime and de-air the entire test, after which 8 L fresh bovine blood (anticoagulated with 120,000 units porcine heparin) was added and normalized for temperature, hematocrit, and blood gases. The final hematocrit was maintained at 27% and was measured with the IEC Microhematocrit Centrifuge (International Equipment Co., Needham Heights, MA). The activated coagulation time (ACT) was maintained >600 seconds, using the Hemochron ACT machine (International Technidyne, Edison, NJ).
The Cobe Century (Cobe, Arvada, CO) positive displacement roller pump was used for delivering blood flow. The rollers were underoccluded at a drop of 1 in every 15–20 seconds to reduce GMEs generated by the rollers themselves. Actual blood flow was maintained at 4.0 LPM and measured after the arterial filter using the HT110 Transonic Bypass Flowmeter (Transonic Systems, Ithaca, NY). Gas flow was maintained at a constant 4.0 LPM with 3.8 LPM of air (93%) and 0.2 LPM of carbon dioxide (7.0%). Using the Radiometer ABL505 blood gas analyzer (Radiometer Medical, Bronshoj, Denmark), blood gases were maintained normoxic (pH 7.42; pCO2,35 mmHg; pO2, 115 mmHg; HCO3, 24.7 mmol/L) to reduce the possibility of GMEs generated through hyperoxic conditions.
The manufacturer’s recommended minimum blood level for all three oxygenators was exceeded by maintaining this level at 500 mL throughout the experiments. Blood temperatures were maintained at a constant 30°C using the Yellow Springs Temperature Module (YSI Incorporated, Yellow Springs, OH) and the Hemotherm 400 cooler/heater (Cincinnati Sub Zero, Cincinnati, OH). Arterial pressures were maintained at 178 mmHg using the CDX3 Dual Pressure Monitor (Mesa Labs, Lakewood, CO) and measured after the arterial filter.
Data Collection
The first question we attempted to address was whether medications would pass through the emboli shunt and get to the patient during bypass. Because this in vitro study did not have the ability to measure physiologic responses to vasoactive agents, we elected to make a visual measurement after the arterial filter, using 10-mL bolus injections of blood and air into a crystalloid perfusate (to simulate drug passage). To test the response time between the venous inlet and the arterial line, we placed the Hatteland bubble sensor 24 in after the arterial filter and injected 10 mL air (×3 tests) through the sampling manifold using the conventional method. To test the response time between the integral cardiotomy and the arterial line, we timed the appearance of blood 24 in after the arterial filter after we injected 10 mL bovine blood (×3 tests) through the sampling manifold using the emboli shunt.
Before the start of the air bolus challenges, the blood perfusate was circulated for a period >30 minutes to allow for test preparation while microbubble activity (recorded by the Hatteland CMD20) remained at baseline for that entire period. The first oxygenator was challenged with a bolus injection of 10 mL of air through the sampling manifold in the conventional way (Figure 4A). Data were continuously collected by the Hatteland CMD20 data collection system for a period of 3 minutes after the challenge, after which a 10- to 15-minute period was maintained with no challenges to allow the emboli activity to once again reach baseline values; the test was then repeated with another 10-mL air challenge. This sequence of challenges using the conventional method was repeated five times for each oxygenator in the study.
Figure 4.
Preparing for 10 mL injection of air through conventional and shunt lines.
Another 10- to 15-minute “settle down time” was used to allow any bubble activity to return to baseline before the second part of the study (shunt) began. At that time, the stopcock was turned, and the emboli shunt was activated (Figure 4B). The same sequence of air bolus challenges was repeated through the manifold, another five times for each oxygenator, using the same protocol that was used with the conventional method.
Statistics
All statistical analysis was performed using the Microsoft Excel 2000 Data Analysis tool pack. Values are expressed as mean ± SD. Data for conventional techniques were compared with the shunt technique using a two-tailed Student t test, assuming equal and unequal variances. For all tests, p ≤ 0.05 was considered statistically significant.
RESULTS
The results of the simulated drug passage tests (n = 3) indicated that, if a substance enters the venous line from the sampling manifold, it will transit the oxygenator and filter and may be detected 24 inches after the arterial filter ∼20 ± 4 seconds later. If a substance enters the oxygenator’s cardiotomy filter, it may be detected 24 inches after the arterial filter ∼22.7 ± 5 seconds later.
