Abstract:
While blood:crystalloid cardioplegia is the clinical standard for patients undergoing cardiopulmonary bypass (CPB), it has been postulated that whole blood minicardioplegia may benefit the severely injured heart by reducing cardioplegic volume, thereby reducing myocardial edema. To test this hypothesis, we compared the cardioprotection of a popular 4:1 blood:crystalloid cardioplegia to whole blood minicardioplegia (WB) in a porcine model of acute myocardial ischemia. Yorkshire pigs (n = 20) were placed on atriofemoral bypass and subjected to 30 minutes of global normothermic ischemia. Animals were randomized to receive either 4:1 cold cardioplegia (n = 10) or WB cold cardioplegia (n = 10) delivered antegrade continuously for 90 minutes. Baseline (BL) echocardiographic determination of left ventricular mass (LVM) was compared within groups for cardiac edema (%) measured by histologic morphometrics. All (100%) animals receiving WB were successfully weaned off CPB, whereas only 40% of animals receiving 4:1 were successfully weaned off CPB. Cardiac edema percentage (p < .004) and LVM (p < .05) were significantly decreased in the WB group compared with 4:1. WB cardioplegia increases the number of hearts successfully weaned from CPB and decreases cardiac edema in our porcine model of acute myocardial ischemia. This finding implies whole blood cardioplegia may be more protective in a select group of patients undergoing extended CPB time by decreasing myocardial edema.
Keywords: myocardial protection, blood cardioplegia, myocardial edema, minicardioplegia, global normothermic ischemia
Results of contemporary cardiac surgery are exceptional. However, changing trends in patient populations and the number of high-risk candidates for heart surgery are prompting further advancement in both technique and technology (1). For instance, more patients present with previous interventional cardiology procedures, advanced age, reoperations, and more urgent surgeries. Christakis et al. (2) noted that, even with the trends to more difficult cases overall, mortality stayed the same, although morbidity increased. They attributed the increased survival to contemporary techniques of myocardial preservation but also postulated that the increasing morbidity may be caused by the same. Such findings highlight a few important trends. First, cardiac surgery patients are older and sicker than ever before. Second, improved myocardial preservation techniques can make a difference in outcomes for these patients. Further innovations are needed if mortality is to remain at the current level and morbidity decreased. One such innovation came a few years ago when Menasché (3) questioned the need to dilute blood: crystalloid cardioplegia. He described the use of minicardioplegia, which diverts small volumes of blood from the arterial port of the oxygenator and supplements it with arresting agents—in practice, only small volumes of potassium and magnesium (4). This greatly decreases the volume of saline solution delivered in a typical bypass procedure and avoids the detrimental consequences of volume overload and hemodilution.
The minicardioplegia concept has gained several proponents over the last few years and has been tested clinically. These clinical observations are supported by laboratory studies documenting the detrimental effects of myocardial edema (5–7). Such studies suggest that myocardial edema can result in postoperative dysfunction and complications. It is also clear from the clinical reports that whole blood (WB) cardioplegia may result in improved outcomes in certain variables and settings. To date, however, no studies have documented whether WB cardioplegia can indeed reduce cardiac edema or whether reduction in cardiac edema is the reason for the observed improvements in outcome. With this in mind, we tested the hypothesis that WB cardioplegia, compared with a standard 4:1 blood:crystalloid cardioplegia, would decrease myocardial edema and that this would in turn result in more hearts weaned from bypass after an ischemic insult.
MATERIALS AND METHODS
Surgical Preparation
Healthy Yorkshire hybrid pigs (30–40 kg) were placed under general anesthesia and instrumented for hemodynamic monitoring with a pulmonary artery catheter and femoral arterial line as previously described (1). Initial ventilator settings were FiO2 = 50%, tidal volume = 12 mL/kg, and a rate of 10 breaths/min. Adjustments were made in the respiratory rate to obtain a baseline PaCO2 = 45–55 mmHg.
Base excesses (BE) less than −3 mEq/L were corrected with intravenous sodium bicarbonate, and adjustments were made in ventilatory rate to maintain PaCO2 within a normal range (40–45 mmHg). Heating pads and warmed intravenous fluids were used to maintain a core temperature between 34 and 38°C. All groups received lactated Ringer solution (25 mL/kg/h) in addition to bolus infusion of dextran 70 after cardiopulmonary bypass (CPB) termination to maintain cardiac output (CO) within 10% of baseline.
