Abstract:
The purpose of this double-blind prospective and randomized study was to examine the effects of surface-modifying additives (SMAs) and poly-2-methoxyethylacrylate (PMEA) circuits on platelet count, platelet function (Sonoclot), postoperative chest tube drainage volume, peri- and postoperative blood product use, extubation time, and intensive care time. Terumo noncoated, Terumo-coated (PMEA), Cobe noncoated, and Cobe coated (SMA) circuits were evaluated to find the most cost-effective way to improve patient outcomes. We aimed to find if an additional charge for a coated CPB circuit would be recovered by reducing other patient costs (blood transfusions, intensive care unit time, and bring back postoperative bleeding). An initial literature review revealed the comparison of PMEA circuits vs. noncoated circuits and SMA circuits vs. noncoated circuits in both adult and porcine models. Both SMA- and PMEA-coated circuits decreased platelet consumption, platelet factor release, and the overall perioperative inflammatory response while on cardiopulmonary bypass (CPB). The question not answered in an initial search was simply, “which coated circuit is best for the patient: SMA or PMEA?” Research comparing the above coated circuits each other was not found. The study was approved by the Institutional Review Board. Thirty patients were scheduled for elective coronary artery bypass grafting and/or valvular repair or replacement surgery. These 30 patients were randomized as 10 patients to Terumo X-Coating (PMEA surface coating) (CT), 10 patients to Cobe Smart-X coating (SMA surface coating; CC), 5 patients to Terumo noncoated tubing (NCT), and 5 patients to Cobe noncoated tubing (NCC). Informed consent was obtained from each patient before surgery. The data showed no statistically significant relationship between platelet counts, platelet function (Sonoclot), postoperative chest tube drainage volume, peri- and postoperative blood products, intensive care unit time, or total hospital length of stay. Analysis revealed statistically significant clinical associations of extubation time and protamine dose with treatment group. This study provided evidence that SMA- and PMEA-coated circuits do not improve platelet consumption or decrease blood product use for patients undergoing CPB. There was statistical significance with a reduction in extubation time and total protamine requirement needed to return activated clotting time (ACT) to baseline post-CPB. Although the use of SMA and/or PMEA circuits during CPB has clinical benefit to the CPB patient, an additional charge for the specialty circuit may not be realized.
Keywords: cardiopulmonary bypass, biocompatible circuits, poly-2-2methoxyethylacrylate, surface-modifying additives
Cardiopulmonary bypass (CPB) damages blood components and activates the coagulation cascade during cardiac surgery. When blood contacts a synthetic surface during CPB, plasma proteins quickly absorb into the biomaterial surface (1). Continued exposure results in contact activation of the blood. This increases the patient’s need for peri- and postoperative blood products (2).
Surface-modifying additives (SMAs) have been incorporated into the polymer used to prepare circuits for CPB. SMA, triblock copolymer with both polar and nonpolar polymer chains, is the additive that migrates to the surface of the tubing during the manufacturing process. This creates alternating hydrophobic and hydrophilic chains, which is hypothesized to bind all sites for potential platelet activation (2–4). SMA-coated CPB circuits reduce complement activation, reduce platelet consumption, and inhibit platelet factor release (5,6).
Poly-2-methoxyethylacrylate (PMEA) is another biocompatible surface for CPB. It is composed of a hydrophobic polyethylene backbone that adheres to the surface and a hydrophilic blood-contacting layer. The hydrophilic layer swells when blood contacts it, creating a water-filled boundary layer that maintains protein conformation and prevents surface activation. PMEA-coated bypass circuits reveal excellent biocompatibility and exhibit better suppression of perioperative inflammatory response (7–9).
Although coated circuits have been compared to a non-coated control CPB circuit, no previous comparison was found for SMA and PMEA coatings. A gap in the literature probably exists because PMEA coating is a relatively new biomaterial. An in vivo comparison of SMA and PMEA coating in addition to control groups for both coatings might reveal a superior platelet-protecting surface and/or a decreased postoperative blood loss (10,11).
MATERIALS AND METHODS
Study Outline
Thirty patients were scheduled for elective coronary artery bypass grafting and/or valvular repair or replacement surgery. Of these 30 patients, four groups of patients were compared: 10 patients Terumo X-Coating (PMEA surface coating), 10 patients Cobe Smart-X coating (SMA surface coating), 5 patients Terumo noncoated tubing, and 5 patients Cobe non-coated tubing. The patients were randomly allocated into this double-blind study.
