Abstract:
Electronic data collection during cardiac surgery creates an enormous data source that has many potential applications. After the introduction of the Stockert Data Management System (DMS; Munich, Germany) to our perfusion practice, we recognized that the data could be used for the purpose of quality control (QC). Our aim was to create an automated technique of data analysis and feedback for cardiopulmonary bypass (CPB) procedures. Using visual basic programming, we created a process by which data from the DMS is analyzed and processed in a Microsoft Access database after a CPB procedure. The processing is designed to transfer the collected data to a research database and create a number of CPB quality indicator (QI) parameters, such as mean arterial pressure being less than 40 mmHg for more than 5 minutes or a venous saturation of less than 60% for more than 5 minutes. In the event of QI parameter detection, a QC report is generated and e-mailed to the senior perfusionist and the perfusionist performing the procedure. The introduction of electronic data collection and subsequent development of electronic data processing techniques has enabled us to transfer the data into a readily accessible database and create a data set of perfusion variables and quality indicators for CPB procedures. This data set may be used for immediate automated QC feedback after CPB procedures and direction of performance improvement initiatives through retrospective or prospective data analysis as part of a continuous quality improvement process.
Keywords: electronic data, quality control, cardiopulmonary bypass
Cardiac surgery has advanced at enormous rates over the last 50 years, and most recently the outcomes have been examined in greater detail with the advancement of alternative surgical and nonsurgical intervention strategies. Outcomes are usually multifactorial in origin; optimal management systems intraoperatively contribute significantly to improving patient outcomes. The perception of data management as an integral component of modern clinical practice has in some areas been slow to evolve, and the potential value of automated data management systems has certainly been underrecognized. Historically, early perfusion electronic data collection systems have offered limited data integration or management (e.g., Cardio Link; Cobe Cardiovascular Inc., Arvada, CO). More recently, however, a number of data management systems have been developed that have facilitated integration of data not only from the heart lung machine (HLM), but also from anesthetic machines and other patient monitors, laboratory data, on-line blood gas measurement devices, and other point of care devices, in addition to all of the mandatory information directly relating to the conduct of bypass (e.g., blood and gas flow rates, line pressures, temperatures).
The collection of data in itself is only one component of the value that can be gained from these integrated systems. The generation of the perfusion record is an obvious example of what we are able to do with this type of system; however, it is important to recognize that this is just the tip of the iceberg of the true power associated with data collection. The data provide an enormous resource for ongoing research activities, and we have previously showed that the use of automated data collection systems provides the opportunity to minimize transcription error and bias and provide a more detailed information base for the clinical procedure (1). Potentially, the most important influence that data management may have on clinical practice is with respect to quality management. The importance and benefits of quality assurance and quality control (QC) in health care delivery are well recognized. In our institution, although quality assurance processes were implemented, our QC processes were not as fully developed. Riley (2), in a recent editorial in the Journal of Extracorporeal Technology, promoted the role of the perfusion profession in recognizing the importance of quality improvement and reporting in perfusion. In a similar editorial, Groom et al. (3) highlighted the value of data collection and analysis in the improvement and management of cardiopulmonary bypass (CPB). We recognized that in addition to the data being a valuable research tool, additional benefits would be gained if we were able to use the information we collected to improve our day to day per-fusion practices via electronic data processing—to develop an automated QC process.
Quality control is defined as a set of activities or techniques whose purpose is to ensure that all quality requirements are being met. To achieve this purpose, processes are monitored and performance problems are solved (4). To implement the QC process requires the identification of quality indicator (QI) variables that can be used to define specified quality outcomes. The routine analysis of the quality indicators is essential in achieving a continuous quality improvement (CQI) process. Rath and Strong (5) have described a five-step structured process (DMAIC) as an approach to standardizing the CQI process. The steps they identified were as follows: define the problem; gather information on the problem; identify root causes of the problem and confirm with the data; try out and implement solutions that address root cause(s) of the problem; and evaluate the solutions and outline and maintain the gains by standardizing the process.
This can be coupled with six sigma reporting to also allow standardization of measurement. Outcome, which in the perfusion setting may be defined as the occurrence of an event (e.g., an accident, an observation, a protocol deviation), is able to be defined as number of events per million opportunities (DPMO). Riley (2) has proposed that six sigma DMAIC methodology be adopted by perfusionists, to allow them to study and meaningfully report and compare improvements in their clinical processes.