Cardiopulmonary Bypass
After median sternotomy, the pericardium was opened, the animals were heparinized, and a 5F transducer-tipped pressure catheter (Mikro-Tip; Millar Instruments, Houston, TX) was inserted through a purse-string suture and threaded into the left ventricle. The right femoral artery was cannulated with a 22F arterial cannula for CPB. The right atrium was cannulated with a 32F two-stage cannula for venous return. The CPB circuit included Cobe oxygenators (Cobe Duo flat plate membrane) (Cobe Cardiovascular Inc., Arvada, CO), tubing pack, an arterial filter (40 μm), and a Sarns (Terumo Cardiovascular Systems, Ann Arbor, MI) roller pump. All animals were initially anticoagulated with 300 units of porcine heparin. During the bypass, anticoagulation was titrated to an activated clotting time (ACT) of >480 seconds with Hemochron 801 and FTC510 ACT tubes. The pump prime solution consisted of lactated Ringer (1500 mL), mannitol (5 g), sodium bicarbonate (35 mEq), porcine lung heparin (5000 units), and diphenhydramine hydrochloride (Benadryl) (10 mg). Nonpulsatile CPB was initiated at a flow rate of 80 mL/kg. Mean arterial blood pressure was maintained (30–70 mmHg) by adjusting blood flow rate. Sweep gas and pump flow were titrated to maintain physiologic blood gases (pH 7.35–7.45, PCO2 35–40 mmHg, and PO2 150–350 mmHg).
The left ventricle was vented by a 20F catheter introduced into the left ventricle through the apex of the heart. The hemiazygos vein was ligated at the left hilum proximal to the confluence with the coronary sinus. The ascending aortic root was instrumented with a cardioplegia cannula for cardioplegia delivery and a 22-gauge needle for monitoring root pressure after cross-clamp placement. As soon as the animal was stabilized on CPB, the aorta was cross-clamped, and a myocardial temperature probe was placed in the ventricular septum. Antegrade cardioplegia was initiated 30 minutes after aortic cross-clamping and delivered with a myocardial protection system (MPS; Quest Medical Inc., Allen, TX).
Experimental Protocol—Myocardial Protection
The protocol (Figure 1) is a modification of models established by Ericsson and Takeshima (8) and Catinella et al. (9). Swine were randomized to continuous 4:1 modified Buckberg cardioplegia or WB cardioplegia. Baseline hemodynamic, mechanical, and metabolic data were recorded before cannulation for CPB. Totally vented CPB was started, and the aorta was clamped. After 30 minutes of “unprotected” global normothermic ischemia, cardioplegia was instituted. Cardioplegia delivery was divided into three distinct phases over a 90-minute period. Animals randomized to the 4:1 group received cardioplegia according to the following protocol:
Phase 1 (induction): Initiated after 30 minutes of unprotected normothermic ischemia. One liter of warm solution (37°C) containing high potassium chloride (KCl) (18–20 mEq/L), D51/4 normal saline (NS) 349–360 mOsm, citrate phosphate dextrose (CPD) 0.5–0.6 mM/L, trishydroxymethyl amino methane (THAM) (pH 7.7–7.8) was delivered at a constant aortic root pressure of 70–80 mmHg, requiring a flow rate of 200–250 mL/min before progressing to phase 2.
Phase 2 (maintenance): This solution was low KCl (8–10 mEq/L), with all other constituents identical to phase 1 and administered cold (10–15°C) continuously until phase 3. The first1Lof this phase was administered at a constant aortic root pressure of 70–80 mmHg, which required flows of 200–250 mL/min. The remainder of the solution delivered during this phase was delivered at 150 mL/min, which yielded an aortic root pressure of 30–40 mmHg.
Phase 3 (hot-shot): After 84 minutes of cardioplegia delivery (phase 1 and phase 2 delivery), phase 3 was initiated. This solution (KCl 8–10 mEq/L, DSW 380–400 mOsm, D50W > 400 mg%, CPD 0.15–1.25 mM/L, THAM pH 7.5–7.6, aspartate 13 mM/L, glutamate 13 mM/L) was delivered warm (37°C) over a 3-minute period. After reperfusate delivery, lidocaine (100 mg) and MgSO4 (1 g) were administered into the CPB reservoir, the reperfusate solution was discontinued, and an additional 3 minutes of warm (37°C) whole blood perfusate was given.