Patient Criteria
Excluded from the study were patients less than 19 years of age, patients with platelet counts less than 50,000 platelets/μL, creatinine greater than 3.0 mg/dL, and a blood urea nitrogen (BUN) level greater than 40 mg/dL, and patients who have used the following antiplatelet drugs within the specified amount of time: Plavix (clopidogel), 7 days; Neop, 8 hours; Ticld (ticlopidine), 10 days. Informed consent was obtained from each patient before surgery. The study was approved by the Institutional Review Board.
Circuit
The uncoated Terumo circuit (NCT) consisted of a hard-shell reservoir and oxygenator (Capio SX 18; Terumo Cardiovascular, Ann Arbor, MI), Capiox arterial line filter (Terumo), and polyvinyl chloride tubing (Terumo). The X-coated Terumo circuit (CT) was identical with PMEA coating throughout the bypass circuit, excluding cannulas and cannula connectors.
The uncoated Cobe circuit (NCC) consisted of a hard-shell reservoir with integrated membrane oxygenator (Monolyth integrated membrane lung; Sorin Biomedical, Irvine, CA), Sorin 40-/m arterial line filter (Sorin Biomedical), and polyvinyl chloride tubing. The Smart-X Cobe circuit (CC) was identical with SMA coating throughout the bypass circuit, excluding cannulas and cannula connectors.
Procedure
Each circuit was primed in the usual fashion with 1100 to 1300 mL Plasmalyte A, 10,000 units porcine heparin sodium, 25 mEq mannitol, 50 mEq sodium bicarbonate, and 100 mL 25% albumin. Arterial blood gases and venous saturations were monitored with the CDI 500 (3M Health Care, Minneapolis, MN). The patients hematocrits were maintained greater than 22%. The patients were heparinized to achieve an activated clotting time (ACT) greater than 480 seconds. During bypass, the pump flow was set at 2.4 L/m2/min, and patients were maintained at a temperature of 37°C. Protamine sulfate was administered to return ACT to baseline. After termination of CPB, the residual volume in the extracorporeal circuit was collected in the cell-saver, washed, and returned to the patient.
Sample Analysis
Arterial blood samples were taken on six occasions: before the start of surgery (S1), 3 minutes after heparin administration (S2), after the start of CPB after the cross-clamp was in place (S3), 1 hour on CPB (S4), 3 minutes after protamine (S5), and after arriving in the intensive care unit (ICU) (S6). S1 and S5 samples were analyzed using ACT (Actalyke; Array Medical, Bridgewater, NJ), as well as platelet function study using the Sonoclot (Sienco, Wheat Ridge, CO). Pre- and postoperative complete blood counts and chemistry panel tests were drawn. Arterial blood gas, electrolyte panel, platelet count, and hematocrit were drawn at S1, S3, S4, S5, and S6.
Clinical Data
Postoperative chest drainage volume, blood products administration, total time on CPB, length of stay in the ICU and hospital, extubation time, operative mortality within 30 days, and incidence of complications were analyzed.
Statistical Methods
The Kruskal-Wallis test was used to compare continuous outcomes between the four groups. Fisher exact test was used to compare categorical variables. p values less than 0.05 are considered to be statistically significant.
RESULTS
There were no statistically significant differences in pre-operative patient characteristics of sex, age, body weight, or height. All groups had similar characteristics of heparin dose, CPB, and cross-clamp times, and use of Amicar or aprotinin.
Median hemoglobin levels are displayed in Figure 1. In all groups, the hemoglobin value decreased from the start of CPB (S3) and remained decreased in the ICU (S6). No significant intergroup differences were found.
Figure 1.