Dickinson et al. (6) examined the use of perfusion data for QC purposes. They were able to show that compliance with the majority of perfusion quality indicators correlates with external validation of superior unit performance. One limitation of this study was that the original perfusion data was manually collected; more recently electronic data collection has been shown to be more accurate than manual data collection during surgery (1,7). Our unit has embraced a strong ethos toward quality of care; however, we recognized that we needed to develop a CQI process, and we identified the need to use a DMAIC process, and eventually six sigma reporting, to achieve our goals. To facilitate the process, we recognized the need to overcome one of the clear limitations of many of the commercial data management systems, that is, the lack of easy access to the raw data that was being collected, for the purpose of data processing. Therefore, the aim of this work has been to develop a method of easy access to the data collected by the data management system and to develop an automated technique of data analysis and feedback for CPB procedures, which may be used as part of a CQI process for our perfusion practice.
MATERIALS AND METHODS
Data Collection Technique
Since the inception of the Flinders Cardiothoracic Surgical Unit in 1992, we used Microsoft Access (Microsoft Corporation, Redmond, WA) to create a cardiac surgery database (CSD) for storing perfusion data, in addition to a comprehensive data set for each cardiothoracic patients clinical course. The perfusion record was created manually on a paper chart and data were manually entered into the CSD. We therefore needed to introduce a medium for the capture of perfusion data electronically and automated transfer to the CSD. In 2000, we implemented the Data Management System (DMS; Stockert, Munich, Germany) to routinely record and generate our CPB records. The data integration hub of the S3 heart-lung machine (Stockert) is a serial interface that provides the facility to communicate data with peripheral devices through an RS-232 connection. Data from these devices are transmitted from the serial interface in one signal to the laptop computer or touchscreen monitor on the HLM (Figure 1). This is a significant advantage because the data from these devices are able to be integrated into the perfusion record. However, this may also be a limitation because it requires multiple communication ports on the HLM and the ability of the devices to communicate with the HLM. It is important for manufacturers to recognize the new generation of interconnectivity required in the market place and to recognize the need to be able to establish communications between the different devices in the operating room.
Figure 1.
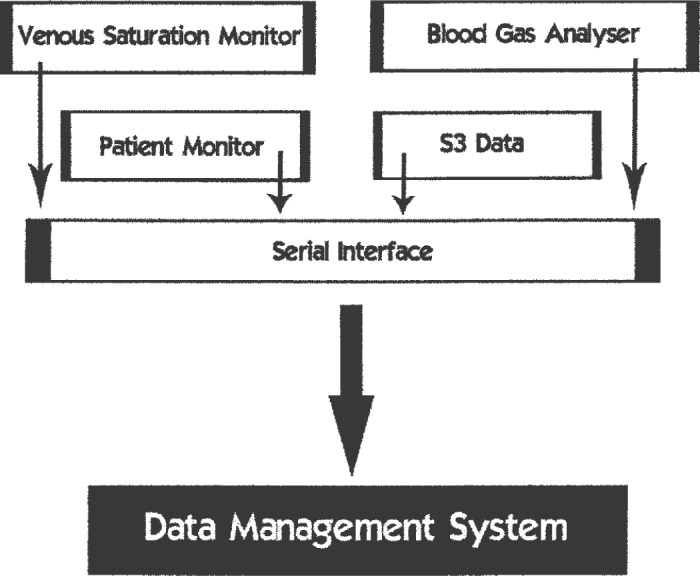
Integration of data communication—peripheral monitoring devices are connected to the serial interface of the S3 heart-lung machine, and the integrated data are collected by the DMS software on the laptop computer.
Data are currently collected from the following peripheral devices: AS3 (Datex-Ohmeda, Helsinki, Finland) and Solar 8000 (GE Healthcare, Waukesha, WI) anesthetic machines, SAT/HCT monitor (Cobe Cardiovascular, Arvada), and the ABL700 blood gas analyser (Radiometer, Bronshoj, Copenhagen, Denmark). Data are collected and stored at 20-second intervals.
Data Integration
The initial step in allowing full data integration was to move the existing CSD from an Access platform to a SQL server platform, requiring conversion of existing database tables (Access) to SQL tables, while retaining the Access user interface. This provided a stable multiuser environment, allowing procedural data to be entered onto the CSD in real time by the perfusionist in the theater on the HLM laptop/touchscreen computers, which are connected through wireless local area network access. After a perfusion procedure, the DMS data are exported from the HLM computer to the perfusion server computer for report storage and printing. During this process, the data are stored as text files on the hospital network. Located on the hospital network is a processing database (transfer database) that was created to process and transfer data from the DMS text files into the CSD. The transfer database is linked to the CSD through open database connection (ODBC). The importing process relies on import specifications that are set using the Access importing wizard. To initiate the automated data transfer process, a button is clicked on the CSD that calls a visual basic programming module in the transfer database to perform the following functions. 1) import DMS text files into the transfer database, according to the import specifications. At the start of the import process, the import tables are empty so that patient records are processed individually. 2) Append the imported data in the transfer database to the CSD and run queries to process and update specific data variables to the CSD. 3) Delete the data in the transfer database tables, ready for the next import process.
