Abstract:
Postcardiotomy failure requiring ventricular assist occurs in about 1% of adult patients undergoing cardiac surgical procedures. One method of support is a short-term ventricular assist device. This incurs the cost of the device, which is substantial, and allows for reduced anticoagulation in the first 24 hours. Another option is a heparin-coated extracorporeal membrane oxygenation (ECMO) circuit. This also allows for reduced anti-coagulation and can support the lungs if necessary. The use of a heparin-coated ECMO circuit requires 24-hour monitoring, but the cost of disposables is considerably less than the cost of ventricular assist devices. This decision analysis uses a Markov model to evaluate the relative outcomes and costs associated with selection between these modalities of support. Data from the past 5 years of patients who received postcardiotomy support will be used to develop the Markov model. The hypothesis is that supporting the patient on heparin-coated ECMO before instituting ventricular assistance will reduce cost and allocate resources in a more cost-effective manner. The model was used to determine the optimal economic time for initiation of ventricular assist devices in postcardiotomy patients. The total costs associated with support begin to level out between postoperative days 6 and 10 using an Abiomed BVS5000 ventricular assist device. The largest decline in costs occurs after postoperative day 3. This model suggests that patients should be supported on heparin-coated ECMO for 2–3 days to evaluate their potential for recovery before instituting more expensive ventricular assist devices.
Keywords: extracorporeal membrane oxygenation, ventricular assist device, cost benefit analysis, Markov model
Ventricular failure occurs in 2% to 6% of patients after cardiac surgical repair (1). Postcardiotomy cardiogenic shock (PCS) is usually manifested as a failure to initially wean from cardiopulmonary bypass. Many of these patients respond favorably to inotropic support and intra-aortic balloon pumping. Despite aggressive management, 1% of these patients will require additional ventricular assistance (2).
Two options for PCS support are extracorporeal membrane oxygenation (ECMO) using a heparin-coated emergency bypass circuit or a ventricular assist device (VAD). Although both modalities can provide support for ventricular failure, the device decision is often based on factors such as coagulation status, pulmonary vascular resistance, and the presence of biventricular failure. In addition, the cost of support can also become a factor if the patient outcomes of the two modalities are similar.
The purpose of this study was to develop a model to look at the costs associated with ECMO and ventricular assist in the postcardiotomy patient. A Markov model was used for statistical evaluation of the support methods. Developed by Andrey Markov, a Russian mathematician, Markov models are used in decision analysis to accurately represent complex processes that involve transition in and out of various states of health. The two different types of states are transition states, also called health states, and absorbing states. Patients are able to move in and out of the transition states freely, but once they enter an absorbing state such as death, they remain there. The steps that construct a Markov model can be seen in Figure 1.
Figure 1.

Outline of steps for completing a Markov model.
As device costs increase, the timing of VAD insertion is critical to maximizing the use of these devices. The aim of this study was to identify the postoperative day of support when the switch from ECMO to a VAD provides the most economic benefit.
MATERIALS AND METHODS
After Institutional Review Board approval, patients receiving postcardiotomy support at the Medical University of South Carolina during the last 5 years were identified through a review of surgical records and the Society of Thoracic Surgeons (STS) database. A cohort of 17 patients matched the criteria for postoperative support. The patient’s charts were reviewed to identify the type of support, the etiology of ventricular failure, length of support, and the outcome. Once the patient data was collected, a Markov model was developed to determine the probabilities for the various outcomes.
The transition states in the current model are ECMO, VAD, and biventricular assist device (BIVAD). The absorbing or final states in the model are either wean from support or death (Figure 2). Patients were entered into the model for each day of support and were tracked by their movement through the transitional states to the absorbing states (Table 1). The transitional probabilities for each state were estimated based on the patient database (Table 2). The model was carried out through day 47 (longest period of support), but cost analysis was preformed on days 1 through 12 because the majority of patient movement between the various states takes place during this time period.
Figure 2.