Figure 1.
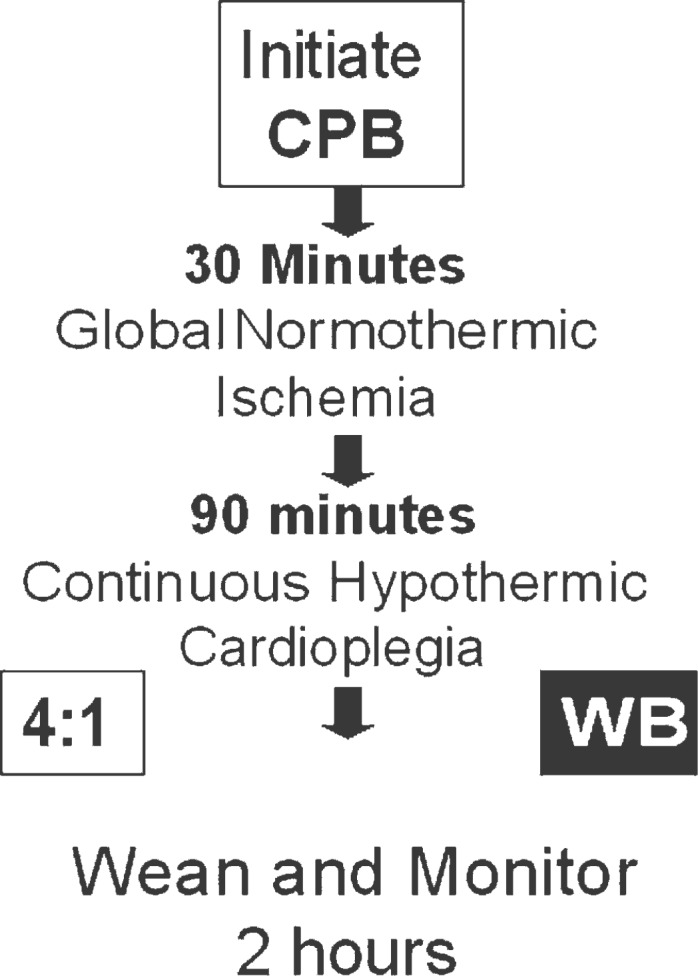
Experimental protocol.
Animals randomized to the WB protocol group were administered cardioplegia according to the following protocol:
Phase 1 (induction): Initiated after 30 minutes of unprotected normothermic ischemia. One liter of warm (37°C) high KCl (whole blood with 18–20 mEq/L KCl and 375 mg MgSO4 added through the MPS arrest and additive cassettes) was delivered at a constant aortic root pressure of 70–80 mmHg, requiring a flow rate of 200–250 mL/min before progressing to phase 2.
Phase 2 (maintenance): Cold (10–15°C) low KCl (whole blood with 8–10 mEq/L KCl and 4.7 g MgSO4 added through the MPS arrest and additive cassettes) was administered continuously until phase 3. The first 1 L of this phase was administered at a constant aortic root pressure of 70–80 mmHg, which required flows of 200– 250 mL/min. The remainder of the solution delivered during this phase was delivered at 150 mL/min, which yielded an aortic root pressure of 30–40 mmHg.
Phase 3 (hot-shot): After 84 minutes of cardioplegia administration (phases 1 and 2), phase 3 was initiated. Normothermic (37°C) low K cardioplegic solution (whole blood with 8–10 mEq/L KCl and 280 mg MgSO4 added through the MPS arrest and additive cassettes) was administered for 3 minutes. MgSO4 (1 g) and lidocaine (100 mg) were administered into the CPB reservoir. Potassium infusion through the MPS additive cassette was discontinued, and 3 additional minutes of warm whole blood (37°C) perfusion was delivered into the aortic root.
In both groups, phase 3 was discontinued and cross-clamp was removed after return of regular myocardial electrical activity. Cardioversion and active left ventricular venting was used as necessary to avoid myocardial distension. Animals were weaned from CPB over a 30-minute period. Intravenous infusion of isoproterenol (4 g/min) was initiated with termination of CPB. Five minutes before discontinuation of CPB, calcium chloride (1 g) was given, and the left ventricular vent was removed. After CPB, all blood within the oxygenator was transfused back into the animal, and heparin was reversed with protamine (1.3 mg/100 units heparin).