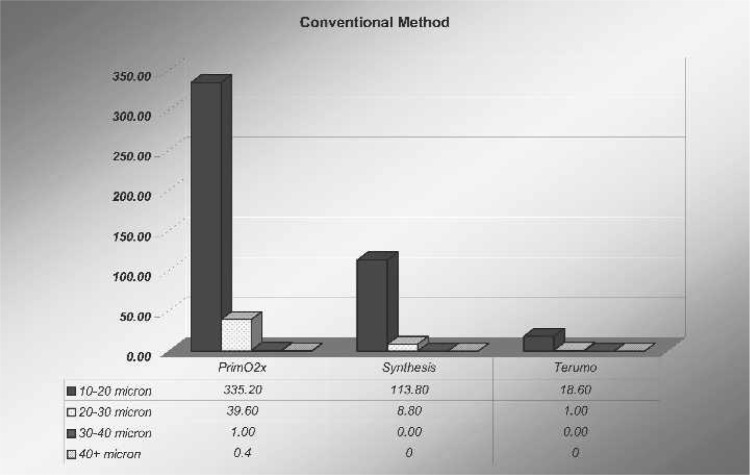
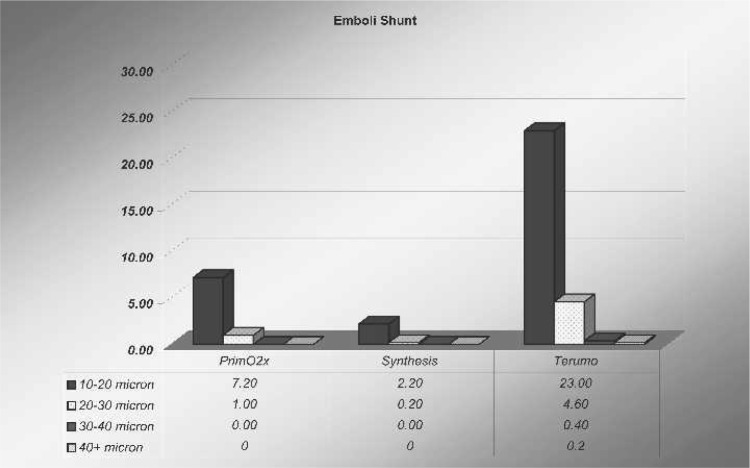
In all oxygenators studied, there were varied amounts of microbubbles and sizes counted after the arterial filter after all 10-mL bolus air injections through the oxygenator’s manifold. After the arterial filter, as shown in Figure 5, the amount of microbubbles in the 10- to 20-μm range using the conventional method was 335.2 ± 200 microbubbles for the PrimeO2x. After the shunt was turned on, microbubble counts of the same size decreased to 7.2 ± 5.5 microbubbles (p = .001). The Synthesis had fewer microbubbles in the 10- to 20-μm range after the arterial filter using the conventional method, with a count of 113.8 ± 55 microbubbles, but when the shunt was used, these bubble counts decreased to 2.2 ± 2.0 microbubbles (p = .001). The Capiox had the least amount of 10- to 20-μm bubbles counted with the conventional method (18.6 ± 7.0 microbubbles). However, when the 10-mL bolus challenge was run with the shunt turned on, the actual microbubble count in this oxygenator was 23.0 ± 10 microbubbles (p = not significant).
Figure 5.
Air challenge (10 mL) from the sample manifold entering the venous inlet of the oxygenator.
Looking at the larger bubbles (Figure 6), the amount of microbubbles in the 20- to 30-μm range using the conventional method was 39.6 ± 25 microbubbles for the PrimeO2x. Microbubbles of the same size after the shunt was used decreased to 1.0 ± 1.0 microbubbles (p = .002). The Synthesis again had fewer bubbles in the larger 20- to 30-μm range using the conventional method, with a count of 8.8 ± 8.0 microbubbles, and when the shunt was used, these numbers decreased to 0.2 ± 0.8 (p = .004). The Capiox had the least amount of 20- to 30-μm bubbles detected with the conventional method (1.0 ± 2.0 microbubbles). However, when the challenge was run with the shunt turned on, the actual microbubble count went up to 4.6 ± 3.0 microbubbles (p = .01).