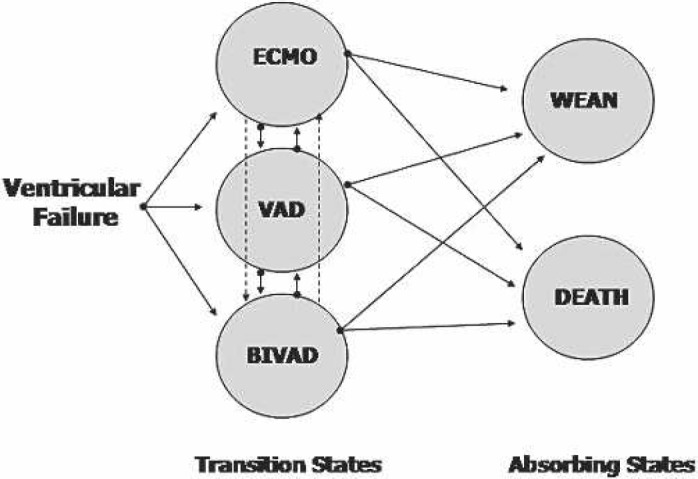
Example of possible patient movement directions for the applied Markov model.
Table 1.
Patient movement days 1–12.
| Day | ECMO | VAD | BIVAD | WEAN | Death | Total |
|---|---|---|---|---|---|---|
| 1 | 5 | 5 | 5 | 0 | 2 | 17 |
| 2 | 4 | 5 | 5 | 0 | 3 | 17 |
| 3 | 4 | 5 | 5 | 0 | 3 | 17 |
| 4 | 4 | 4 | 6 | 0 | 3 | 17 |
| 5 | 4 | 4 | 6 | 0 | 3 | 17 |
| 6 | 5 | 3 | 4 | 1 | 4 | 17 |
| 7 | 5 | 3 | 3 | 1 | 5 | 17 |
| 8 | 4 | 3 | 3 | 2 | 5 | 17 |
| 9 | 3 | 3 | 2 | 3 | 6 | 17 |
| 10 | 1 | 3 | 2 | 4 | 7 | 17 |
| 11 | 1 | 3 | 1 | 5 | 7 | 17 |
| 12 | 0 | 3 | 1 | 6 | 7 | 17 |
Table 2.
Calculated transitional probabilities.
| ECMO | VAD | BIVAD | Wean | Death | |
|---|---|---|---|---|---|
| ECMO | 0.58 | 0.00 | 0.00 | 0.34 | 0.08 |
| VAD | 0.00 | 0.95 | 0 | 0.02 | 0.029 |
| BIVAD | 0.00 | 0.00 | 0.58 | 0.15 | 0.27 |
| Wean | 1.00 | 0 | |||
| Death | 1.00 |
This model uses either the Abiomed BVS5000 or the AB5000 VAD (Abiomed, Danvers, MA). The cost for the device was based on the manufacturer’s list price. The Abiomed costs were limited to the cost of the device itself. ECMO costs were defined as the cost of the circuit plus the labor associated with 24-hour monitoring. Labor cost was calculated at a rate of $50.00/h or $1200 for each 24-hour period (Table 3). No additional blood pumps or circuits were allotted for change out. The majority of patients had a balloon pump inserted during the weaning attempts, but this was not included in any part of the cost model.
Table 3.
Device and labor cost.
| BVS5000 | AB500 | ECMO | |
|---|---|---|---|
| VAD | 18,000 | 45,000 | — |
| BIVAD | 36,000 | 90,000 | — |
| ECMO | 19,000 | 46,000 | 1000 |
| Labor | 0 | 0 | 1200 |
On day 1, the model showed the estimated cost based on the actual treatment modality of the study patients. However, the model on day 2 began with all patients being placed on ECMO for day 1, and the patients in the two VAD groups transitioned to either univentricular support (VAD) or biventricular (BIVAD) support on day 2 based on the previously derived transitional probabilities. Day 3 began with all patients being place on ECMO for the first 2 days, with the VAD groups transitioned to either VAD or BIVAD on day 3. This trend was carried out until day 12.The patient distribution, transitional probabilities, and associated cost with each treatment modality were combined to produce the cost of treatment per day. With each successive day that the model was carried out, the cost of support was adjusted using the probability of patient survival and transition for that day (Table 4). The total cost of treatment was divided by the overall survival rate to calculate a cost per survivor.