One hundred twenty minutes after termination of CPB, hemodynamic, mechanical, and metabolic parameters were measured. The experiment was terminated, and samples of the left ventricle were placed in 10% buffered formalin for morphometric analysis. Sections of tissue (10–18 g) from all three major coronary distributions were also removed for wet to dry weight analysis.
Left Ventricular Mass (Echocardiography)
Two-dimensional echocardiography was done before institution of CPB and compared with echocardiograms repeated 15 minutes after animals were weaned from bypass. In those cases where animals were unable to be weaned, mass measurements were obtained within 15 minutes of failure to wean. Echocardiography was selected for its ability to provide reproducible measurements both before surgery and during the perioperative period. Images were obtained from the open chest using a Hewlett-Packard SONO 5500 ultrasound system with a 5.0/3.7 MHz transesophageal transducer (Phillips Medical Systems, Andover, MA). The probe was placed in a sterile protective sheath with the tip of the sheath filled with ultrasound gel. The tip of the probe was placed within the open chest at the right ventricular apex, aortic root, and right ventricular free wall. From these locations, the probe could be mechanically steered and electronically rotated to allow optimal long axis, short axis, four chamber, and two chamber images to be obtained (10). Instrument settings were optimized for each data set to obtain the best endocardial and epicardial delineation.
From the data sets collected it was determined that images were of excellent quality with distinct endocardial and epicardial border definition. The modified Simpson’s rule was used to determine left ventricular endocardial and epicardial volumes (11). The following formula was used to calculate left ventricular mass (LVM) (12). The formula takes into account the specific gravity of myocardial tissue (1.05).
All images were stored on VHS tapes and analyzed after the procedure. The Hewlett Packard SONO 5500 ultrasound system was used to analyze each data set. A single interpreter was chosen to make all measurements to insure consistency in method and analysis. Three to five end diastolic frames were measured and averaged.
Quantitative Histology and Morphometrics
Tissue blocks (2 × 2 × 1 cm) obtained from the left ventricle were fixed in formalin and processed for histology. Paraffin sections were made at 7 mm and stained with routine hematoxylin and eosin. One section was randomly chosen from each pig for evaluation. Histological sampling of each section was systematic and unbiased, carried out on photomicrographs obtained at points that were equidistant along a random sampling probe. The sampling points along the probe were located by their x and y coordinates on the microscope stage. Thus, the microscopic field was seen only after it was chosen as a sample. There were five sampling points per animal. The photomicrographs were made at high magnification, each covering 320 × 250 mm of myocardium. The photomicrographs were analyzed with Image Pro Plus (Media Cybernetics, Silver Spring, MD) to determine the percentage of area occupied by myocardium, percentage of area occupied by nuclei, and percentage of area occupied by connective tissue. Variability among pigs subjected to similar treatment was assessed with ANOVAs, while differences between treatment conditions were determined with t tests using the MINITAB statistical package. Significance was set at the 95% level.
All experiments described in this study were performed in accordance with the National Institutes of Health guidelines for the use of experimental animals in research. The protocol was approved for the Committee for the Humane Use of Animals at our institution.
RESULTS
Survival (Wean From CPB, 2 Hours)
Our primary endpoint was to test which hearts came off bypass and how many of those were viable at 2 hours (Figure 2). All animals (100%) in the WB group were able to be weaned from bypass. In comparison, less than one half of the 4:1 hearts were able to be weaned from bypass after ischemic injury (40%). However, at 2 hours, survival was equal in both groups (40%).
Figure 2.

Hearts weaned from CPB and survival at 2 hours.
Hemodynamic/Metabolic Parameters
Of the animals that survived 2 hours after bypass, there was no difference in cardiac index or urine output between the two groups. In addition, there was no difference in hemoglobin levels or troponin levels between the two groups at any time-point.
Myocardial Water Content
There were no differences in water content between the various major coronary distributions in any group—left anterior descending (LAD), right coronary (RC), and circumflex (CX) (Figure 3). Likewise, there were no overall statistical differences between the 4:1 and WB groups when water content was averaged over all samples (4:1, 73.2 ± 1.5%; WB, 71.1 ± 0.8%).
