Abstract:
Cardiopulmonary bypass (CPB) is associated with surgical stress, hypothermia, hyperoxia, enhancement of neuroendocrine outflow, and administration of glucogenic catecholamines that are associated with glucogonolysis and glucogenesis that result in hyperglycemia. The hyperglycemic state during CPB has been associated with adverse outcomes, such as infection, neurological impairment, cardiac dysfunction, prolonged hospitalization, and higher mortality rates. This report justifies vigilant monitoring of blood glucose levels and a rational protocol for the treatment of hyperglycemia of all open heart surgical patients that may improve post-CPB surgical outcomes.
Keywords: glucose, glycogen, GLUT2, GLUT4, hyperoxia, hypothermia, insulin, hyperglycemia
In this report our goal is to discuss basic physiological mechanisms of glucose metabolism, discuss issues related to cardiopulmonary bypass (CPB) management with a focus on potential mediators of hyperglycemia, and suggest a rational protocol for glucose management during intraoperative and post-operative periods. The aim of this report is to give reason for attentive monitoring of blood glucose levels and integration of perfusion techniques that may reduce the risk of hyperglycemia.
Hyperglycemia is defined as glucose levels above the normal physiological range. Normal physiological fasting blood glucose levels are 70–120 mg/dL and levels >120 mg/dL are the diagnostic levels for diabetes. During CPB and off-pump coronary artery bypass (OPCAB), most patients tend to have elevated blood glucose levels despite a lack of previous medical history of diabetes (1,2). Recent anesthesia protocols suggest intra-operative blood glucose levels >200 mg/dL are considered to be hyperglycemic and should be treated (3). However, we will justify why this treatment threshold should be lower. Because most studies have examined hyperglycemia associated with CPB, our discussion will be primarily focused on CPB factors that may induce hyperglycemia; however, hyperglycemia is seen with most major surgical procedures including OPCAB.
High glucose levels during and after CPB is an independent predictor of morbidity and mortality in both diabetic and non-diabetic patients (4). Patients with persistently elevated glucose levels >200 mg/dL (i.e., insulin resistance) have increased post-operative mortality (5). Hyperglycemia has also been associated with stroke (6), myocardial infarction (7), and infection (8). The treatment of hyperglycemia while on CPB has been controversial because of the post-operative risk of hypoglycemia. However, because of the potential adverse outcomes with elevated levels, the threshold for treatment must be reappraised. Carr et al. (9) suggested that the threshold should be adjusted to 110 mg/dL (9). The remaining sections of this discussion will focus on eight potential mechanisms of cardiac surgical induced hyperglycemia.
To describe the mechanism of CPB hyperglycemia, the normal homeostasis control of glucose will be first described. The main dietary sources of glucose are through the consumption of carbohydrates, such as starch, sugars, and lactose. Two main areas for glucose digestion are the mouth and the small intestine. The enzymes released by the salivary glands, mainly a-amylase, are responsible in breaking starch molecules into maltose. The lining of the small intestine releases enzymes such as maltase, lactase, and sucrase, which converts the carbohydrates into glucose, fructose, and galactose. The glucose is absorbed into the circulation in the duodenum and upper jejunum and is transported to the liver. Obviously, a major exogenous source of glucose in the surgical patient is through intravenous and CPB prime solutions.
The detection of variations in blood glucose levels by pancreatic β cells and a subsequent secretion of insulin are key events in the control of glucose homeostasis. Figure 1 shows the glucose transport into the pancreas by the GLUT2 transporter followed by the enzyme glucokinase that conversion of glucose into glucose-6 phosphate. Glucose-6 phosphate then enters the citric acid cycle for production of ATP. The increase of ATP concentrations secondary to glucose metabolism in the pancreas inhibits the KATP channel, and in contrast, activation of the α2-adrenergic receptor opens the KATP channel. The inhibition of the KATP channel by ATP causes membrane de-polarization, activates the voltage-gated Ca2+ channel, and thereby promotes Ca2+ influx into the cell, whereas α2-adrenergic stimulation inhibits membrane depolarization. Membrane activation induces transcription of insulin resulting in preproinsulin synthesis, which is converted into proinsulin consisting of A, B, and C domains. These domains are cleaved into A and B chains joined by two disulfide bonds, which is insulin, and the C domain, which is termed the C-peptide. The insulin and C-peptide are packaged in the secretory granule and secreted at a 1:1 molar ratio. The elevated intra-cellular Ca2+ stimulates the exocytosis and release of secretory granules with the release of both insulin secretion and C-peptide. The Cpeptide is used as a marker for insulin secretion (10).