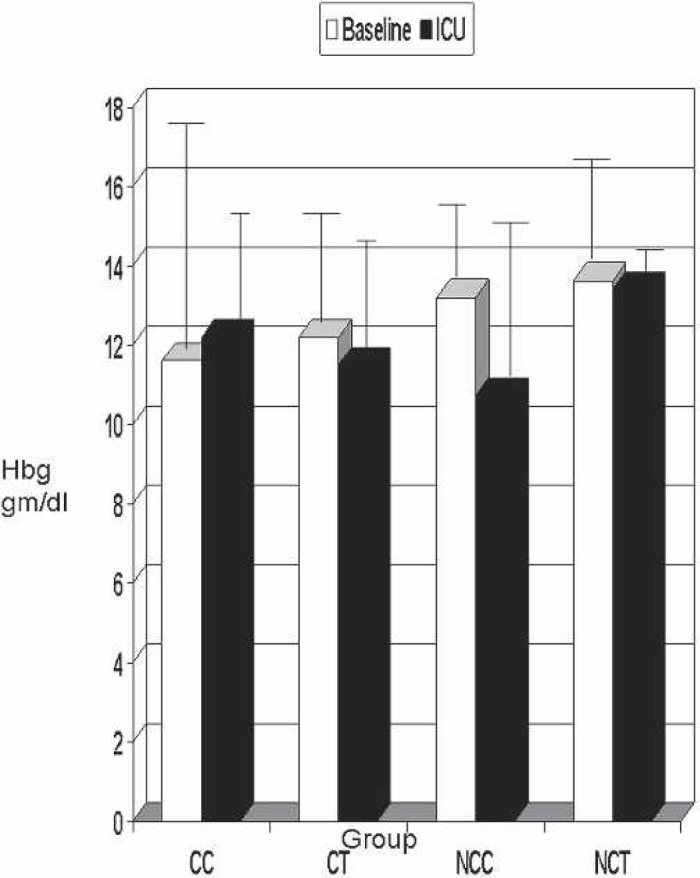
Baseline vs. ICU hemoglobin. Hbg, hemoglobin; CC, coated Cobe; CT, coated Terumo; NCC, noncoated Cobe; NCT, noncoated Terumo.
Results of the platelet count are shown in Figure 2. When all groups were compared, the platelet count showed a decrease after CPB (S3) and remained decreased in the ICU (S6) (Figure 2). The patients not receiving platelets and packed red blood cells peri- or post-operatively are shown in Figure 3. Again, platelet counts decreased from baseline to in the ICU in all groups. The CC group decreased platelets from only 213 to 180 × 103/μL, whereas the CT group decreased platelets 285 to 127 × 103/μL.
Figure 2.
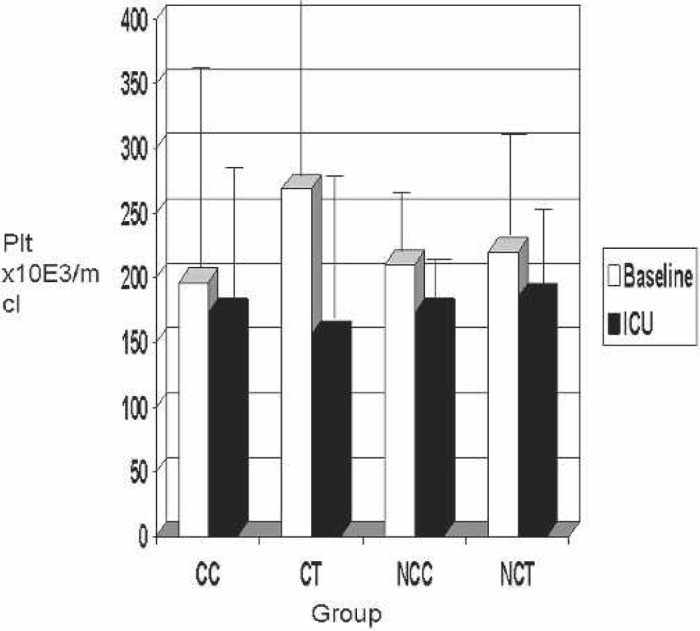
Baseline vs. ICU platelet counts. Plt, platelet count; CC, coated Cobe; CT, coated Terumo; NCC, noncoated Cobe; NCT, non-coated Terumo.
Figure 3.