Figure 3.
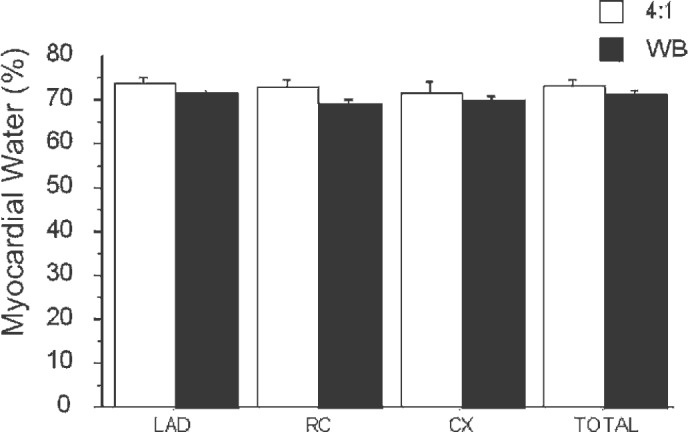
Myocardial water content (%).
LVM (Echocardiography)
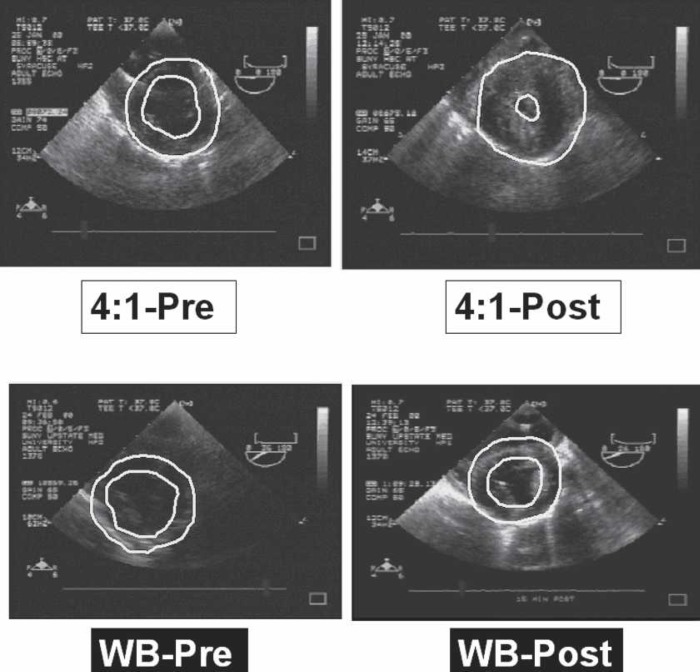
Baseline LVM in both groups was −97 g (Figure 4). This increased to >130 g in the WB group. However, an even greater increase in LVM was noted in the 4:1 group (168 g). Thus, postbypass LVM showed statistically significant differences between the 4:1 and WB groups (p < .05). This change represents a 73% increase in LVM in the 4:1 group compared with a 34% increase in the WB group. Illustrative echocardiographic still frames of pre- and postbypass hearts for both 4:1 and WB groups are shown in Figure 5.
Figure 4.
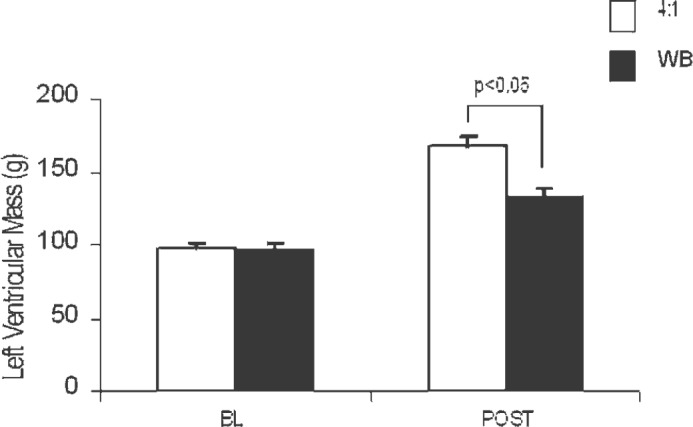
LVM, measured in grams. BL, baseline measurement before injury; POST, 15 minutes after weaning from bypass or failure to wean from bypass.
Figure 5.