Figure 1.
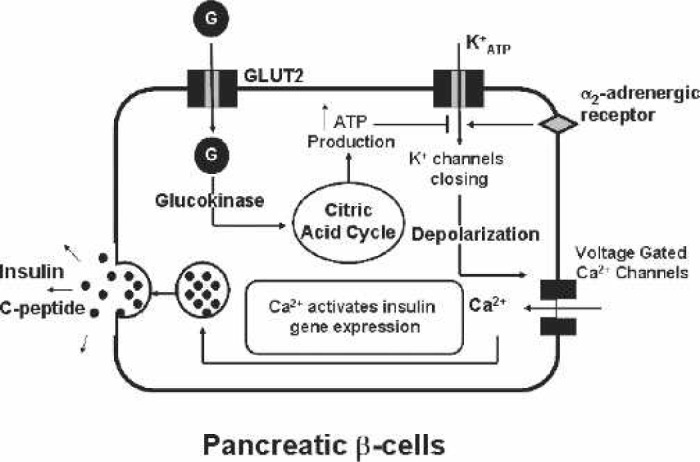
Pancreatic β cell. Glucose (G) is transported into the β cell by the glucose transporter, GLUT2. The resulting generation of ATP closes the K+ATP channel and induces membrane depolarization and Ca2+ influx. This Ca2+ stimulates the gene expression, synthesis, and secretion of insulin and C-peptide. The stimulation of the α2-receptor inhibits the Ca2+ influx and thus insulin synthesis.
The three key target tissues for insulin are the liver, adipocyte, and muscle. When glucose enters the hepatocyte by the GLUT2 transporter, insulin induces glucose storage in the form of glycogen or conversion to triglycerides (Figure 2). In mammals, the major stores of glycogen are in muscle and the liver. Insulin-stimulated glycogen synthesis involves activation of both glucose transport and glycogen synthase. With stimulation by glucagon or β2-adrenergic agonists, the glycogen is converted to glucose and transported out of the liver by the GLUT2 transporter. Unlike the liver, glucose is transported by the insulin-sensitive transporter, GLUT4, in the adipocyte and muscle (Figure 3). Importantly, insulin markedly stimulates the GLUT4 activity, which is not the case for GLUT2. The glucose taken up into the adipocyte by the GLUT4 transporter is converted to triglycerides. The muscles, in addition to the brain and kidney, are key insulin-sensitive tissues and the primary site of insulin-mediated glucose uptake. GLUT4 predominates as the glucose transporter in heart tissue. GLUT4 is localized mainly on intracellular membrane vesicles in the basal metabolic state and translocates to the plasma membrane in response to stimuli such as insulin (11), ischemia and hypoxia (12), and α1-adrenergic stimulation (13). GLUT1 is constitutively found in the heart muscle plasma membrane and is generally considered to be responsible for basal glucose transport. The glucose can either be converted into glycogen or shuttled through the glycolytic citric acid cycle for the generation of ATP in muscular tissues.
Figure 2.