Table 4.
Cost effectiveness analysis.
| Day 1 | VAD on Day 2 | VAD on Day 3 | VAD on Day 4 | VAD on Day 5 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | ECMO | VAD | Labor | ECMO | VAD | Labor | ECMO | VAD | Labor | ECMO | VAD | Labor | ECMO | VAD |
| 18,000 | 5,000 | 306,000 | 18,000 | 15,000 | 0 | 18,000 | 15,000 | 0 | 18,000 | 15,000 | 0 | 18,000 | 15,000 | 0 |
| 16,800 | 0 | 4,800 | 0 | 270,000 | 16,800 | 0 | 0 | 16,800 | 0 | 0 | 16,800 | 0 | 0 | |
| 11,988 | 0 | 2,784 | 0 | 2,784 | 0 | 190,260 | 11,988 | 0 | 0 | 11,988 | 0 | 0 | ||
| 9,094 | 0 | 1,615 | 1,615 | 1,615 | 0 | 142,462 | 9,094 | 0 | 0 | |||||
| 7,317 | 0 | 937 | 937 | 937 | 937 | 0 | 113,263 | |||||||
| 6,192 | 0 | 543 | 543 | 543 | 543 | |||||||||
| 5,450 | 0 | 315 | 315 | 315 | 315 | |||||||||
| 4,934 | 0 | 183 | 183 | 183 | 183 | |||||||||
| 4,554 | 0 | 106 | 106 | 106 | 106 | |||||||||
| 4,255 | 0 | 61 | 61 | 61 | 61 | |||||||||
| 4,007 | 0 | 36 | 36 | 36 | 36 | |||||||||
RESULTS
The breakdown of patients with PCS is as follows; five were supported on ECMO, five were placed on a VAD, and five were placed on a BIVAD. Additionally, two patients died within 1 hour of support initiation and were not included in the analysis.
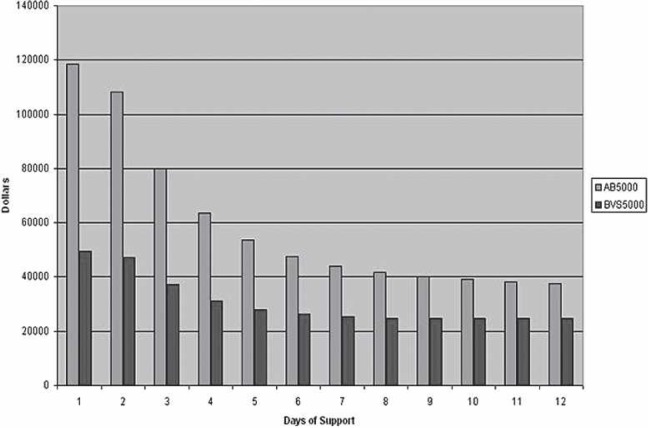
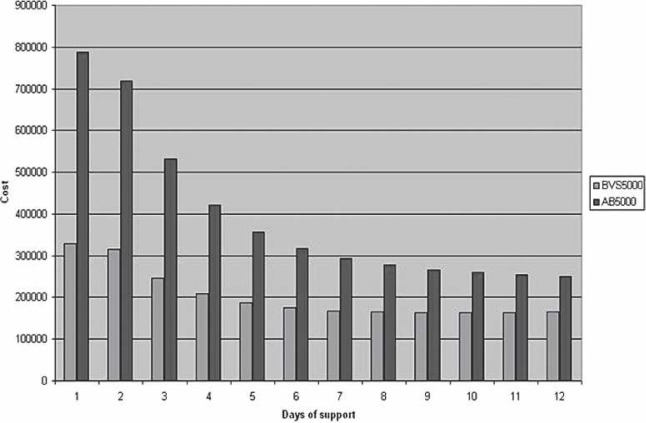
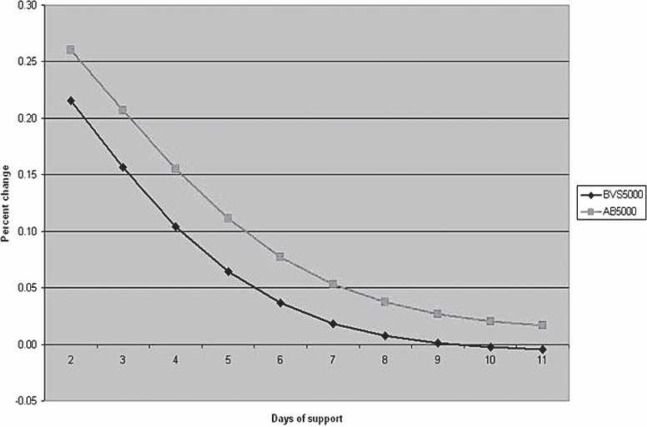
The cost per life and total costs follow a downward trend as the day of VAD initiation is extended postoperatively (Figures 3 and 4). This reduction applies to both the BVS5000 and AB5000 groups. The BVS5000 cost decrease began to level out on day 6 (<10% change/day). Higher costs associated with delayed BVS5000 insertion were seen as day 10 approached. This is represented by the trend line crossing the x-axis (Figure 5). The increase was caused by the labor costs associated with ECMO. When the AB5000 data were analyzed, a leveling of the cost reduction was not seen until day 7 (<10% change/day). The model was not carried out far enough to see an increase in cost from delayed AB5000 insertion. The greatest reduction in per patient and total cost for both the BVS5000 and AB5000 groups was seen by delaying VAD insertion until after days 2 and 3 (Figure 5). By day 3, there was a 42% reduction in total cost for the BVS5000 and a 56% reduction for the AB5000.