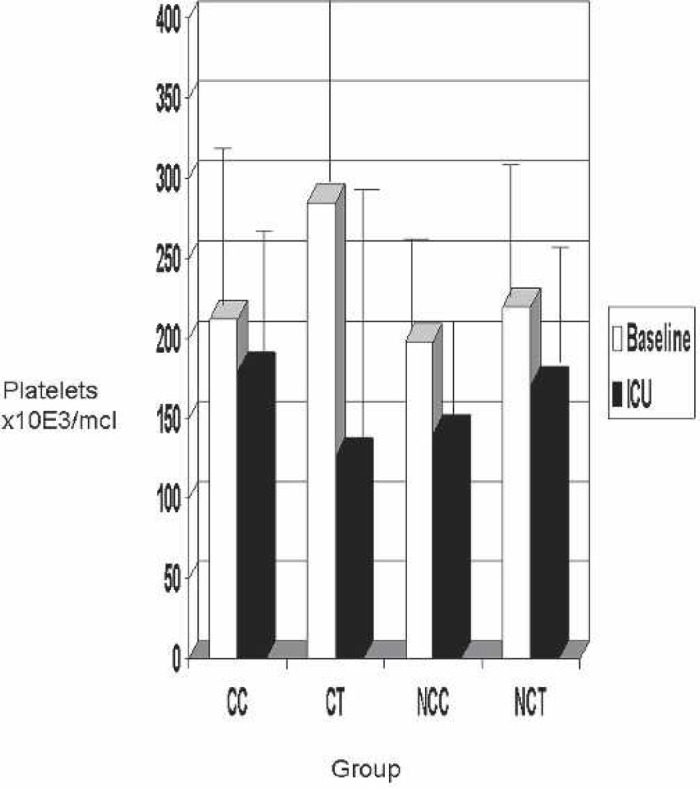
Baseline vs. ICU platelet counts in patients not transfused with platelets. Plt, platelet count; CC, coated Cobe; CT, coated Terumo; NCC, noncoated Cobe; NCT, noncoated Terumo.
The clot rate (S1 and S5) and platelet function (S1 and S5) are shown for each group in Figures 4 and 5. Normal clot rate is 7 to 25 clot signal units/min. Clot rates in all groups were within range. The platelet function on native whole blood in a normal population with no heparin administered was greater than 1.2. Although the post-CPB clot rate in the CC and CT groups tended to be more similar to base clot rate in both groups, no statistical significance was found (baseline, p = 0.15; post-CPB, p = 0.67).
Figure 4.
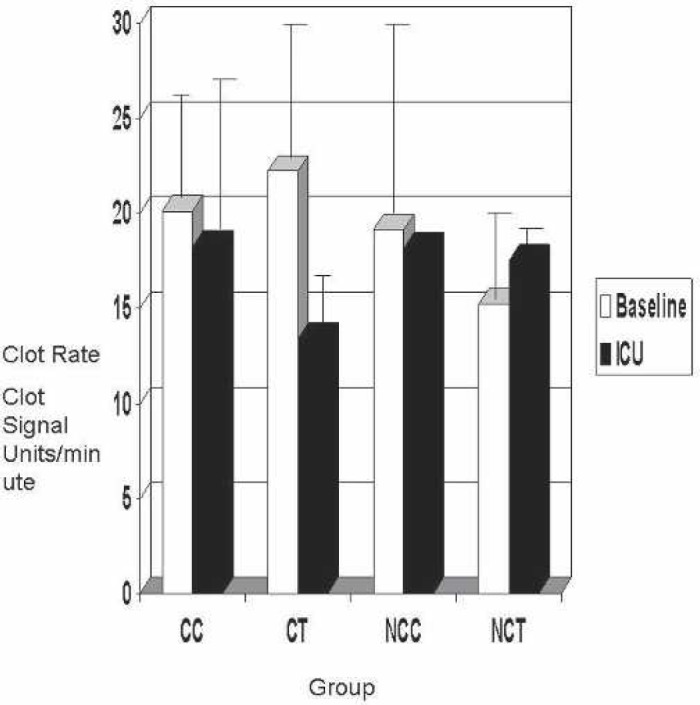
Baseline vs. ICU Sonoclot clot rate. CC, coated Cobe; CT, coated Terumo; NCC, noncoated Cobe; NCT, noncoated Terumo.
Figure 5.
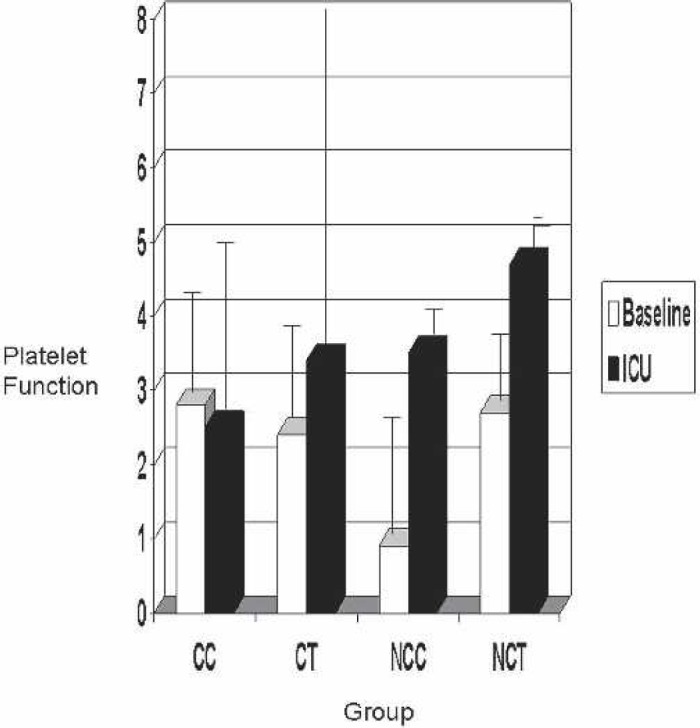
Baseline vs. ICU Sonoclot platelet function. CC, coated Cobe; CT, coated Terumo; NCC, noncoated Cobe; NCT, noncoated Terumo.