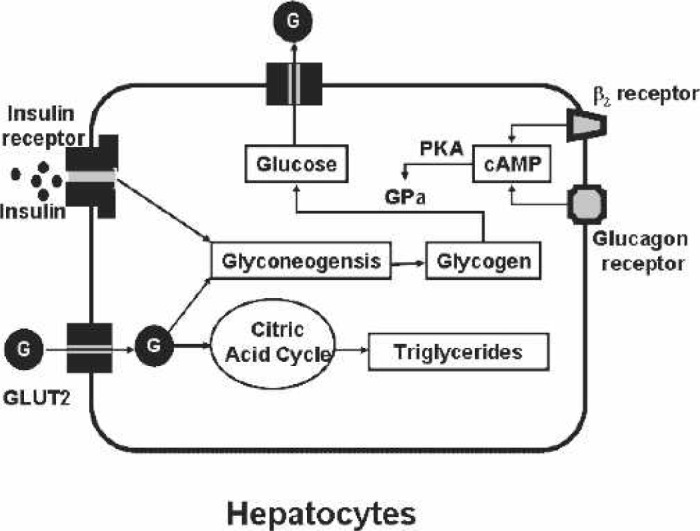
Hepatocyte. Glucose (G) is transported into the hepatocyte by the glucose transporter, GLUT2. Insulin receptor stimulation induces the formation of glycogen. Stimulation of either the β2-adrenergic or glucagon receptors induces cyclic-adenosine monophosphate (cAMP) and thereby protein kinase A (PKA). PKA activates glycogen phosphorylase (GPa) that induces the production of glucose from glycogen.
Figure 3.
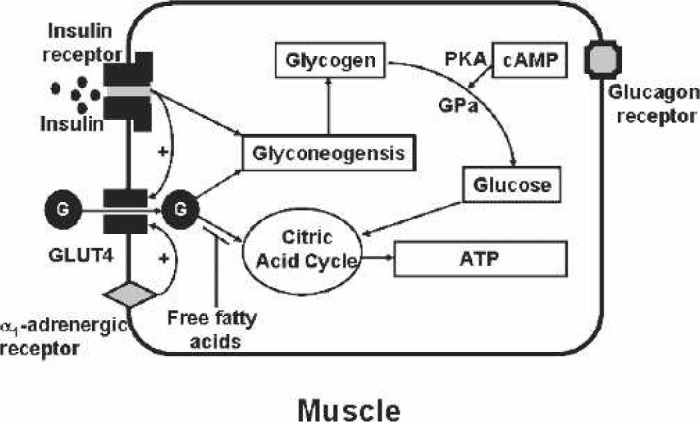
Muscle. Glucose (G) is transported into muscle by the insulin-sensitive glucose transporter, GLUT4. Either insulin receptor or α1-receptor stimulation can cause the translocation of the GLUT4 from the cytosol to the membrane. The glucose can be converted into ATP by the citric acid cycle or to glycogen. Increased free fatty acids inhibit the glucose conversion to ATP. Glucagon receptor stimulation induces cyclic-adenosine monophosphate (cAMP) and thereby protein kinase A (PKA). PKA activates glycogen phosphorylase (GPa) that produces glucose from glycogen.
In the event of hypoglycemia, glucagon is released by pancreatic α cells. Glucagon acts primarily on the liver to mobilize glucose from the glycogen stores and also induces release glucose from muscle, fat, and hepatic tissues. From a clinical standpoint, glucagon is released during periods of fasting—as during pre-operative NPO periods—in response to low plasma glucose levels. Glucagon also induces the phosphorylation of glycogen phosphorylase (GPa) and GPa, which converts glycogen to glucose. In the liver and muscle, β2-receptor stimulation by catecholamines also induces GPa. The result of GPa activation is the breakdown of glycogen to glucose, which thereby counters a hypoglycemic state. In the liver, intracellular glucose concentrations can exceed blood glucose concentration and export of glucose from liver occurs through GLUT2. Thus, any factors that interrupt or stimulate the described pathways can result in hypoglycemic or hyperglycemic states. Clearly the disruption of glucose homeostasis is observed in diabetes in addition to the populations presenting with metabolic syndrome and in select cases of pregnancy. In the context of this discussion, CPB procedures are associated with a disruption of the glucose homeostasis frequently resulting in hyperglycemia—despite the lack of underlying risk factors.