Representative echocardiograms. Baseline echocardiograms before injury or bypass are designated “Pre,” and echocardiograms of the same heart taken after injury and plegia are designated “Post.”
Histologic Morphometrics
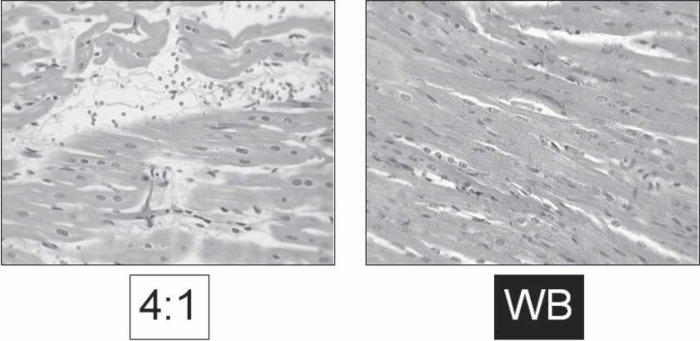
Random sections of myocardium were analyzed as outlined in the Materials and Methods section. Percentage of myocardium was significantly increased in the WB group (81.2 ± 0.9%) compared with the 4:1 group (75.2 ± 1.6%, p < .002; Table 1). The percentage of interstitial edema was significantly higher in the 4:1 group (21.6 ± 1.7%) compared with the WB group (15.5 ± 0.9%, p < .002). Illustrative histologic sections of both groups are shown in Figure 6.
Table 1.
Histologic morphometric data.
| WB | 4:1 | |
|---|---|---|
| Myocardium (%) | 81.2 ± 0.9 | 75.2 ± 1.6 |
| Edema (%) | 15.5 ± 0.9 | 21.6 ± 1.7 |
Figure 6.
Representative histology. Random sections from left ventricle on routine hematoxylin and eosin stain showing differences in myocardial edema.
Cardioplegia Volumes
Total cardioplegia volumes for the 4:1 and WB groups were similar (16,730 ± 1001 and 17,759 ± 475, respectively) and were not statistically significantly different. However, the crystalloid volume delivered in both groups was significantly different (4:1, 2875 ± 54; WB, 164 ± 8; p < .05). This represents 95% less crystalloid delivered to the heart in the WB group compared with the standard 4:1 cardioplegia.
DISCUSSION
The main findings of these experiments can be summarized as follows regarding the comparison of WB to 4:1 blood:crystalloid cardioplegia in our model. First, there was an increase in the number of hearts that survived to be weaned off bypass in those receiving WB cardioplegia (WB, 10/10; 4:1, 4/10). Second, postbypass LVM assessed by echocardiography was significantly decreased in hearts receiving WB cardioplegia. Third, myocardial edema, assessed by histologic morphometrics, was significantly decreased in hearts receiving WB cardioplegia. Fourth, the crystalloid volume of delivered cardioplegia was significantly decreased in hearts receiving WB cardioplegia. Taken together, these findings suggest that WB cardioplegia may be superior in some instances to 4:1 cardioplegia. Certainly in our model, there was a demonstration of decreased myocardial edema and a correlated increase in the number of hearts weaned of bypass.
There is wide variability and controversy among surgeons regarding cardioplegia and myocardial protection strategy. The spectrum ranges from those who use cold intermittent antegrade crystalloid cardioplegia to those who use warm continuous retrograde blood cardioplegia and various combinations in between. In general, results are excellent regardless of myocardial protection strategy used. There is room for improvement in higher risk patients, namely those who require more complex cardiac surgery that necessitates a longer cross-clamp time. There are clear advantages of blood cardioplegia compared with crystalloid cardioplegia in terms of improved oxygen delivery, decreased rates of infarction, and decreased creatinine phosphokinase-MB release (3). Furthermore, evidence from the CABG Patch Trial (Studying Prophylactic Implantable Cardioverter Defibrillators in Patients Who Are Having Ventricular Dysfunction and a Positive Signal-Averaged ECG) suggests that blood cardioplegia and combined antegrade and retrograde cardioplegia decreases postoperative morbidity compared with crystalloid and antegrade cardioplegia alone in a high-risk group of patients with an ejection fraction (EF) < 36% (12). At issue is whether further modifications of the standard 4:1 blood:crystalloid formula would further improve myocardial protection.