Median total chest drainage volumes 6 hours after surgery are shown in Figure 6. Although the volume tended to be lower in the CC and NCC groups, the difference did not reach statistical significance (p = 0.075).
Figure 6.
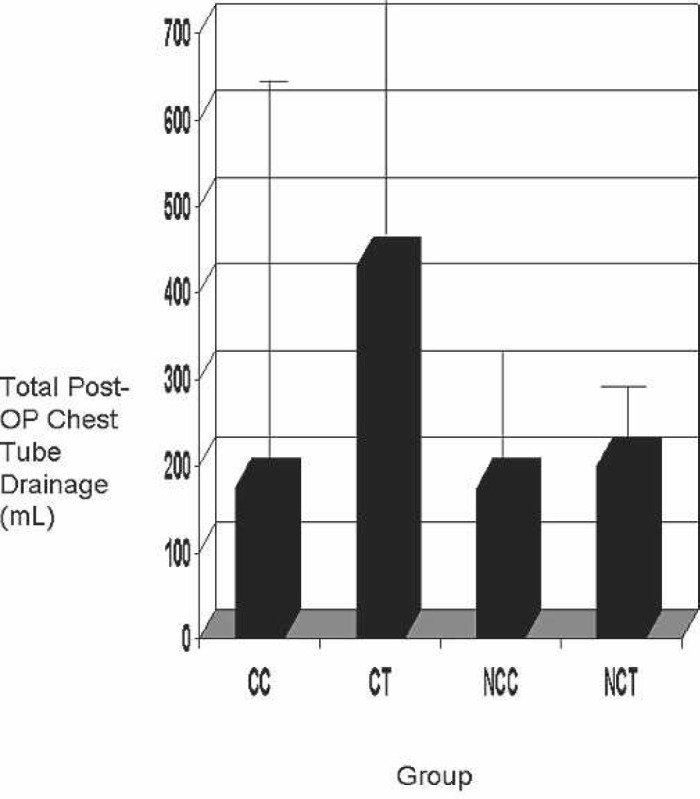
Postoperative chest tube drainage. Op, operative; CC, coated Cobe; CT, coated Terumo; NCC, noncoated Cobe; NCT, non-coated Terumo.
The extubation time is shown in Figure 7. The NCT group had the greatest percentage (100%) of patients extubated in less than 8 hours. The CC group followed next with only 50% of the patients extubated in less than 8 hours. Fewer patients in the NCC (20%) and CT (22%) groups were extubated in less than 8 hours. This was statistically significant (p = 0.025).
Figure 7.
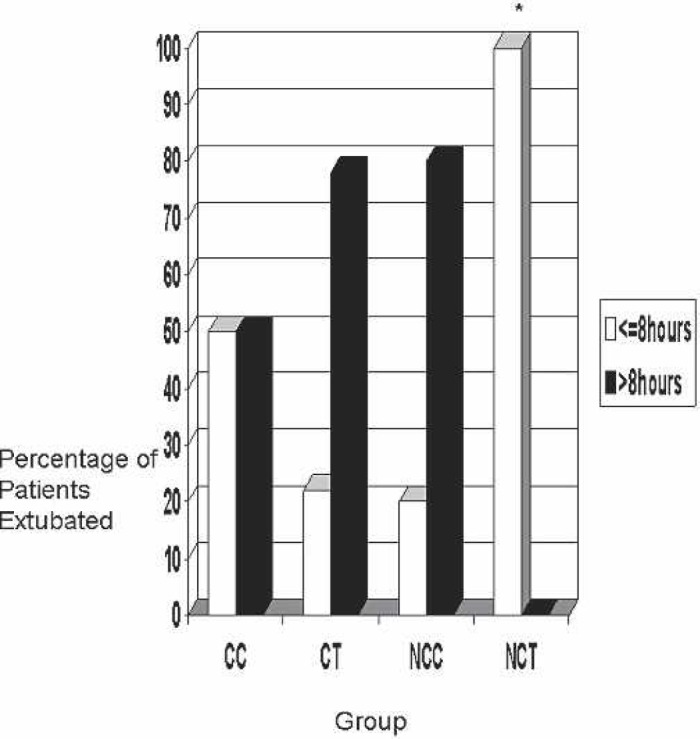
Patient extubation time. CC, coated Cobe; CT, coated Terumo; NCC, noncoated Cobe; NCT, noncoated Terumo.