SURGICAL STRESS AND HYPERGLYCEMIA
Hyperglycemia and glucose resistance are common to all types of severe acute trauma, injury, and illness—even in patients without diabetes (14). Related to surgical stress, it has been well established that stress hormones such as glucogenic catecholamines (i.e., epinephrine), glucagon, and cortisol are released into the circulation, which are associated with altered insulin and glucose metabolism. Glucogenic catecholamines exert effects by inhibiting insulin secretion and augmenting hepatic glucose output by stimulating gluconeogenesis and glycogenolysis (15). Cortisol-induced insulin resistance is caused by decreased insulin receptor response by hepatic and peripheral tissues (16). The induction of stress hormones, such as cortisol, is not different when comparing OPCAB with on-CPB (1,2). As mentioned above, glucagon stimulates the conversion of glycogen to glucose. Therefore, surgical stress increases levels of glucogenic catecholamines, cortisol and glucagon, and thus increases the overall plasma glucose concentrations by increasing blood glucose production and reducing tissue glucose uptake. Compounding these endogenous effects during open heart surgery is the exogenous use of catecholamine-based inotropic agents and in some cases administration of exogenous glucocorticoids.
HYPOTHERMIA AND HYPERGLYCEMIA
Hypothermia indirectly increases the glucose levels in the blood stream, resulting in hyperglycemia. As hypothermia is initiated, sympathetic activity is increased resulting in elevated levels of catecholamines and free fatty acids, which as described above, results in decreased insulin secretion and increased tissue resistance to insulin. The increase in catecholamine release is compounded by impaired peripheral glucose uptake at the tissue level because of hypothermia. The insulin decrease is also caused by cooling of the islets of Langerhans, responsible for insulin secretion (17). A third effect of hypothermia is hypokalemia (18). Hypokalemia is associated with insulin resistance and hyperglycemia (19). Therefore, three factors play a role in increasing glucose levels in hypothermic conditions: increased catecholamine levels, hypothermic effect on the pancreas, and hypothermic induction of hypokalemia.
HYPEROXIA AND HYPERGLYCEMIA
Hyperoxia in CPB procedures is an important determinant of glucose levels. In a porcine model, a direct correlation was shown between PaO2 on CPB and blood glucose (r = 0.7, p = 0.02). A 2-fold increase in the blood glucose levels were observed as the PaO2 reached levels >300 mmHg during normothermic CPB. These same findings were observed with pigs being ventilated on high FIO2 without CPB. A return to normoxia conditions only partially restored the blood glucose levels toward control values (20). In a separate study, it was shown that ventilation with hyperoxia conditions (PaO2 of 434 mmHg) increased glucagon by 3-fold (p < 0.001) and plasma insulin levels by 2-fold (p < 0.003) and significantly reduced GLUT4 levels by >70% (21). Thus, the increase in glucose level was attributed to the influential effects of hyperoxia in reducing the total GLUT4 transporters. Furthermore, elevated PaO2 increases the hepatic glucagon receptor expression, thus increasing glycogenolysis (22). The implication is that hyperoxia during cardiovascular surgery can induce a hyperglycemic state. Therefore, because PaO2 can be easily controlled by anesthesia and during CPB, maintaining lower PaO2 should proportionally reduce blood glucose levels.
HEPARIN AND HYPERGLYCEMIA
It is well established that heparin is known to increase the total free fatty acid levels in the blood on administration in a dose-dependent manner (23,24). It is established that increased blood free fatty acids induces insulin resistance in humans through its inhibition of glucose transport activity (25) and muscle glycolysis (26). Moreover, the elevated free fatty acids effectively compete with glucose for uptake by peripheral tissues, regardless of the presence of hyperglycemia as shown in Figure 3. The initial dose of heparin infused before the initiation of CPB in combination with the heparin added to the circuit prime indirectly causes hyperglycemia by increasing the free fatty acids in the circulation and thus impairing the reuptake of glucose by the peripheral tissues. Because heparin dose dependently increases free fatty acid levels and free fatty acid levels influence glucose homeostasis, it may be, therefore, justified to titrate heparin administration with frequent activated clotting time (ACT) measurements.