Menasché (3) described a method of diverting blood from the arterial circuit and adding only a small amount of arresting agents. The result was a dramatic reduction in the amount of crystalloid given with cardioplegia. He termed this technique miniplegia. There are several theoretic advantages to this approach. The technique itself is simple and likely to be cost effective. Using WB cardioplegia should avoid volume overload and minimize hemodilution, which may lead to decreased blood product use. Also, the increased number of blood cells present in WB cardioplegia should improve oxygen delivery. The combination of lower crystalloid volume and improved oxygen delivery should in theory lead to reduced myocardial edema and improved ventricular function (3). There is clear evidence that myocardial edema negatively impacts both systolic and diastolic ventricular function. Starr et al. (13) showed that a lower osmolarity cardioplegia solution is associated with higher myocardial water content and impaired diastolic filling. One goal of any myocardial protection strategy should be to minimize myocardial edema.
Our model represents a severe model of global normothermic myocardial ischemia meant to simulate a high-risk surgical patient requiring a long cross-clamp time. In lower-risk patients who require a shorter cross-clamp time, it is unlikely that there would be a significant difference between cardioplegia strategies. Our central hypothesis was that myocardial edema causes and exacerbates impaired ventricular function after an ischemic insult. Given this, we chose an experimental model, as described by Ericsson and Takeshima (8), which would provide a strong edemagenic insult. It is clear that a major ischemic insult will increase microvascular permeability and cause myocardial edema. The combination of this strong ischemic insult and a cardioplegia strategy that minimizes myocardial edema should, theoretically, accentuate any difference between WB and 4:1 cardioplegia.
In our model, we found that postbypass LVM and myocardial edema as assessed by histologic morphometrics were significantly decreased in hearts receiving WB cardioplegia. There was no difference in wet to dry ratios between the two groups, but this apparent contradiction is not unexpected because there is evidence that the relationship between myocardial water content and LVM is nonlinear. That is, a small potentially insignificant change in myocardial water content will correspond to a much larger change in LVM as seen on echocardiography (11).
There is clear evidence that myocardial edema negatively impacts both systolic and diastolic ventricular function. Excess myocardial fluid impairs both isovolumetric relaxation and chamber stiffness, the two phases of diastolic function (7). In a model of acute and chronic myocardial edema, Laine and Allen (5) showed that an elevation of myocardial extravascular fluid led to a 30% reduction in cardiac output. An important finding of that study was that the increased cardiac edema levels were not readily apparent without careful quantification and that only small changes in myocardial edema led to dramatic reductions in ventricular function. The edema levels in their study represented only a 3% change in myocardial water content and only a 12% change in heart weight. Furthermore, in a chronic model of pure myocardial edema, increased interstitial fluid accumulation led to increased myocardial interstitial collagen deposition and subsequent development of myocardial fibrosis (5). The postulated mechanism involves displacement and breaking of interstitial collagen secondary to increased interstitial volume and pressure that compromises ventricular function in the short term and leads to fibrosis in the long term (14).
There is evidence that myocardial edema can occur secondary to ischemia. There is also evidence that myocardial edema may exacerbate pre-existing ischemia. With accumulation of interstitial edema, the diffusion distance of oxygen to myocytes increases. This, coupled with the fact that the myocardium operates at near maximum oxygen extraction rates, can lead to an imbalance between oxygen supply and demand and resultant ischemia. Laboratory evidence that myocardial infarctions grow more rapidly in the presence of myocardial edema and left ventricular hypertrophy supports this proposed mechanism (15).
Limitations of this study relate to the severity of our experimental model. This is a very severe model of global ischemia that may in fact be too severe to highlight a difference between cardioplegia strategies in terms of hemodynamic function. There was an improvement in weaning in the WB cardioplegia group, but survival between the two groups was the same at 2 hours. This suggests that some element of irreversible ischemia occurred that limited the effectiveness of both cardioplegia strategies. By using weaning from CPB as a surrogate marker for ventricular function, we can say that WB cardioplegia transiently improved ventricular function, but this effect was short-lived, likely because of the severity of the ischemic insult.