Creation of Custom Perfusion Quality Indicator Variables
The data collection and data integration processes allowed us to achieve our goals of collecting and processing the data. To progress to data analysis and feedback, we recognized the need to define what we wished to report from the CSD. To achieve this, we created a customized set of perfusion data variables such as the number of transfusions, minimum and maximum values, fluid balance, and pressure and temperature cumulative time values. We defined a number of QI parameters, based on the unit per-fusion protocols. Our QI parameters were activated clotting time (ACT) less than 400 seconds, cardiac index less than 1.6 L/min/m2 for more than 5 minutes, mean arterial pressure less than 40 mmHg for more than 5 minutes, venous saturation less than 60% for more than 5 minutes, hemoglobin less than 7 g/dL, pCO2 less than 35 or more than 45 mmHg, pO2 less than 100 mmHg, and arterial blood temperature of less than 37.5°C for more than 2 minutes. Time for all QI parameters is defined as cumulative time. The QI parameters have also been used in conjunction with data from procedural events to identify inappropriate responses from the perfusionist according to our unit protocols, such as when a blood transfusion is given when the patient hemoglobin is more than 7 g/dL or if no blood transfusion occurred when the hemoglobin was less than 7 g/dL. These parameters allow us to monitor a broad range of clinical responsibilities, including anticoagulation, blood gas management, hemodilution, rewarming, and perfusion adequacy.
Automated Quality Control Feedback
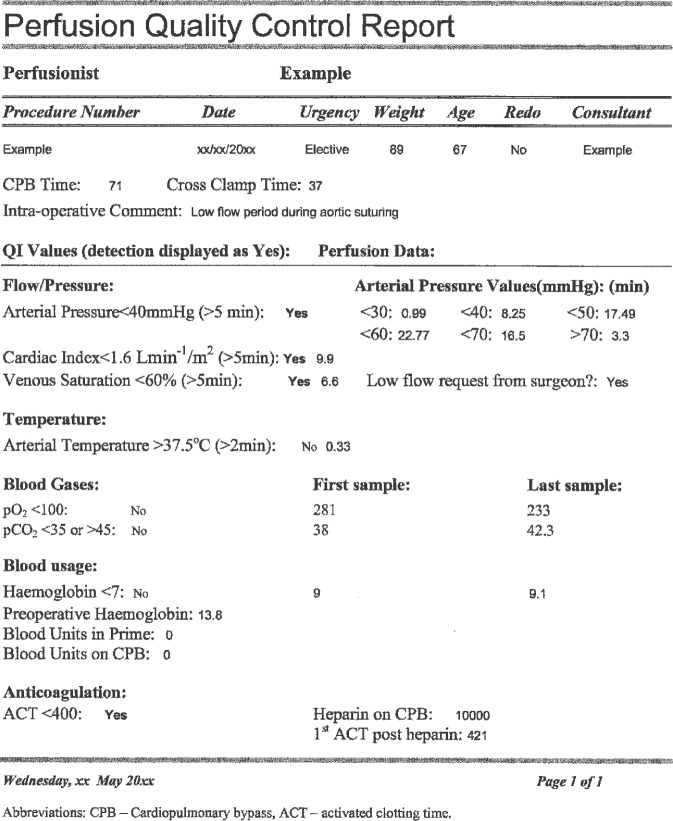
The automated feedback process was created by generating a report template in the transfer database and modification of the visual basic programming module so that in the event that any of the QI parameters are detected, a QC report was generated. The report was automatically e-mailed to both the senior perfusionist and the perfusionist performing the CPB procedure. Figure 2 is an example of a perfusion quality control report and shows the data variables currently included.
Figure 2.
Perfusion quality control report.
Report Interpretation
The quality control report is designed to highlight the QI parameters and display related data and certain procedural events such as a whether there was a low flow request from the surgeon, which makes interpretation of the report more meaningful (Figure 2). The report shows that there was an arterial pressure reported of less than 40 mmHg for more than 5 minutes; this is also reflected in the cardiac index and venous saturation. There is a qualifying comment, “low flow request from the surgeon” that accompanied these parameters, which may assist in interpreting the context in which these variations occurred. The intraoperative comment verifies that a low flow period was required during aortic suturing. As indicated, the arterial pressure for the majority of the case was between 40 and 70 mmHg. An ACT of less than 400 seconds is also reported, along with the event, administration of 10,000 units of heparin. In this particular example, the perfusionist should consider why these QI parameters occurred, and how to improve performance in the future. From an administrative point of view, although, there is sufficient information to suggest that no immediate action is required; feedback and guidance could assist the performance improvement process.