DECREASED LEVELS OF INSULIN AND CARDIOPULMONARY CPB
One of the logical causes of hyperglycemia is reduced levels of blood insulin. As the cardiopulmonary bypass is initiated, the non-pulsatile flow may result in hypoperfusion of some vital organs such as the pancreas, thereby reducing the production of insulin by pancreas (27). However, Doenst et al. (4) showed that plasma Cpeptide levels remained unchanged throughout CPB procedures, suggesting the pancreatic insulin secretion is not reduced during CPB. An alternate explanation for the insulin decrease during CPB might be the binding of insulin protein to the CPB circuit. However, this latter hypothesis has yet to be tested.
SEQUELI OF HYPERGLYCEMIA
Hyperglycemia and Infection
Post-surgical infections are undesirable outcomes that markedly affect surgical outcomes and patient costs. Hyperglycemia reduces neutrophil activity by impairing its adhesion, chemotaxis, phagocytosis, oxidative activity, and bactericidal activity. However, infusion of insulin not only reverses these effects but also increases the peripheral neutrophil count (28). In a report on the impact of infection caused by hyperglycemia, it was noted that with each 50-mg/dL blood glucose increase, there was an associated 0.76 days increase in hospital stay and $2824 increase in hospital cost per patient, which adds up to a total of $12,542 increase in the cost of patient care on average (29). In support of the concept of hyperglycemia and the immune function, mediastinal infections have been reduced from 1.6% to 0% with maintaining the blood glucose to 110 mg/dL or lower in a study of 1800 patients (9). Therefore, tight control of blood glucose in cardiac surgery patients can significantly reduce infectious processes.
Hyperglycemia and Cardiac Function
The main energy source for the heart during normal physiologic condition is free fatty acids. However, during CPB and myocardial ischemia, glucose rather than free fatty acids is the choice of energy substrate for the heart (30). Glucose transport into myocardial cells is mediated by the isoforms GLUT4 and GLUT1. It has been shown that peri-operative glucose-insulin-potassium markedly improved cardiac performance and reduced the requirement for inotropes (31) and neurological function (32). Free fatty acids increase during CPB as a function of heparin-induced lipoprotein lipase. Increased levels of Free fatty acids can be toxic to an ischemic heart and result in arrhythmias. Insulin and glucose are known to reduce free fatty acid levels, therefore reducing the effect of high free fatty acids in an ischemic heart (33). The presence of elevated glucose levels is detrimental to patient outcomes; insulin seems to have protective effects (31).
Hyperglycemia and Coagulation
Diabetic hyperglycemia causes hyperfibrinogenemia, thereby stimulating the synthesis of interleukin (IL)-6 and tumor necrosis factor (TNF)-α cytokines (34). Moreover, diabetic hyperglycemia may also result in elevated clotting factors VII, factor VIII, factor XI, factor XII, kallikrein, von Willebrand factor, and platelet hyperactivity. This condition, as seen in hyperglycemic diabetic patients, results in a hypercoagulable state (35). Hyperglycemia also affects the coagulation system and platelet function by increasing platelets activation and adhesion and increasing levels of von Willebrand factor in the circulation (36). Even though these factors are altered during the chronic state of hyperglycemia in the diabetic patient, it is not clear the role of transient hyperglycemia on these factors in the non-diabetic patient during open heart surgery.