In conclusion, despite equal cardioplegic time periods, WB cardioplegia was associated with a clear decrease in myocardial edema and improved weaning from CPB compared with standard 4:1 cardioplegia. As outlined, we hypothesize that the combination of reduced crystalloid volume and improved oxygen delivery caused by increased red blood cells in WB cardioplegia led to a decrease in myocardial edema. This in turn improved ventricular function and minimized the ischemic insult, leading to improved weaning from CPB. Taken together, these findings suggest that WB cardioplegia may be superior to blood: crystalloid cardioplegia in procedures where a long cross-clamp time is anticipated. Future studies involving a less severe ischemic model and a longer postbypass observation period could better elucidate differences between the cardioplegic strategies.
ACKNOWLEDGMENTS
We gratefully acknowledge Quest Medical for the use of the MPS cardioplegia delivery system and Andrew Paskanik for expert technical assistance.
REFERENCES
- 1.Cohen G, Borger MA, Weisel RD, et al. Intraoperative myocardial protection: current trends and future perspectives. Ann Thorac Surg. 1999;68:1995–2001. [DOI] [PubMed] [Google Scholar]
- 2.Christakis GT, Ivanov J, Weisel RD, et al. The changing pattern of coronary artery bypass surgery. Circulation. 1989;80(suppl I):I-151–61. [PubMed] [Google Scholar]
- 3.Menasché P.. Blood cardioplegia: do we still need to dilute? Ann Thorac Surg. 1996;62:957–60. [DOI] [PubMed] [Google Scholar]
- 4.Menasché P, Touchot B, Pradier F, et al. Simplified method for delivering normothermic blood cardioplegia. Ann Thorac Surg. 1993;55:177–8. [DOI] [PubMed] [Google Scholar]
- 5.Laine GA, Allen SJ.. Left ventricular myocardial edema: lymph flow, interstitial fibrosis, and cardiac function. Circ Res. 1991;68:1713–21. [DOI] [PubMed] [Google Scholar]
- 6.Spotnitz HM, Hsu DT.. Myocardial edema: importance in the study of left ventricular function. Adv Cardiac Surg. 1994;5:1–25. [PubMed] [Google Scholar]
- 7.Geissler HJ, Allen SJ.. Myocardial fluid balance: pathophysiology and clinical implications. Thorac Cardiovasc Surg. 1998;46(suppl):242–7. [DOI] [PubMed] [Google Scholar]
- 8.Ericsson AB, Takeshima SV.. Simultaneous antegrade and retrograde delivery of continuous warm blood cardioplegia after global ischemia. J Thorac Cardiovasc Surg. 1998;115:716–22. [DOI] [PubMed] [Google Scholar]
- 9.Catinella FP, Cunningham JN, Spencer F.. Myocardial protection during prolonged aortic cross-clamping. J Thorac Cardiovasc Surg. 1984;88:411–23. [PubMed] [Google Scholar]
- 10.Wyatt HL, Heng MK, Meerbaum S, Hestenes JD, Cobo JM, Davidson RM, Corday E.. Cross-sectional echocardiography: I. Analysis of mathematical models for quantifying mass of the left ventricle in dogs. Circulation. 1979;60:1104. [DOI] [PubMed] [Google Scholar]
- 11.Weyman AE.. Principles and Practice of Echocardiography, 2nd ed. Philadelphia, PA: Lippincott Williams Wilkins; 1994. [Google Scholar]
- 12.Flack JE, Cook JR, May SJ, et al. Does cardioplegia type affect outcome and survival in patients with advanced left ventricular dysfunction? Results from the CABG patch trial. Circulation. 2000;102(suppl III):III-84–9. [DOI] [PubMed] [Google Scholar]
- 13.Starr JP, Jia C, Amirhamzeh MMR, et al. Coronary perfusate composition influences diastolic properties, myocardial water content, and histologic characteristics of the rat left ventricle. Ann Thorac Surg. 1999;68:925–30. [DOI] [PubMed] [Google Scholar]
- 14.Capasso JM, Robinson TF, Anversa P.. Alterations in collagen cross-linking impair myocardial contractility in the mouse heart. Circ Res. 1989;65:1657–64. [DOI] [PubMed] [Google Scholar]
- 15.Dellsperger K. Clothier J, Hartnett J, Haun L, Marcus M.. Acceleration of the wavefront of myocardial necrosis by chronic hypertension and left ventricular hypertrophy in dogs. Circ Res. 1988;63:87–96. [DOI] [PubMed] [Google Scholar]