Figure 6.
Air challenge (10 mL) from the sample manifold entering the integral cardiotomy of the oxygenator.
DISCUSSION
Arterioles can range in size between 5 and 100 μm, which eventually branch into capillaries that are in the range of 5 to 10 μm in diameter (13). Microbubbles are generally <300 μm and tend to gather a protein coating while circulating in blood. The latter results in a larger wall thickness, which makes the bubbles harder to break or adsorb onto surfaces. By their very nature, they are much less buoyant than larger bubbles. The significance of microbubbles is the fact that they have the ability to create morbidity in the same manner as solid microemboli (14–16).
The literature unquestionably indicates the potential harm that can originate from microemboli that are allowed to enter the microcirculation (14–17).
On a biological level, bubbles do not travel alone. There is a constant process of smaller bubbles fusing with other smaller bubbles to create larger ones, and even larger bubbles breaking up into many smaller ones (16). Even the shape of a microbubble changes in the biological model. As the bubble traverses the circulation, it becomes more elongated as it reaches capillary vessels, therefore making the length greater than the width. The consequences of this shape change increase the bubble dissolution time by 50% compared with the calculated dissolution time of a round bubble of the same volume (14,17).
Reducing emboli and neurologic injury during CPB requires a multidisciplinary approach that includes several simple diagnostic and therapeutic strategies, such as the method presented in this paper. These are presently the objectives for many centers research in the fields of cardiac anesthesia, surgery, and perfusion. Clinical experience has shown that agents do traverse the membrane in a timely manner when using the shunt properly. However, one of the limiting factors of this study was the method used to detect simulated pharmacologic intervention.
CONCLUSION
The results found in this study indicate that shunting the sample manifold to the integral cardiotomy of the Sorin Synthesis and Sorin PrimeOX2 will dramatically reduce or eliminate GMEs that are being inadvertently produced by perfusionists withdrawing blood samples for analysis and administering bolus injections of drugs during CPB. This was dramatically shown in this study by a 98% decrease (p = .001) in GMEs (10–20 μm) when using the shunt with the PrimeOX2 and a 98% decrease (p = .001) when using the Synthesis (Table 1). Similar statistically significant results were shown with GME reduction in the 20- to 30-μm range when using these two oxygenators (Table 2).
Table 1.
Amount of bubbles (10- to 20-μm range).
| Oxygenator | Current | Shunt | p |
|---|---|---|---|
| PrimeO2X | 335.2 ± 200 | 7.2 ± 5.5 | .001 |
| Synthesis | 113.8 ± 55 | 2.2 ± 2.0 | .001 |
| Capiox | 18.6 ± 7.0 | 23.0 ± 10 | NS |
Table 2.
Amount of bubbles (20- to 30-μm range).
| Oxygenator | Current | Shunt | p |
|---|---|---|---|
| PrimeO2X | 39.6 ± 25 | 1.0 ± 1.0 | .002 |
| Synthesis | 8.8 ± 8.0 | 0.2 ± 0.8 | .004 |
| Capiox | 1.0 ± 2.0 | 4.6 ± 3.0 | .01 |
Results found with the Capiox indicated a nonsignificant increase of GMEs in the 10- to 20-μm range when using the shunt (Table 1), followed by a significant increase (p = .01) of GMEs in the 20- to 30-μm range when the shunt was again used (Table 2). Unfortunately, no reason was found for the latter increase in bubble activity.
Although many papers have examined the handling of air entering an oxygenator’s venous line, results found in this study clearly indicate that shunting the sampling manifold fluids away from the venous line and into the oxygenator’s integral filter will reduce or eliminate GME transmission into the arterial line. Further study is warranted for the handling of any fluid (vent, suction, fluid additives, purge lines) that enters an oxygenator’s integral cardiotomy reservoir and has the potential to generate GMEs in the extracorporeal circuit.