Hyperglycemia and Neurologic Function
Last, and most importantly, hyperglycemia during CPB significantly increases cerebral lactate levels without adversely affecting cerebral blood flow and metabolism, cerebrospinal fluid pH, or cerebral energy charges. Glucose is the single essential energy substrate for the brain and enters the cells by the GLUT1 transporter. Anaerobic glycolysis occurring during cerebral ischemia is the primary cause of lactic acidosis in the brain, and its severity is directly associated with the pre-ischemic glucose levels. Hyperglycemia, therefore, is associated with neurological injury after cerebral ischemia (37). This is especially important in patients undergoing total circulatory arrest, where cerebral ischemia is a major risk. Also, in the context of diabetes, diabetics are known to lack autoregulatory blood flow to the brain and consequently possess an increased risk for cerebral ischemia during CPB if the perfusion pressures are not maintained >70 mmHg (38). Therefore, maintaining normal glucose levels plus elevated perfusion pressures in this population is highly critical.
Despite all the benefits of monitoring glucose levels while on CPB, this topic still remains controversial in the context of treatment with insulin. The counter argument in treating hyperglycemia while on CPB possibly originates from the concern in possible development of hypoglycemia after surgery. Hypoglycemia, after CPB, may result in neurological problems such as diminished level of consciousness, syncope, or seizures, and the latter are a greater concern because most patients are sedated, and these symptoms may not be apparent (39).
TREATMENT OF HYPERGLYCEMIA
Optimal glucose levels pre-operatively and postoperatively for CPB patients is suggested to be 110 mg/dL (9). In general, we administer 0.1 U/kg of insulin, and it is adjusted accordingly to whether the glucose levels reach higher levels. Also, insulin treatments have been known to cause electrolyte disturbances, specifically lowering potassium and magnesium levels, and in some cases, insulin administration has resulted in cardiac arrhythmias (40). Therefore, corresponding with close monitoring of blood glucose–electrolyte monitoring is crucial with insulin therapy.
CONCLUSION
Cardiac vascular surgery with or without CPB is associated with hyperglycemia in non-diabetics and can be detrimental to coronary artery bypass grafting outcomes (1). Multiple factors influence the levels of serum glucose levels that result in hyperglycemia in almost all patients, diabetic and non-diabetic. These factors include glucogenic drugs (catecholamines), insulin receptor desensitization (endogenous cortisol and exogenous glucocorticoids), exogenous glucose (D5W), hyperoxia, hypothermia, exogenous heparin, biosurfaces (CPB system), hemodilution, and hypoperfusion (CPB perfusion). Many of the listed factors can not be alleviated during open heart surgical procedures, and therefore, the key is frequent glucose and electrolyte monitoring coupled with appropriate intraoperative and post-operative insulin and glucose treatment.
ACKNOWLEDGMENTS
This study was supported by NIH Grant R01 HL079206-01, the James and Linda Lee Heart Failure Research Award, and the Steinbronn Heart Failure Research Award to D.F.L.
REFERENCES
- 1.Anderson RE, Brismar K, Barr G, et al. Effects of cardiopulmonary bypass on glucose homeostasis after coronary artery bypass surgery. Eur J Cardiothorac Surg. 2005;28:425–30. [DOI] [PubMed] [Google Scholar]
- 2.Velissaris T, Tang AT, Murray M, et al. A prospective randomized study to evaluate stress response during beating-heart and conventional coronary revascularization. Ann Thorac Surg. 2004;78:506–12. [DOI] [PubMed] [Google Scholar]