Figure 3.
Cost per life saved comparing the BVS5000 and AB5000. The day of support initiation is represented on the x-axis, with the cost on the y-axis.
Figure 4.
Total cost comparing the BVS5000 and AB5000. The day of support initiation is represented on the x-axis, with the cost on the y-axis.
Figure 5.
Percent change in cost per day per life saved comparing the BVS5000 and the AB5000. A negative change represents an increase in cost for that day.
DISCUSSION
PCS is a devastating complication of cardiac surgery. Even with aggressive treatment, our experience with PCS yields a combined wean rate of 41%. This is similar to the 39% wean rate for ECMO and 55% wean rate for VADs reported by Smedira and Blackstone (3). DeRose et al. (4) reported a survival rate of 82% when using the TCI Heart-Mate (Thermo Cardiosystems, Woburn, MA) left VAD, with eight of the nine surviving patients receiving a transplant. Nationally, the Extracorporeal Life Support Organization Registry provided a 33% survival rate for 1994 in patients that require ECMO to be weaned from cardiopulmonary bypass (5). With regards to VADs, the U.S. VAD Registry has reported wean rates of 50% and discharge rates of 25% for patients with PCS (6).
With the exception of DeRose et al. (4), the outcomes using either ECMO or VADs are similar. What is not similar is the cost associated with both treatments. The model that was used in this report shows decreasing costs as the day of VAD initiation is delayed. The leveling out of cost that was seen with the BVS5000 was not seen until further into the model with the AB5000. This suggests that the model is driven primarily by the cost of the devices and not by other factors. In a report by Couper et al. (7), it was found to be more economical for patients to be placed on VADs for long-term support. The costs associated with VAD devices have increased significantly since that time, and even then, the authors also noted that for short-term PCS, the difference was small (7).