ACKNOWLEDGMENTS
The author thanks the Sorin Group for the use of research facilities and the time their research staff provided to allow this project to become possible. A special thanks goes to Cheri Voorhees, BAH, (ASCP) SH, CPT (Senior Research Scientist), Rob Haynes (Senior Engineer) and research assistants Bob Eke and Barry Kaup for experience and assistance in this project. The author also thanks RenRen Johnson for excellent work on the figures included with this paper.
REFERENCES
- 1.Taylor RL, Borger MA, Weisel RD, et al. . Cerebral microemboli during cardiopulmonary bypass: Increased emboli during perfusionist intervention. Ann Thorac Surg. 1999;68:89–93. [DOI] [PubMed] [Google Scholar]
- 2.Nijhoff M.. Microembolization: Etiology and prevention. In: Hilberman M, ed. Brain Injury and Protection During Heart Surgery. Martinus-Nijhoff Publishing, Boston, MA; 1998; 67–83. [Google Scholar]
- 3.Pugsley W.. The use of Doppler ultrasound in the assessment of microemboli during cardiac surgery. Perfusion. 1989;4:115–22. [Google Scholar]
- 4.Clark RE, Brillman J, Davis DA, et al. . Microemboli during coronary artery bypass grafting: Genesis and effect on outcome. J Thorac Cardiovasc Surg. 1995;109:249–58. [DOI] [PubMed] [Google Scholar]
- 5.Stock UA, Miller T, Bienek R, et al. . Deairing of the venous drainage in standard extracorporeal circulation results in a profound reduction of arterial micro bubbles. Thorac Cardiovasc Surg. 2006;54:39–41. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell SJ, Willcox T, Gorman DF.. Bubble generation and venous air filtration by hard-shell reservoirs: A comparative study. Perfusion. 1997;12:325–33. [DOI] [PubMed] [Google Scholar]
- 7.Padayachee TS, Parsons S, Theoboldt R, et al. . The effect of arterial filtration on reduction of gaseous microemboli in the middle cerebral artery during cardiopulmonary bypass. Ann Thorac Surg. 1988;45: 647–9. [DOI] [PubMed] [Google Scholar]
- 8.Schoenburg M, Kraus B, Muehling A, et al. . The dynamic air bubble trap reduces cerebral microembolism during cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2004;128:154–62. [DOI] [PubMed] [Google Scholar]
- 9.Stump DA, Rogers AT, Hammon JW, et al. . Cerebral emboli and cognitive outcome after cardiac surgery. J Cardiothorac Vasc Anesth. 1996;10:113–19. [DOI] [PubMed] [Google Scholar]
- 10.Borger MA, Peniston CM, Weisel RD, et al. . Neuropsychologic impairment after coronary bypass surgery: Effect of gaseous microemboli during perfusionist interventions. J Thorac Cardiovasc Surg. 2001;121:743–9. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez RA, Williams KA, Babaev A, et al. . Effect of perfusionist technique on cerebral embolization during cardiopulmonary bypass. Perfusion. 2005;20:3–10. [DOI] [PubMed] [Google Scholar]
- 12.Rudolph JL, Tilahun D, Treanor PR, et al. . Use of a large bore syringe creates significantly fewer high intensity transient signals (HITS) into a cardiopulmonary bypass system than a small bore syringe. Perfusion. 2006;21:67–71. [DOI] [PubMed] [Google Scholar]
- 13.Berne RM, Levy MN.. The microcirculation and lymphatics. In: Cardiovascular Physiology. 4th ed. CV Mosby, St. Louis, MO; 1981;109–22. [Google Scholar]
- 14.Muth CM, Shank ES.. Gas embolism. N Engl J Med. 2000;342:476–82. [DOI] [PubMed] [Google Scholar]
- 15.Barak M, Katz Y.. Microbubbles: Pathophysiology and clinical implications. Chest. 2005;128:2918–32. [DOI] [PubMed] [Google Scholar]
- 16.Lindner JR, Song J, Jayaweera AR, et al. . Microvascular rheology of definity microbubbles after intra-arterial and intravenous administration. J Am Soc Echocardiogr. 2002;15:396–403. [DOI] [PubMed] [Google Scholar]
- 17.Branger AB, Eckmann DM.. Theoretical and experimental intravascular gas embolism absorption dynamics. J Appl Physiol. 1999;87:1287–95. [DOI] [PubMed] [Google Scholar]