Quality Indicator Data Analysis
In addition to individual feedback, group analysis of the incidence of QI parameters can be performed. This can be done in both a prospective and retrospective manner, allowing data analysis to monitor compliance of quality standards. Retrospective analysis enables identification of practice areas where performance improvement initiatives should be directed (root cause analysis), while prospective analysis provides assessment of the efficacy of these initiatives (root cause analysis solutions).
DISCUSSION
The processes that we have described have allowed us to achieve our goals of developing a method of easy access to the data collected by the DMS and to develop an automated technique of data analysis and feedback for CPB procedures, thereby achieving the first three steps of the DMAIC process; the ability to define the areas or problems that we wish to look at, the ability to gather the appropriate information, and the ability to analyze the data to allow root causes to be identified. Thus, we are well positioned to fulfill our goal of a DMAIC-guided CQI process.
This undertaking has shown the enormous advantage of creating and storing perfusion data in a separate database that is purpose designed to allow immediate access to the data, thus providing advantages from both an administrative and a research perspective. In addition to facilitating access to the created variables, it provides immediate access to the data collected by the DMS including all data obtained from the patient monitor. In contrast, accessing the perfusion data in the DMS requires the data to be exported into another application and linked before it can be analyzed and/or processed. One of the unplanned benefits that we identified has been in relation to the data processing technique that we established, because this has enabled us to analyze QIs on CPB procedures performed before the introduction of the automated feedback process. Therefore, we will be able to ask the question “is there a performance improvement effect caused by automated feedback alone?”
The data obtained from our technique can provide us with valuable feedback on perfusionist performance and adherence to protocols and also provides us with data to challenge the adequacy of our protocols. Even though we may attempt to practice an evidence-based medicine approach, not all aspects of perfusion management have sufficient evidence available to support all of our protocols. The analysis and interpretation of our QI data has become a routine part of our clinical practice and will assist us in the process of protocol evaluation and modification. Furthermore, this implements the final stages of the DMAIC process because it allows us to undertake a professional performance improvement strategy and a new direction for the further development of new clinical processes. Prospective analysis of these variables after the introduction of the automated report generation feedback process is currently in progress. Our final challenges with this project will be to introduce six sigma reporting. Through the application of this methodology, we look forward to reporting our CQI process. The technology that we have used to achieve this is available to all users of HLM systems that allow collection and integration of electronic data; however, to simplify the process and promote broader use of perfusion QC, we are assisting the Stockert program development team with including similar quality management features within their DMS software.
CONCLUSIONS
The introduction of electronic data collection and subsequent development of electronic data processing techniques has enabled us to transfer the data into a readily accessible database and create a data set of perfusion variables and QIs for CPB procedures. This data set may be used for immediate automated QC feedback after CPB procedures, and direction of performance improvement initiatives through retrospective or prospective data analysis as part of an evolving CQI process.
REFERENCES
- 1.Ottens J, Baker RA, Newland RF, Mazzone A.. The future of the perfusion record: automated data collection vs manual recording. J Extra Corpor Technol. 2005;37:355–9. [PMC free article] [PubMed] [Google Scholar]
- 2.Riley JB.. Are perfusion technology and perfusionists ready for quality reporting employing six-sigma performance measurement? J Extra Corpor Technol. 2003;35:168–71. [PubMed] [Google Scholar]
- 3.Groom R, Likosky DS, Rutberg H.. Understanding variation in cardiopulmonary bypass: statistical process control theory. J Extra Corpor Technol. 2004;36:224–30. [PubMed] [Google Scholar]
- 4.ISO 9000. Definitions Translated into Plain English. Alberta, Canada: Praxiom Research Group. [Google Scholar]
- 5.Rath and Strong Management Consultants. Six Sigma Pocket Guide. AON Consulting Worldwide, Lexington, MA, 2002. [Google Scholar]
- 6.Dickinson T, Riley J, Zabetkis PM.. External validation of compliance to perfusion quality indicators. Perfusion. 2004;19:295–9. [DOI] [PubMed] [Google Scholar]
- 7.Hollenburg JP, Pirraglia PA, Williams-Russo P, et al. . Computerised data collection in the operating room during coronary artery bypass surgery: a comparison to the hand–written record. J Cardiothorac Vasc Anesth. 1997;11:545–51. [DOI] [PubMed] [Google Scholar]