The ICU median stay is shown in Figure 8. With a p = 0.56, no statistical significance was found.
Figure 8.
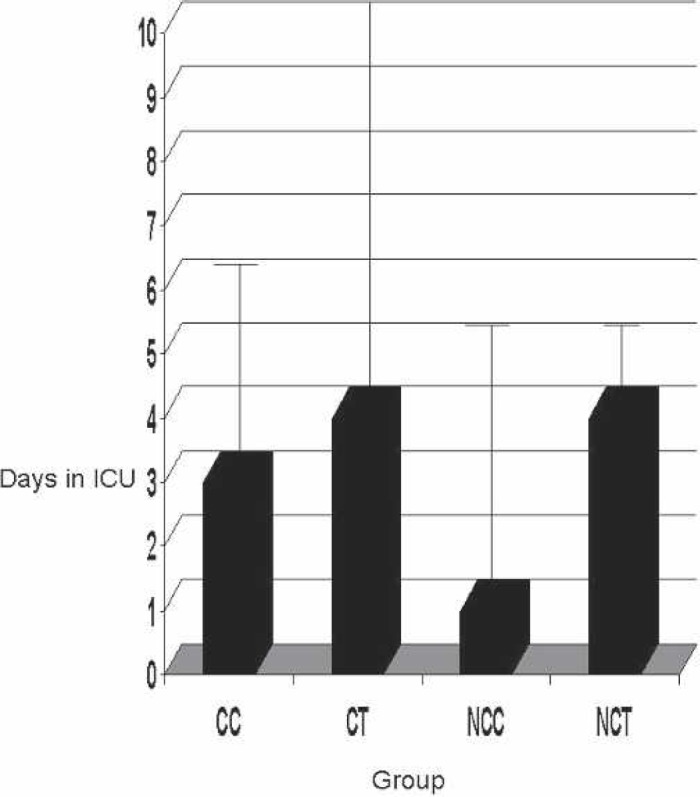
ICU stay. CC, coated Cobe; CT, coated Terumo; NCC, non-coated Cobe; NCT, noncoated Terumo.
Figure 9 shows the length of hospital stay for the patients in each group. The median length was shown to be lowest in the NCC group and greatest in the NCT group. However, no statistical significance was found (p = 0.39).
Figure 9.
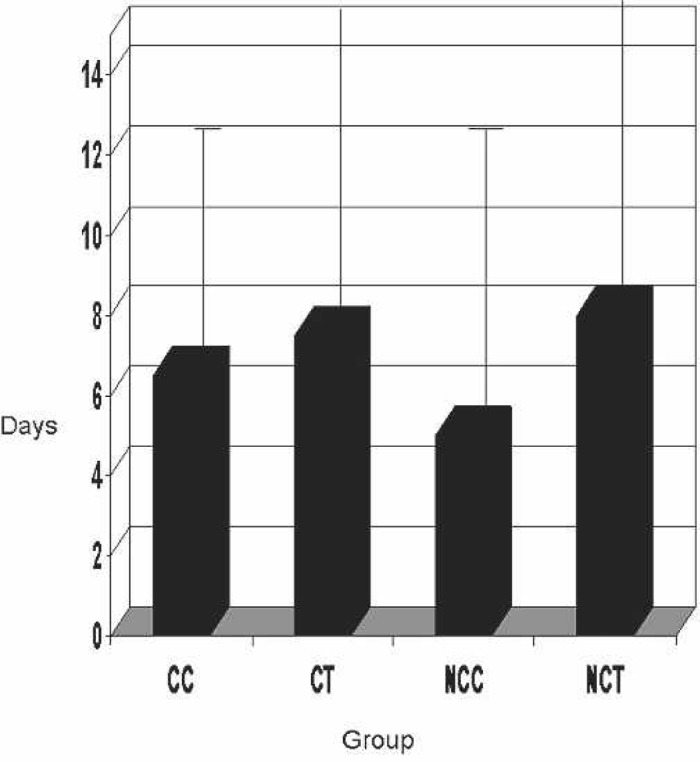
Patient length of stay in hospital. CC, coated Cobe; CT, coated Terumo; NCC, noncoated Cobe; NCT, noncoated Terumo.