- 3.Ruesch S, Levy J.. The postcardiopulmonary bypass period: A systems approach. In: Hensley F, Martin D, Gravlee G, eds. A Practical Approach to Cardiac Anesthesia. Philadelphia, PA: Lippincott Williams and Wilkins; 2003; 235–49. [Google Scholar]
- 4.Doenst T, Wijeysundera D, Karkouti K, et al. Hyperglycemia during cardiopulmonary bypass is an independent risk factor for mortality in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2005;130:1144. [DOI] [PubMed] [Google Scholar]
- 5.Lazar HL.. Hyperglycemia during cardiac surgery. J Thorac Cardiovasc Surg. 2006;131:11–3. [DOI] [PubMed] [Google Scholar]
- 6.Parsons MW, Barber PA, Desmond PM, et al. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. Ann Neurol. 2002;52:20–8. [DOI] [PubMed] [Google Scholar]
- 7.Fonseca VA.. Management of diabetes mellitus and insulin resistance in patients with cardiovascular disease. Am J Cardiol. 2003;92:50J– 60J. [DOI] [PubMed] [Google Scholar]
- 8.Trick WE, Scheckler WE, Tokars JI, et al. Modifiable risk factors associated with deep sternal site infection after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2000;119:108–14. [DOI] [PubMed] [Google Scholar]
- 9.Carr JM, Sellke FW, Fey M, et al. Implementing tight glucose control after coronary artery bypass surgery. Ann Thorac Surg. 2005;80:902–9. [DOI] [PubMed] [Google Scholar]
- 10.Thorens B.. GLUT2 in pancreatic and extra-pancreatic glucodetection (review). Mol Membr Biol. 2001;18:265–73. [DOI] [PubMed] [Google Scholar]
- 11.Zaninetti D, Greco-Perotto R, Assimacopoulos-Jeannet F, et al. Effects of insulin on glucose transport and glucose transporters in rat heart. Biochem J. 1988;250:277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun D, Nguyen N, DeGrado TR, et al. Ischemia induces translocation of the insulin-responsive glucose transporter GLUT4 to the plasma membrane of cardiac myocytes. Circulation. 1994;89:793–8. [DOI] [PubMed] [Google Scholar]
- 13.Fischer Y, Thomas J, Holman GD, et al. Contraction-independent effects of catecholamines on glucose transport in isolated rat cardiomyocytes. Am J Physiol. 1996;270:C1204–10. [DOI] [PubMed] [Google Scholar]
- 14.Martinez-Riquelme AE, Allison SP.. Insulin revisited. Clin Nutr. 2003;22:7–15. [DOI] [PubMed] [Google Scholar]
- 15.Huang MT, Lee CF, Dobson GP.. Epinephrine enhances glycogen turnover and depresses glucose uptake in vivo in rat heart. FASEB J. 1997;11:973–80. [DOI] [PubMed] [Google Scholar]
- 16.Rizza RA, Mandarino LJ, Gerich JE.. Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor detect of insulin action. J Clin Endocrinol Metab. 1982;54:131–8. [DOI] [PubMed] [Google Scholar]
- 17.Mallet ML.. Pathophysiology of accidental hypothermia. QJM. 2002;95:775–85. [DOI] [PubMed] [Google Scholar]
- 18.Zydlewski AW, Hasbargen JA.. Hypothermia-induced hypokalemia. Mil Med. 1998;163:719–21. [PubMed] [Google Scholar]
- 19.Plavinik FL, Rodrigues CI, Zanella MT, et al. Hypokalemia, glucose intolerance, and hyperinsulinemia during diuretic therapy. Hypertension. 1992;19:II26–9. [DOI] [PubMed] [Google Scholar]
- 20.Bandali KS, Belanger MP, Wittnich C.. Is hyperglycemia seen in children during cardiopulmonary bypass a result of hyperoxia? J Thorac Cardiovasc Surg. 2001;122:753–8. [DOI] [PubMed] [Google Scholar]
- 21.Bandali KS, Belanger MP, Wittnich C.. Does hyperoxia affect glucose regulation and transport in the newborn? J Thorac Cardiovasc Surg. 2003;126:1730–5. [DOI] [PubMed] [Google Scholar]
- 22.Krones A, Kietzmann T, Jungermann K.. Periportal localization of glucagon receptor mRNA in rat liver and regulation of its expression by glucose and oxygen in hepatocyte cultures. FEBS Lett. 1998;421:136–40. [DOI] [PubMed] [Google Scholar]