Using the cost for the BVS5000, VAD initiation after only 1 day of support on ECMO would cost $47,285 per life saved, whereas initiation on day 6 would decrease the cost to $26,228 per life saved. The treatment algorithm reported by the Cleveland Clinic is conversion from ECMO to an implantable device after 48–72 hours if the patient is an appropriate transplant candidates and myocardial function has not improved (3). Using this model would result in a decrease in the cost per life saved by $12,292 for the BVS5000 and by $38,401 for the AB5000. The use of ECMO as the initial support has an added benefit over VADs because many of these patients’ eligibility for transplantation has not been established (8). By using a less expensive method of support until the patient’s status can be determined, institutions can save hundreds of thousands of dollars annually.
This study does have limitations, such as only including one blood pump per patient, not including the cost and logistics of maintaining an ECMO program, and not including the capital equipment or preventative maintenance costs associated with the consoles. Related cost associated with the use of each device such as blood product use and treatment for complications arising from the individual device were not part of this study but warrants further investigation.
Using a modeling approach, individual centers can reduce PCS costs while taking into account their own survival rates and not those of other larger centers. Magovern and Simpson (9) described the ideal strategy for short-term cardiac assist as combining “low-cost, adaptability to diverse applications and patient requirements, and feature rapid and easy deployment.” ECMO, as the initial support for PCS, meets all of these requirements. The cost savings associated with PCS can vary greatly with the type and length of support. In these times where fiscal responsibility and asset allocation are of the utmost importance, choosing the right support method will allow funds to be directed to other areas of patient care.
ACKNOWLEDGMENTS
The authors thank Kit N. Simpson and Arthur J. Crumbley, MD, for assistance with this project.
REFERENCES
- 1.Muehrcke DD, McCarthy PM, Stewart RW, et al. . Extracorporeal membrane oxygenation for postcardiotomy cardiogenic shock. Ann Thorac Surg. 1996;61:684–91. [DOI] [PubMed] [Google Scholar]
- 2.Doll N, Kiaii B, Borger M, et al. . Fiver year results of 219 consecutive patients treated with extracorporeal membrane oxygenation for refractory postoperative cardiogenic shock. Ann Thorac Surg. 2004;77:151–7. [DOI] [PubMed] [Google Scholar]
- 3.Smedira NG, Blackstone EH.. Postcardiotomy mechanical support: risk factors and outcomes. Ann Thorac Surg. 2001;71:S60–66. [DOI] [PubMed] [Google Scholar]
- 4.De Rose JJ, Umana JP, Argenziano M, et al. . Improved results for postcardiotomy cardiogenic shock with the use of implantable left ventricular assist devices. Ann Thorac Surg. 1997;64:1757–63. [DOI] [PubMed] [Google Scholar]
- 5.Tracy TR, Delosh T, Bartlett RH.. Extracorporeal life support organization 1994. ASAIO Trans. 1994;40:1017–9. [PubMed] [Google Scholar]
- 6.Pae WE, Miller CA, Matthews Y, et al. . Ventricular assist devices for postcardiotomy cardiogenic shock. J Thorac Cardiovasc Surg. 1992;104:541–53. [PubMed] [Google Scholar]
- 7.Couper GS, Dekkers RJ, Adans DH.. The logistics and cost effectiveness of circulatory support: advantages of the abiomed BVS 5000. Ann Thorac Surg. 1999;68:646–9. [DOI] [PubMed] [Google Scholar]
- 8.Smedira NG, Moazami N, Golding CM, et al. . Clinical experience with 202 adults receiving extracorporeal membrane oxygenation for cardiac failure: survival at five years. J Thorac Cardiovasc Surg. 2001;121:92–102. [DOI] [PubMed] [Google Scholar]
- 9.Magovern GJ, Simpson KA.. Extracorporeal membrane oxygenation for adult cardiac support: the Allegheny experience. Ann Thorac Surg. 1999;68:655–61. [DOI] [PubMed] [Google Scholar]