Protamine sulfate reversal dose is shown in Figure 10. A statistically significant value (p = 0.027) was found. A greater amount of protamine was given in both the CT (365 mg) and CC (325 mg) groups. Figure 10 also shows the total porcine heparin given throughout CPB. No statistical significance was found (p = 0.33). Although no more heparin was given in the CC and CT groups, a significantly greater amount of protamine was given for reversal of heparin.
Figure 10.
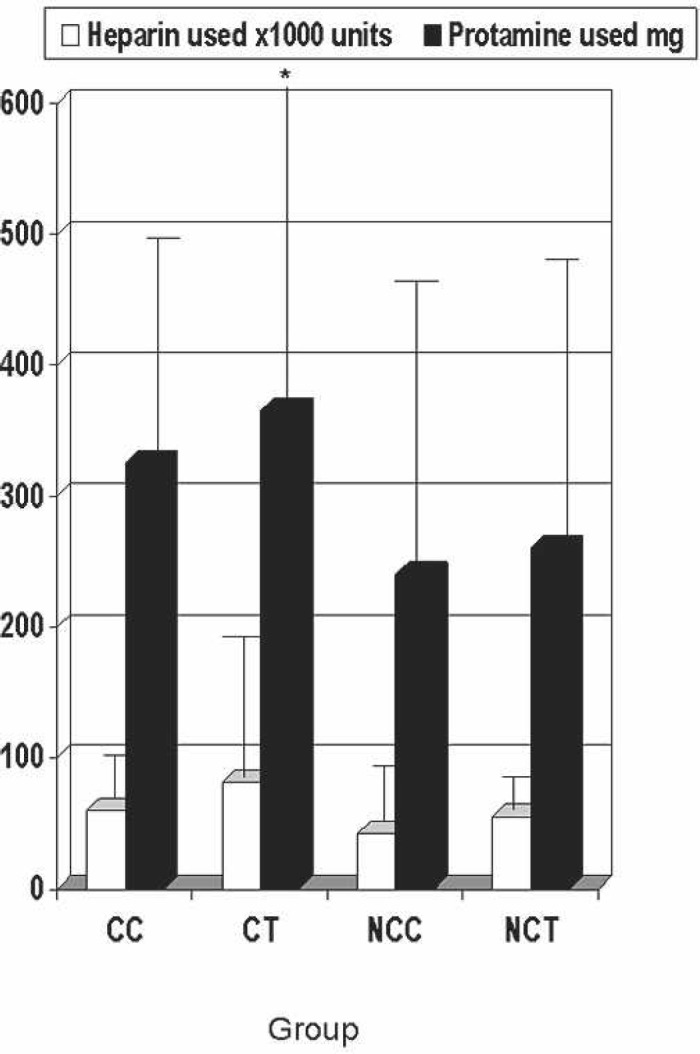
Protamine and heparin used. CC, coated Cobe; CT, coated Terumo; NCC, noncoated Cobe; NCT, noncoated Terumo.
Biocompatible circuits used in our institution were not found to reduce postoperative chest drainage, blood products administration, length of stay in the ICU, or hospital length of stay. Statistically significant differences were found in the dose of protamine sulfate and extubation time.
DISCUSSION
Exposure to foreign surfaces leads to activation of the clotting cascade and coagulation using essential blood components. Although heparin-treated circuits have shown decreased complement activation, platelet activation, and reduced postoperative blood loss, a second generation of biocompatible surfaces has been developed for CPB (11).
SMA, a triblock copolymer with polar and nonpolar polymer chains, was found to decrease platelet activation, decrease B-thromboglobulin, and decrease thrombin generation (12).
An even more physiologically biocompatible circuit was created with a hydrophobic background and a hydrophilic surface. PMEA treatment created a structure that forms a molecular mesh. Compared with uncoated circuits, PMEA-coated circuits have shown decreased platelet absorption and plasma proteins related to fibrinolysis and complement activation (13–15).