- 23.Wittnich C, Dewar ML, Chiu RC.. Myocardial protection: heparin-induced free fatty acid elevation during cardiopulmonary bypass and its prevention. J Surg Res. 1984;36:527–31. [DOI] [PubMed] [Google Scholar]
- 24.Jaume JC, Mendel CM, Frost PH, et al. Extremely low doses of heparin release lipase activity into the plasma and can thereby cause artifactual elevations in the serum-free thyroxine concentration as measured by equilibrium dialysis. Thyroid. 1996;6:79–83. [DOI] [PubMed] [Google Scholar]
- 25.Dresner A, Laurent D, Marcucci M, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103:253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JK, Wi JK, Youn JH.. Plasma free fatty acids decrease insulin-stimulated skeletal muscle glucose uptake by suppressing glycolysis in conscious rats. Diabetes. 1996;45:446–53. [DOI] [PubMed] [Google Scholar]
- 27.Herreros J, Berjano EJ, Sola J, et al. Injury in organs after cardiopulmonary bypass: A comparative experimental morphological study between a centrifugal and a new pulsatile pump. Artif Organs. 2004;28:738–42. [DOI] [PubMed] [Google Scholar]
- 28.Rassias AJ, Givan AL, Marrin CA, et al. Insulin increases neutrophil count and phagocytic capacity after cardiac surgery. Anesth Analg. 2002;94:1113–9. [DOI] [PubMed] [Google Scholar]
- 29.Estrada CA, Young JA, Nifong LW, et al. Outcomes and perioperative hyperglycemia in patients with or without diabetes mellitus undergoing coronary artery bypass grafting. Ann Thorac Surg. 2003;75:1392–9. [DOI] [PubMed] [Google Scholar]
- 30.Stanley WC, Recchia FA, Lopaschuk GD.. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–129. [DOI] [PubMed] [Google Scholar]
- 31.Quinn DW, Pagano D, Bonser RS, et al. Improved myocardial protection during coronary artery surgery with glucose-insulinpotassium: a randomized controlled trial. J Thorac Cardiovasc Surg. 2006;131:34–42. [DOI] [PubMed] [Google Scholar]
- 32.Quinn DW, Pagano D, Bonser RS.. Glucose and insulin influences on heart and brain in cardiac surgery. Semin Cardiothorac Vasc Anesth. 2005;9:173–8. [DOI] [PubMed] [Google Scholar]
- 33.Trence DL, Kelly JL, Hirsch IB.. The rationale and management of hyperglycemia for in-patients with cardiovascular disease: Time for change. J Clin Endocrinol Metab. 2003;88:2430–7. [DOI] [PubMed] [Google Scholar]
- 34.Dalla VM, Mussap M, Gallina P, et al. Acute-phase markers of inflammation and glomerular structure in patients with type 2 diabetes. J Am Soc Nephrol. 2005;16(Suppl 1):S78–82. [DOI] [PubMed] [Google Scholar]
- 35.Carr ME.. Diabetes mellitus: A hypercoagulable state. J Diabetes Complications. 2001;15:44–54. [DOI] [PubMed] [Google Scholar]
- 36.Yamagishi S, Imaizumi T.. Diabetic vascular complications: Pathophysiology, biochemical basis and potential therapeutic strategy. Curr Pharm Des. 2005;11:2279–99. [DOI] [PubMed] [Google Scholar]
- 37.Murkin JM.. Pro: tight intraoperative glucose control improves outcome in cardiovascular surgery. J Cardiothorac Vasc Anesth. 2000;14:475–8. [DOI] [PubMed] [Google Scholar]
- 38.Pallas F, Larson DF.. Cerebral blood flow in the diabetic patient. Perfusion. 1996;11:363–70. [DOI] [PubMed] [Google Scholar]
- 39.Chaney MA, Nikolov MP, Blakeman BP, et al. Attempting to maintain normoglycemia during cardiopulmonary bypass with insulin may initiate postoperative hypoglycemia. Anesth Analg. 1999;89:1091–5. [DOI] [PubMed] [Google Scholar]
- 40.Binder G, Bosk A, Gass M, et al. Insulin tolerance test causes hypokalaemia and can provoke cardiac arrhythmias. Horm Res. 2004;62:84–7. [DOI] [PubMed] [Google Scholar]