In an effort to make CPB more biocompatible, treated surface circuits have been used. The circuits in this study used SMA, PMEA, and uncoated models to examine blood product use, postoperative chest tube drainage, extubation, ICU time, and hospital length of stay. This study showed that SMA- and PMEA-coated circuits do not improve platelet consumption or decrease blood product use for patients undergoing CPB. There was statistical significance with a reduction of extubation time and total protamine requirement for heparin reversal. Although the use of SMA and/or PMEA circuits during CPB has clinical benefit to the patient with CPB, an additional charge for the specialty circuit may not be realized. Further study on a larger patient population may be indicated to evaluate whether SMA or PMEA circuits contribute to the improvement of clinical outcomes.
REFERENCES
- 1.Gravlee GP, Davis RF, Kurusz M, Utley JR.. Cardiopulmonary Bypass Principles and Practices. Philadelphia, PA: Lippincott Williams and Wilkins; 2000. [Google Scholar]
- 2.Rubens FD.. Cardiopulmonary bypass technology transfer: musings of a cardiac surgeon. J Biomater Sci Polymer Educ. 2002;13:485–99. [DOI] [PubMed] [Google Scholar]
- 3.Colman RW.. Platelet and neutrophil activation in cardiopulmonary bypass. Ann Thorac Surg. 1990;49:32–4. [DOI] [PubMed] [Google Scholar]
- 4.Wenger RK, Lukasiewicz H, Mikuta S, Niewiarowski S, Edmunds LH Jr.. Loss of platelet fibrinogen receptors during clinical cardiopulmonary bypass. Ann Thorac Surg. 1989;97:235–9. [PubMed] [Google Scholar]
- 5.Defraigne JO, Pincemail J, Dekoster G, et al. SMA circuits reduce platelet consumption and platelet factor release during cardiac surgery. Ann Thorac Surg. 2000;70:2075–81. [DOI] [PubMed] [Google Scholar]
- 6.Gu YJ, Boonstra PW, Rijnsburger AA, Haan J, van Oeveren W.. Cardiopulmonary bypass circuit treated with surface-modifying additives: a clinical evaluation of blood compatibility. Ann Thoracic Surg. 1998;65:1342–7. [DOI] [PubMed] [Google Scholar]
- 7.Ninomiya M, Miyaji K, Takamoto S.. Influence of PMEA-coated bypass circuits on perioperative inflammatory response. Ann Thorac Surg. 2003;75:913–7. [DOI] [PubMed] [Google Scholar]
- 8.Ikuta T, Fujii H, Shibata T, et al. A new poly-2-methoxyethylacrylate-coated cardiopulmonary bypass circuit possesses superior platelet preservation and inflammatory suppression efficacy. Ann Thorac Surg. 2004;77:1678–83. [DOI] [PubMed] [Google Scholar]
- 9.Suhara H, Sawa Y, Nishimura M, et al. Efficacy of a new coating material PMEA, for cardiopulmonary bypass circuits in a porcine model. Ann Thorac Surg. 2001;71:1603–8. [DOI] [PubMed] [Google Scholar]
- 10.Vijay V, McCusker K.. Recent advances in biocompatible surface-modifying additives for cardiopulmonary bypass. Perfusion. 2003;18:41–5. [DOI] [PubMed] [Google Scholar]
- 11.Aldea GS.. Heparin bonded circuits decrease thromboembolitic complications and improve outcomes in patients undergoing coronary artery bypass graft surgery. Semin Cardiothorac Vasc Anesth. 1999;3:9–16. [Google Scholar]
- 12.Rubens FD, Labow RS, Lavillee GR, et al. Hematologic evaluation of cardiopulmonary bypass circuits prepared with a novel block copolymer. Ann Thorac Surg. 1999;67:689–96. [DOI] [PubMed] [Google Scholar]
- 13.Anzai T, Okumura A, Kawaura M, et al. Evaluation of the biocompatibility of an in vitro study test using poly2-methoxyethylacrylate coated oxygenator. Jpn J Artif Organs. 2000;29:73–9. [Google Scholar]
- 14.Tanaka M, Motomura T, Kawada M, et al. Blood compatible aspects of poly (2-methoyethylacylate) (PMEA) relationship between protein absorption and platelet adhesion on PMEA surface. Biomaterials. 2000;21:1471–81. [DOI] [PubMed] [Google Scholar]
- 15.Saito N, Motoyama S, Sawamoto J.. Effects of new polymer-coated extracorporeal circuits on biocompatibility during cardiopulmonary bypass. Artif Organs. 2000;24:547–54. [DOI] [PubMed] [Google Scholar]


